Abstract
An environmentally friendly microextraction method which includes determination of trace levels of rhodamine B in cosmetic and detergent samples by using UV–vis spectrophotometric determination after enrichment with has been established heat-induced homogeneous liquid–liquid microextraction method. As extraction solvent, cyclohexylamine has been used, and the rhodamine B concentration in extraction phase was determined at 550 nm by using UV–vis spectrophotometry. The major parameters influencing in the method including pH, volume of cyclohexylamine, amount of NaCl, sample volume have been assessed. The influences of the matrix components were also investigated. The limit of detection, the limit of quantitation and linear range were found as 0.014, 0.047 and 0.047–4.79 μg mL−1, respectively. The relative standard deviation was 1.03%. The extraction procedure was applied to determination of rhodamine B in the cosmetic and detergent samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Some synthetic coloring dyes caused serious health problems if used differently from the safety conditions specified by the food and agriculture organization and the world health organization. According to the Regulation (EC) No 1223/2009, published in the Official Journal of the European Union, has prohibited the use of 1328 compounds in cosmetic products. Rhodamine B (Rh-B), 9-(2carboxyphenyl)-3,6-bis(diethylamino) xanthylium chloride, is among the oldest and most widely used synthetic dyes identified as additives. Rh-B, a synthetic hydrophilic xanthene dye, is an organic pollutant commonly used as a colorant in the leather, dyeing, paper, plastic and textile industries, foodstuffs, biological studies, analytical chemistry, a pigment in drug and cosmetic preparations. Also used in the food industry as a colorant to increase the visual appeal of the product due to its low cost, Rh-B belongs to the class of xanthan dyes and is highly soluble in water and alcohol. Rh-B is harmful to humans and animals, causing serious damage to skin, eyes and respiratory system. In recent years, it has been proven by research that long-term exposure of Rh-B due to its high toxicity causes various potential disorders such as carcinogenic effect on humans and animals, irritation of eyes and skin. Due to the serious health problems caused by Rh-B, it is desirable to develop simple, highly accurate and reliable methods for determining Rh-B in real samples such as food and cosmetic products with detrimental effects [1,2,3,4,5,6,7,8,9,10,11]..
So far, for the determination of Rh-B, high-performance liquid chromatography (HPLC) [12,13,14], liquid chromatography-mass spectrometry (LC–MS) [15, 16], LC—tandem mass spectrometry [17, 18], voltammetry [19,20,21] and spectrophotometry, fluorescence spectrophotometry [22, 23], and electrokinetic capillary chromatography [24] have been used. Spectrophotometric techniques are a good choice, with wide availability, such as the analysis of organic compounds, including measurements in ultraviolet and visible wavelengths as the most accurate, low cost, most accessible device. Spectrophotometry is a good choice, especially for the evaluation of species by routine laboratories, large peaks overlap and serious analytical problems are solved only by curve analysis techniques, and accordingly such approaches are highly recommended for quantitative analysis of food dyes. Different food matrices due to low instrumentation cost and high molar absorptivity values complementing the ability to work with non-experts.
The sample preparation step in an analytical process typically consists of an extraction procedure resulting in the isolation and enrichment of the respective components from a sample matrix. The extraction can vary in selectivity, speed and degree of suitability and depends not only on the approach and conditions used, but also on the geometric configurations of the extraction step [25,26,27]. Separation-preconcentration methods such as coprecipitation [28], cloud point extraction (CPE) [29,30,31], liquid–liquid extraction (LLE) [32, 33] and solid-phase extraction (SPE) [34,35,36,37] are among the enrichment methods used for the determination and determination of low concentrations of analytes by separating from the complex matrix medium in extraction studies. However, these techniques are time consuming and require the use of large amounts of toxic and hazardous organic solvents. In recent studies, it has been aimed to minimize the disadvantages of traditional approaches with the development of economical and miniaturized sample preparation methods, and to protect the environment against the use of high amounts of solvents and to reduce costs through miniature studies. Therefore, there is a tendency to fast, simple and miniaturize extraction methods by reducing the consumption of organic solvents. In recent years, different liquid-phase microextraction (LPME) methods have been developed with good extraction efficiency. LPME is the preferred enrichment method for separating analytes from the sample matrix with a simple and powerful enrichment method that reduces extraction time, simplicity, low reagent consumption and organic solvent exposure [38, 39]. Therefore, including single drop microextraction, floating organic drop microextraction, homogeneous liquid–liquid extraction and dispersive liquid–liquid microextraction (DLLME) technique are the fundamentals of LLE which get same miniaturized separation-preconcentration techniques have been developed [40,41,42,43,44,45,46,47].
Homogeneous liquid–liquid extraction (HLLE) is a simple and excellent separation-preconcentration method which reduces extraction time, cost, consumption, green and exposure to the organic solvent to bring the desired analyte present in the homogeneous solution to the organic phase immiscible with water by the organic phase separation phenomenon [48,49,50,51,52]. The HLLE method is simple and rapid, and only the reagent needs to be added. In HLLE during the extraction procedure as compared to LLE and DLLME, there is no interface between the water phase and the organic phase water-immiscible, namely before phase separation the surface area of the interface is extremely large [52,53,54,55,56]. Therefore, no vigorous shaking is compulsory and provides the advantage of rapid extraction by facilitating mass transfer [56,57,58,59,60].
The main aim of this study is to develop a new sample preparation method by applying the heat-induced HLLME method to determine rhodamine B in real samples. Different analytical parameters were studied and optimized to achieve high extraction efficiency of Rh-B and analyzed using a UV–vis spectrophotometer.
Experimental
Instrumentation
The instrumental detection system used Hitachi model UH 5300 spectrophotometer was utilized for the evaluation of under study of Rh-B content in extraction solvent phase. A centrifuge Hettich Rotofix 32 model centrifuge (Buckinghamshire, England) was performed using for complete phase separation. Additionally, the pH meter with Nel pH 900 (Ankara-Turkey) supplied with a combined glass-electrode was used for the pH measurements in the aqueous phase.
Chemicals and reagents
All chemicals and analytical reagents used were of analytical grade in this work. Before the experimental parameters began, the stock solution of Rh-B had been prepared the dissolving by appropriate amount of Rh-B in ethanol. The working solutions were obtained by appropriate diluting of stock solution. The buffer solutions given in the literature were used in the presented work. Cyclohexylamine was obtained from Merck (S21253 729 Merck-Schuchardt, Germany) and except if otherwise stated, analytical-grade sodium chloride and other chemicals used in this study were obtained from Merck (Darmstadt, Germany). All the experiments were performed using ultrapure water system (Bedford, MA, USA), Resistivity 18.2 MΩ cm−1).
Homogeneous liquid-phase microextraction procedure
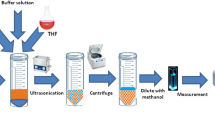
Figure 1 offers a scheme for the extraction method of Rh-B by the improved methodology. In the present study for Rh-B preconcentration, an aliquot of 10 mL aqueous solution containing 0.9 μg mL−1 Rh-B of that adjusted with appropriate volume of desired buffer was placed in a 50 mL centrifuge tube. After adjusting the pH 11.0, then, 3.0 g of NaCl salt was added into to a solution containing the Rh-B, and the solution was shaken for 10 s to obtain a homogeneous solution. After shaking the centrifuge tube, 350 µL of cyclohexylamine was added to the tube and immersed in a heated ultrasonic water bath for 8 min. at 40 ◦C. From the homogeneous solution, a cloudy solution was conducted so that Rh-B was extracted into the extraction phase. This cloudy solution was subjected centrifugation for 10 min at 4000 rpm so that, fine droplets the dispersed was accumulated in the upper phase containing the cyclohexylamine. Then, aqueous phase was carefully removed by a syringe and the total volume of the extraction phase was adjusted up to 1 mL with ethanol. Rh-B content in the final solution was determined by UV–vis spectrophotometer at 550 nm.
Applications
The developed method was applied to 2 pieces of lipstick, 1 bottle of acetone (nail polish remover), 1 bottle of cologne and 1 glass cleaner solution from Kayseri markets. Preparation of lipstick samples; 10 mL of ethyl alcohol was added to the samples, taken from 0.1 g of lipstick samples. The mixture was vibrated in the ultrasonic bath for about 30 min and shaken in the rinse bath for 1 h [50]. This mixture was then centrifuged to separate between the insoluble material and the Rh-B in ethyl alcohol. The insoluble material will appear at the bottom, and Rh-B solution will be on the top. 0.1 mL was taken from this solution, and the method was applied. 10 mL of cologne and 10 mL of nail polish remover solutions were taken, and the acetone and the alcohol in these solutions were removed in a hot water bath (~ 50 °C). The obtained volume from each sample was approximately 0.5 mL and was filled up until 10 mL with ethyl alcohol and water. Then, the microextraction method applied in 1 mL to each sample [10].
Results and discussion
The presented method is based on the use of a water-soluble organic solvent with low dielectric constant, such as cyclohexylamine, to obtain a homogeneous solution. Cyclohexylamine dissolves here at low temperatures. Afterward, when the solution is heated, its solubility decreases, resulting in a two-phase system as extraction solvent, and is distributed to all parts of the solution. At this stage, rhodamine B is extracted into the extraction solvent and determined by using UV–vis spectrophotometer.
The recovery values calculated by using
formulae. In the formulae, Cext and Cref are concentration of rhodamine B in final solution, and concentration of rhodamine B in reference solution, respectively.
Effect of pH
The effect of the pH of the sample solution on extraction efficiency was investigated by preconcentration of Rh-B with liquid-phase microextraction method. The developed method to model solutions ranging from pH 2.0–12.0 was applied. The change in recovery efficiency with pH is given in Fig. 2. As the pH value shifted from the acidic region to the base region, it was observed that the extraction efficiency increased and the quantitative recovery of pH 10.0–12.0 was obtained. As working pH for further works, pH11.0 was selected as 11.0 with the buffer solution. The quantitative recoveries of rhodamine B under alkaline conditions may be related to structure of cyclohexylamine as extraction solvent.
Optimization of salt amount
Cyclohexylamine, which is soluble in the aqueous medium at room temperature, is converted to nonpolar form and thus forms an extraction phase by changing the solution temperature and the mediums ionic strength. In this study, NaCl salt was used to adjust the ionic strength. In 10 ml model solution that contains 1.0–5.0 g of NaCl salt was used to the developed method. In low salt addition (1 g), it was observed that the phases of nonpolar extraction solvent did not occur. Therefore, increasing the amount of additional salt was necessary to obtain significant extraction phases. It was observed that an effective extraction phase was formed in 2.0, 3.0 and 4.0 g salt additions, and the quantitative results for rhodamine B were obtained (Fig. 3). According to the results, optimum amount of NaCl was chosen as 3.0 g. It is shown that the salt is necessary for the quantitative recoveries of analyte. The recoveries of rhodamine B were not quantitative without and too low salt concentrations. Also, the recoveries were decreased and not quantitative after 4.0 g of NaCl may be due to high ionic strength of the working media.
Effect of temperature
When a solvent is added to the solution medium, the kinetic energy of the solvent molecules and the interactions between the solute and the solvent molecules influence the attractive forces between the soluble particles. If the solvent is heated, the average kinetic energy of its molecules increases and it causes form more micelles. Increasing the temperature usually increases the solubility of the substances. As temperature increases, the solubility of cyclohexylamine decreases in water, which is not very common. The interaction between cyclohexylamine and water molecules is affected by hydrogen bonds. At high temperatures, hydrogen bonds were weakened, and therefore, making the solubility of cyclohexylamine in water decreases at high temperatures compared to low temperatures. The effect of temperature on the formation of nonpolar cyclohexylamine phase and extraction efficiency was studied. The effect of the sample solution temperature on the extraction efficiency was studied in 40–80 °C range, and the results are shown in Fig. 4. Further studies were performed at 40 °C to improve the proposed method.
Effect of cyclohexylamine volume
For Rh-B extraction, 3 parallel model solutions that have been prepared then added cyclohexylamine in volumes ranging from 200–500 µL and the method was applied. The results are given in Fig. 5. The quantitative results are obtained in 300–500 µL cyclohexylamine. The cyclohexylamine volume is chosen as 350 µL for further works.
Effect of sample volume
The developed method was applied to the model solutions varying in volumes, and the recovery values were calculated from the obtained results. Based on the observation, the recovery values of 10 mL sample volume were quantitative, but at higher volumes, the recovery values were not quantitative. As the results, the preconcentration factor was obtained as 10 when the sample volume was 10 mL and the final volume was 1 mL.
Matrix effects
In the separation-preconcentration techniques, the possible interfere effects of the matrix components of real samples should be investigated prior to their instrumental detections [61,62,63,64,65,66,67]. In the developed homogeneous liquid–liquid microextraction method, some ions and dyes in different concentrations that may have a disruptive effect were added to the model solutions containing Rh-B and their effects on the method were examined. The recovery values obtained after applying the method are given in Table 1. At the values given in Table 1, it was observed that Rhodamine B was quantitatively recovered in the presence of matrix species in the homogeneous liquid–liquid extraction method.
Analytical performance
The microextraction method developed under optimum conditions was applied to the containing solutions Rh-B concentration in the final volume was determined by UV–vis spectrophotometer. The calibration curve obtained for the method is y = 0.0214 + 0.1476x (y = means the absorbance, x = concentration of Rh-B) with a regression coefficient of 0.996 (r2).
The developed method was applied to 10 parallel blind samples to determine the limit of detection (LOD) and limit of quantification (LOQ). The value of the detection limit of the method calculated by dividing the 3 times (3 s) of the standard deviation of the absorbance values obtained from blank analyzes by the slope of the calibration curve (m) and the limit of quantification was also calculated by dividing the 10 times (10 s) of the standard deviation of the absorbance values obtained from the blind analyzes by the slope of the calibration line (m). The limit of detection, the limit of quantitation and linear range were found as 0.014 , 0.047 and 0.047–4.79 μg mL−1, respectively. The relative standard deviation (RSD) was calculated to be 1.03% (N = 7). The preconcentration factor was calculated by calculating the ratio of the high model solution volume (10 mL) to the final volume (1 mL), which was found to be 10.
Applications
In order to test the accuracy of the method that we applied, analytes were added to the real samples and their recoverability was evaluated. The method was applied with 3 parallel of each sample, and the final volume of Rh-B concentration was measured by UV–vis spectrophotometer. The results are given in Table 2. Quantitative recovery values are obtained for all additions. The results show that the liquid-phase microextraction method that we applied is working quantitatively in matrix mediums.
Conclusion
In the present work, with a facile and competent version of (CHA-based) liquid–liquid microextraction based on heat-induced homogeneous liquid-phase microextraction method was proposed for separation and preconcentration of Rh-B in cosmetic products and cleaning products in advance being analyzed by UV–vis spectrophotometry. It is possible to analysis by use of UV–vis spectrophotometer, which is cheap and easy to use instead of expensive instruments such as HPLC, LC–MS, which are used in the determination of Rh-B. This method is environmentally sensitive due to even though the use of organic solvents cannot be completely eliminated, and it is simple and fast and it has a low cost and short extraction period. The possible matrix effects were investigated in the method, and no significant interference was not found. HLPME method and UV–vis spectrophotometry analysis combination indicate comparison of other literature methods in terms of analytical performance parameters and applicability that our method is simple, inexpensive and has comparable detection limit, extraction time, repeatability (RSD, %) and fast and that can be successfully applied for separation, preconcentration and determination of the Rh-B in different real samples (Table 3). The main advantages of the method provide accurate analysis, high reproducibility and high extraction efficiency by using a low amount of samples. Also, we have some separation and preconcentration procedure including solid-phase extraction [10] and dispersive liquid–liquid microextraction [68] for traces Rhodamine B in different matrices prior to its spectrophotometric detection. The presented procedure is easier and faster than both these procedures [10, 68].
References
R. Ji, Z. Zhao, X. Yu, M. Chen, Optik (Stuttg). 181, 796–801 (2019)
M. Wang, X. Nie, L. Tian, J. Hu, D. Yin, H. Qiao, T. Li, Y. Li, Food Addit. Contam. B. 12, 59–64 (2019)
X. Zhu, G. Wu, C. Wang, D. Zhang, X. Yuan, Measurement 120, 206–212 (2018)
T. Zhang, J. Huang, J. Taiwan Inst. Chem. Eng. 80, 293–300 (2017)
M.-F. Hou, C.-X. Ma, W.-D. Zhang, X.-Y. Tang, Y.-N. Fan, H.-F. Wan, J. Hazard. Mater. 186, 1118–1123 (2011)
R. Khani, S. Sobhani, T. Yari, Microchem. J. 146, 471–478 (2019)
M.R. Khan, J.M. Khan, A.A. Alqadami, Spectrochim. Acta A 206, 72–77 (2019)
T.A. Khan, S. Dahiya, I. Ali, Appl. Clay Sci. 69, 58–66 (2012)
H. Li, N. Li, J. Jiang, D. Chen, Q. Xu, H. Li, J. He, J. Lu, Sensors Actuators B Chem. 246, 286–292 (2017)
M. Soylak, Y.E. Unsal, E. Yilmaz, M. Tuzen, Food Chem. Toxicol. 49, 1796–1799 (2011)
N. Xiao, J. Deng, K. Huang, S. Ju, C. Hu, J. Liang, Spectrochim. Acta A 128, 312–318 (2014)
J. Chen, X. Zhu, Food Chem. 200, 10–15 (2016)
T. Chiang, Y. Wang, W. Ding, J. Chinese Chem. Soc. 59, 515–519 (2012)
X. Xu, M. Zhang, L. Wang, S. Zhang, M. Liu, N. Long, X. Qi, Z. Cui, L. Zhang, Food Anal. Methods 9, 1696–1705 (2016)
Y.-Y. Cheng, T.-H. Tsai, J. Pharm. Biomed. Anal. 125, 394–399 (2016)
A. Müller, S.C. Weiss, W. Schulz, W. Seitz, R. Albert, W.K.L. Ruck, W.H. Weber, Rapid Commun. Mass Spectrom. 24, 659–666 (2010)
J. Li, X. Ding, J. Zheng, D. Liu, F. Guo, H. Liu, Y. Zhang, J. Sep. Sci. 37, 2439–2445 (2014)
J. Li, X.-M. Ding, D.-D. Liu, F. Guo, Y. Chen, Y.-B. Zhang, H.-M. Liu, J. Chromatogr. B 942, 46–52 (2013)
G. Koelbl, K. Kalcher, A. Voulgaropoulos, Fresenius J. Anal. Chem. 342, 83–86 (1992)
H.T. Ngo, V.T. Nguyen, T.D. Manh, T.T.T. Toan, N.T.M. Triet, N.T. Binh, N.T.V. Hoan, T.V. Thien, D.Q. Khieu, J. Nanomater. 2020, 4679061 (2020)
M. Golestaneh, S.M. Ghoreishi, Anal. Bioanal. Electrochem. 12, 81–92 (2020)
M. Alesso, G. Bondioli, M.C. Talío, M.O. Luconi, L.P. Fernández, Food Chem. 134, 513–517 (2012)
A.A.A. Bakheet, X. Shi Zhu, J. Fluoresc. 27, 1087–1094 (2017)
M. Gładysz, M. Król, K. Mystek, P. Kościelniak, Forensic Sci. Int. 299, 49–58 (2019)
H. Birtane, O.A. Urucu, N. Yıldız, A.B. Çiğil, M.V. Kahraman, Mater. Today Commun. 30, 103144 (2022)
A. Alham, A. Ibraimov, M. Alimzhanova, M. Mamedova, Food Anal. Methods 15, 707–716 (2022)
M. Soylak, H.H. Gorucu, E. Yilmaz, EuroBiotech. J. 4, 89–96 (2020)
S. Kagaya, Y. Araki, N. Hirai, K. Hasegawa, Talanta 67, 90–97 (2005)
A. Karatepe, C. Akalin, M. Soylak, Turk. J. Chem. 41, 256–262 (2017)
H. Filik, Z. Yanaz, R. Apak, Anal. Chim. Acta 620, 27–33 (2008)
H. Filik, I. Sener, S.D. Cekic, E. Kilic, R. Apak, Chem. Pharm. Bull. 54, 891–896 (2006)
S. Cerutti, M.F. Silva, J.A. Gasquez, R.A. Olsina, L.D. Martinez, Spectrochim. Acta B 58, 43–50 (2003)
J. Wang, E.H. Hansen, Anal. Chim. Acta. 456, 283–292 (2002)
Z.T. Babaei, A. Larki, K. Ghanemi, J. Iran Chem. Soc. 19, 95–107 (2022)
A.B. Çiğil, O.A. Urucu, H. Birtane, M.V. Kahraman, Cellulose 28, 6439–6448 (2021)
S.M. Turp, N. Ekinci, Fresen. Environ. Bull. 30, 13058–13071 (2021)
M. Soylak, U. Sahin, L. Elci, Anal. Chim. Acta. 322, 111–115 (1996)
M. Rezaee, Y. Assadi, M.R.M. Hosseini, E. Aghaee, F. Ahmadi, S. Berijani, J. Chromatogr. A. 1116, 1–9 (2006)
S. Dadfarnia, A.M. Salmanzadeh, A.M.H. Shabani, Anal. Chim. Acta. 623, 163–167 (2008)
N. Altunay, A. Elik, A. Demirbas, S. Kaya, M.M. Maslov, J. Food Compos. Anal. 102, 104042 (2021)
M.R.K. Zanjani, Y. Yamini, S. Shariati, J.Å. Jönsson, Anal. Chim. Acta. 585, 286–293 (2007)
E.Z. Jahromi, A. Bidari, Y. Assadi, M.R.M. Hosseini, M.R. Jamali, Anal. Chim. Acta. 585, 305–311 (2007)
A.R. Ghiasvand, S. Shadabi, E. Mohagheghzadeh, P. Hashemi, Talanta 66, 912–916 (2005)
F. Ahmadi, Y. Assadi, S.M.R.M. Hosseini, M. Rezaee, J. Chromatogr. A 1101, 307–312 (2006)
A. Bidari, E.Z. Jahromi, Y. Assadi, M.R.M. Hosseini, Microchem. J. 87, 6–12 (2007)
G. Ozzeybek, T. Borahan, M. Nesterkina, I. Kravchenko, S. Bakırdere, Anal. Lett. 54, 2376–2386 (2021)
T. Shahryari, P. Singh, P. Raizada, A. Davidyants, L. Thangavelu, S. Sivamani, A. Naseri, F. Vahidipour, A. Ivanets, A. Hosseini-Bandegharaei, Colloids Surf. A 641, 128528 (2022)
L. Tavakoli, Y. Yamini, H. Ebrahimzadeh, S. Shariati, J. Chromatogr. A 1196, 133–138 (2008)
S.D. Abkenar, Z. Dahaghin, H.B. Sadeghi, M. Hosseini, M. Salavati-Niasari, J. Anal. Chem. 66, 612–617 (2011)
N. Baroumand, A. Akbari, M. Shirani, Z. Shokri, Water Air Soil Pollut. 226, 2254 (2015)
M.A. Farajzadeh, M. Bahram, S. Zorita, B.G. Mehr, J. Hazard. Mater. 161, 1535–1543 (2009)
M.A. Farajzadeh, A.S. Hojaghan, M.R.A. Mogaddam, Food Anal. Methods 10, 3738–3746 (2017)
N. Sheijooni-Fumani, J. Hassan, S.R. Yousefi, J. Sep. Sci. 34, 1333–1337 (2011)
N. Yazdanfar, Y. Yamini, M. Ghambarian, Chromatographia 77, 329–336 (2014)
M.R. Jamali, Y. Assadi, R.R. Kozani, F. Shemirani, E-Journal Chem. 6, 1077–1084 (2009)
X. Wang, X. Zhao, X. Liu, Y. Li, L. Fu, J. Hu, C. Huang, Anal. Chim. Acta. 620, 162–169 (2008)
S.M. Sorouraddin, M.A. Farajzadeh, T. Okhravi, J. Iran Chem. Soc. 16, 1537–1543 (2019)
N. Ozkantar, E. Yilmaz, M. Soylak, M. Tuzen, Int. J. Environ. An. Ch. 99, 1135–1147 (2019)
S.A. Anvar, M. Torbati, M.A. Farajzadeh, M.R.A. Mogaddam, Food Anal. Methods 13, 1282–1291 (2020)
S.M. Sorouraddin, M.A. Farajzadeh, T. Okhravi, Talanta 175, 359–365 (2017)
A. Karatepe, C. Akalin, M. Soylak, Desalin. Water Treat. 57, 25822–25829 (2016)
Z. Li, D. Wang, F. Lv, J. Chen, C. Wu, Y. Li, J. Shen, Y. Li, Materials 15, 970 (2022)
S. Saracoglu, M. Soylak, L. Elci, Anal. Lett. 35, 1519–1530 (2002)
H. Filik, D. Giray, B. Ceylan, R. Apak, Talanta 85, 1818–1824 (2011)
S. Saracoglu, M. Soylak, L. Elci, Trace Elem. Electroly. 18, 129–133 (2001)
A.T. Biskin, I. Narin, M. Ucan, M. Soylak, Oxid. Commun. 38, 232–240 (2015)
N. Pourreza, S. Rastegarzadeh, A. Larki, Talanta 77, 733–736 (2008)
Y.E. Unsal, M. Tuzen, M. Soylak, Desalin. Water Treat. 55, 2103–2108 (2015)
M. Taziki, F. Shemirani, B. Majidi, Sep. Purif. Technol. 97, 216–220 (2012)
H. Bagheri, R. Daliri, A. Roostaie, Anal. Chim. Acta. 794, 38–46 (2013)
L. Yu, Y. Mao, L. Qu, Food Anal. Methods 6, 1665–1670 (2013)
C.C. Wang, A.N. Masi, L. Fernández, Talanta 75, 135–140 (2008)
Acknowledgements
The authors are grateful for the financial support of the Unit of the Scientific Research Projects of Erciyes University (TDK-2018-8318). The authors also thank to Erciyes University Technology Research and Application Center (ERU-TAUM) for instrumental facilities. Dr. Mustafa Soylak thanks to Turkish Academy of Sciences for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is a part of PhD thesis of Zeliha Erbas.
Rights and permissions
About this article
Cite this article
Erbas, Z., Soylak, M. Determination of Rhodamine B by UV–Vis spectrophotometry in cosmetics after microextraction by using heat-induced homogeneous liquid–liquid extraction method. J IRAN CHEM SOC 19, 3935–3942 (2022). https://doi.org/10.1007/s13738-022-02579-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02579-8