Abstract
The imidazolinium chloride salts have improved as a potential new material for a wide variety of industrial operations, due to their exceptional structures and properties. Therefore, recognition of the surface proprieties and thermodynamic criteria of this product is extremely important for fundamental and industrial operations. In this investigation a mixture of fatty imidazolinium chlorides was synthesized dealing with a novel method using sunflower oil as a promoter of fatty acids. The reaction of ethane-1,2 diamine with a fatty acid yields a fatty imidazoline molecule. Hydrochloric acid was used to obtain fatty imidazolinium hydrochlorides with cationic surfactant properties. A FT-IR and NMR spectroscopic technique was using to establish the synthetic structure. The surface tension and conductivity as a function of the surfactant concentration in aqueous solution were measured at distinct temperatures, to figure out the micellization, adsorption and aggregation characteristics in aqueous solutions. Formerly the effect of temperature on the CMC was investigated. The HLB values were assumed for our surfactant and the results achieved indicate that it is O/W emulsifier. The Krafft point was identified at 288.45 K. All Surface adsorption parameters like, the maximum surface excess concentration (Γmax), minimum area per molecule (Amin), effectiveness (πCMC), and efficiency of surface tension reduction (pC20) were calculated using Gibb’s equation. Finally, an attentive study of the thermodynamic aspects of air-ionic surfactant aqueous system reveals that the process of both micellization and adsorbtion was spontaneous and that adsorption is more favored than micellization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are organic molecules, having a surface activity owing to their amphiphilic structure. This type of molecule contains a hydrophilic polar head part and a nonpolar hydrophobic part. The duality polar and nonpolar, which can give the surfactant of molecules their capacity to adsorb on the interface and to make micellar aggregate in the bulk solution. For their remarkable ability to influence the bulk and interfacial properties, surfactants are employed in a very large number of areas.
Cationic surfactants represent an important class of surface-active agents that has diverse operations in various industries [1,2,3]. Fatty imidazolinium surfactants are particularly used as an ingredient in several products, like cosmetics, corrosion inhibitors, drug excipients and pesticide formulations [4, 5].
Surfactants are used in industrial applications and are widely made up by mixtures [6]. These kinds of products are less expensive and offer many cases of uncommon behavior starting from pure surfactants. Their own superior surface-active properties than surfactants taken individually, which is earned to the so-called synergism [7, 8].
It is to highlight that a mixture of fatty acid based on surfactants has been previously synthesized. These are the salts of fatty imidazoline, which consists of a long fatty acid chain as the nonpolar part, while an imidazolinium group as the polar head. The imidazolinium chloride is a cationic surfactant which was already synthesized by various methods [9,10,11]. Several works deal with the synthesis of fatty imidazolinium based surfactants and studied their surface activity [9, 10, 12]. The method we propose in this paper is original. It is based on the use of a natural product which is Sunflower oil. The analysis we carried out on the Sunflower oil showed that it consists essentially of four fatty acids, linoleic, stearic, meristic and palmitic acids. Fatty acids are employed as precursors of these surfactant compounds. Indeed, the reaction of ethane-1,2 diamine with a fatty acid gives an imidazoline. The reaction of hydrochloric acid with imidazoline gives a hydrochloride imidazolinium, which presents surface properties.
The physicochemical properties of the surfactant mixture solution were studied at a different range of temperatures. The surface properties of this cationic surfactant mixture were investigated employing both tensiometric and conductometric techniques [13].
The purpose of this work is to report an alternative synthesis method of cationic surfactant mixture derived from Sunflower oil and its characterization as well as the determination of the surface properties and thermodynamic parameters of adsorption and micellization.
Experimental
Materials
In this study, the chemical reagent such as sodium hydroxide (mass fraction purity 99%), sulfuric acid (mass fraction purity 98%), ethanol (mass fraction purity 95%), ethylenediamine (mass fraction purity 99%), silica gel 60 (grade for column chromatography, particle size ≤ 0.063 mm), chloroform (mass fraction purity ≥ 99%), sodium lauryl sulfate solution (mass fraction purity ≥ 98%), sodium sulfate (mass fraction purity 0.995), benzethonium chloride (hyamine solution, mass fraction purity ≥ 98%) and methylene blue (mass fraction purity ≥ 97%). The solution used in sample processing was all purchased from Sigma-Aldrich. Deionized and distilled water is used with a surface tension of 71.5 mN m−1 at 293 K, while Sunflower oil was supplied by the Algerian Cevital group.
Synthesis route
Hydrolysis of sunflower oil
-
To extract the mixture of fatty acids from Sunflower oil, an amount of 10 g of Sunflower oil was mixed first with sodium hydroxide (20% by weight) in ethanol as solvent. Secondly, heating the solution in a double boiler at 318.15 K during 120 min, throughout the half period of warming, ultra-pure water has been adding drop to drop until the product becomes a homogeneous mixture. Thirdly, once the mixture was lukewarm (air-cooled), a volume of hydrochloric acid solution (15 mL with 37% by weight) was added continuously with stirring for 60 min. When the operation was finished, the mixture was detached into a separating funnel allowed to temper at room temperature for phases separations. Two phases appeared. The organic phase was cutoff and washed by bidistilled water to remove the excess acid and salts. Finally, we dried the output under vacuum at 313.15 K for 24 h.
-
The last step consists of analyses the extract in gas chromatography to determinate the composition of the fatty acids which are: linoleic acid (70%), stearic acid (16%), palmitic acid (10%) and oleic acid (4%). The molecular weight average of the extracted fatty acids was determined from the ratio achieved for these acids was (278,64 g/mol) [14, 15].
-
To determine the acid value of fatty acids, an amount of 2 g of the mixture was replied in a flask with 25 mL of hot ethanol, followed by five drops of phenolphthalein as an indicator solution. The last step consists to heat the mixture until boiling for three minutes, kept by titration with sodium hydroxide solution (1 N).
The acid value was calculated with the following equation [16].
when V is the volume of the sodium hydroxide solution, N the normality and Pe represent the Sunflower oil-weighted.
The values obtained for the fatty acid mixture were reported in Table 1.
An amount of 2 g of fatty acids was employed to establish saponification value, was mixed into 25 mL of alcoholic hydroxide potassium (KOH) and heated on an oil bath during 60 min, until the absence of any oily matter and appearance of a clear solution, which indicates that all the fatty matter were saponified. After cooling, the condenser was cleaned with a hot ethanol solution. The product collected was titrated with hydrochloric acid (1 N), it should be noted that the phenolphthalein was used as an indicator [16].
Using the following relationship, the saponification value was calculated:
V1 and V2 are the HCl volumes in mL required for the blank and the sample, respectively.
Table 1 summarized the acid and saponification values got for the mixture of acids extracted from Sunflower oil.
Preparation of quaternary imidazolinium
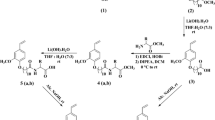
The synthesis of the imidazolinium surfactant involves two steps. The first step (Scheme 1) consists on the synthesis of a fatty imidazoline. In the purpose to obtain stable fatty imidazoline observe a new heterocyclization reaction. For this in a two-necked flask, an amount of 0.1 mol of the fatty acid mixture was dissolved in o-xylene via a dropping funnel, a volume of 4 mL of pure ethylenediamine (EDA 99%) was added slowly during. The reaction mixture was heated (at 404.15 K) under stirring for 2 h. It was refluxed with a continuous separation by a dean-Stark trap [17]. Afterward the product was dried over an anhydrous magnesium sulfate (MgSO4), followed by decantation in ethylene ether [18]. After the finished filtration step, a white to yellowish-brown substance was obtained which has an aspect of paste. It was noted that the fatty imidazoline presents a yield of almost 90%. Scheme 1 shows the synthesis path of the imidazoline mixture.
The second step of synthesis comprises the preparation of 2-alkyl imidazolinium (scheme 2). Nitrogen heterocyclic of formula C3H6N2 (2-alkylimidazoline) was used for this synthesis. Firstly, an amount of 10 g of the heterocyclic was dissolved in dichloromethane, followed by chlorination (scheme 2) to bubble the Hydrochloric acid through the mixture at room temperature for 3 h. Finally, the solvent was removed via vacuum evaporation and the product dried under a high vacuum at 323.15 K to get a pale white solid. In Table 2 we reported the synthesized surfactant along with their physical properties.
Characterization of the synthesized surfactant
The structures of fatty imidazolinium mixture were characterized using Fourier transform infrared spectrum (FT-IR) and nuclear magnetic resonance (NMR of 1H, 13C, and 2D with two methods heteronuclear single quantum correlation (HSQC) and Heteronuclear Multiple Bond Connectivity (HMBC).
FT-IR spectra (KBr) were acquired by a Bruker Alpha-p spectrophotometer using the KBr disk method, while the NMR spectra were recorded using a Bruker Avance 300 spectrometer operating at 300 MHz using deuterated chloroform (CDCl3) as a solvent, with Tetramethylsilane (TMS) as the internal standard. Melting points were determined utilizing the BUCHI M-560/565 Melting Point Apparatus.
Hydrophilic–lipophilic balance (HLB)
The physico-chemical properties of ionic surfactants are intimately associated with the balance between the polarity of the head group and non polarity of the hydrocarbon chain. This property is closely affected by various structural factors such as chain length, structure of the hydrophobic group, nature of hydrophilic group and its position in the molecule. The changes can be described and explained in terms of the hydrophilic-lipophilic balance (HLB). Its knowledge facilitates the choice of the best surfactant in such processes as detergents, emulsification, flotation, flocculation, foam separation and corrosion inhibition. It is also useful in interpretation of interfacial phenomena as the reduction in surface tension, adsorption, micellization, etc. HLB value of FICM was calculated by applying the Davies’ equation and given in Table 2 [19].
Krafft temperature
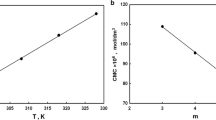
For the speak of the Krafft temperature (TK) in pure water, a saturated aqueous solution of FICM was stored in the refrigerator at 273.15 K for 24 h until the measurement was carried out. The system was then taken out of the refrigerator, followed by raising gradually the temperature of the precipitated system under constant agitation. The electrical conductivity was registered at every 2 K on a Tacussel CD 61 conductimeter using a platinum electrode with a cell constant of 62.82 m−1 (The cell constant was calibrated using KCl solution). The accuracy of the conductance measured was less than 0.01 μS/m (Fig. 1).
Surface tension measurements
Air–water interfacial tension was carried out through a surface tension meter (Gibertini Elettronica™ TSD), via the Wilhelmy plate technique with a polished platinum plate as sensors.
The tensiometer was calibrated with certified weights (0.4 g). The platinum ring was carefully washed with acetone, and flame dried before any measurement. Each concentration was prepared at least one day preceding the measure. After each dilution, the solution allowing 15 min for equilibration than the surface tension was measured. The experimentations were triplicated for reproducibility purposes. The (γ) values are accurate within ± 0.1 mNm−1. All the measurements were registered in the temperature interval of 293 to 318 K.
The surface tension of fatty imidazolinium chloride mixture (FICM) solutions was registered at various concentrations with ultrapure water and was accomplished at diverse temperatures 293, 298, 303, 308, 313 and 318 Kelvin (K).
Conductivity measurements
The electrical conductivity was registered on a Tacussel CD 61 conductimeter using a platinum electrode with a cell constant of 62.82 m−1 (the cell constant was calibrated by using KCl solution). The electrical conductivity of FICM solutions was determined at various concentrations and carried at a different temperature from 293 to 318 K. The conductivity assessed was correct to within 0.01 μS/m.
Foaming property
The foamability or foaming power of a surfactant is the surfactant ability to form foam. The Ross-Miles method measured the foaming properties of the imidazolinium chloride mixture synthesized at a temperature of 298.15 K as described by Wang and al [3]. A volume of 50 mL surfactant solution (0.1 M) was placed in the bottom of a flask and another volume of 200 mL of the same solution was placed in a funnel poured into the flask from the top. Once the solution had run out of the funnel, the volume was recorded at 30 s. The height of the foam was taken after 30 s for the determination of the foaming ability of the surfactants. The height of the foam formed is a measure of the foamability of the solution at the temperature of the experiment.
Results and discussion
Characterization
The FT-IR spectra of the synthesized product confirmed the formation of imidazoline ring characterized by the presence of the bands at 1650.05 cm−1 C=N stretching and at 3290 cm−1 for secondary amine stretching (N–H) of the N–C=N groupe. (Figure 2a). The absorption bands were seen at 2863, 2922, 3290, 1550 cm−1 and 724 cm−1 confirm the presence of aliphatic –C–H symmetric and asymmetric (CH3, CH) stretching,
While, in Fig. 2b the presence of some characteristic functional groups belongs to the formed cationic surfactants and disappearance some functional groups belongs to the reactants, For example, at bands 3301 cm−1 belong to (N–H) stretching (secondary amine) and 1638 cm−1 (C–N+–C) stretching (imidazolinium ring).
Whereas, the nuclear magnetic resonance (NMR) confirmed our interpretation [20]. Figure 3a shows the 1H NMR spectrum (300 MHz, Chloroform-d) of imidazoline.
1H NMR (300 MHz, Chloroform-d) δ (ppm): δ = 0.75–1.03 (m, 3H, CH3), δ = 1.19–1.43 (m, 26H, CH3-(CH2)n), δ = 1.52–1.66 (t, J = 7.4 Hz, 2H, CH3–CH2 –(CH2)n), δ = 2.33–2.42 (s, 2H, C-CH2-(CH2)n), δ = 3.25–3.40 (m, 2H, N–CH2–CH2–NH), 3.40–3.58 (m, 2H, N–CH2–CH2–NH), δ = 6.38–6.47 (s, 1H,–CH2–NH–C).
Figure 3b presents the 13C NMR spectrum (75 MHz, Chloroform-d) of imidazoline [20].
13C NMR (75 MHz, Chloroform-d) δ (ppm): δ = 14.01–14.22, CH3, δ = 25.59–25.95, CH3–CH2–(CH2)n, δ = 27.10–31.60 CH3-(CH2)n, 39.90–40.28, N–CH2–CH2–NH, 41.35–41.63, N–CH2–CH2–NH, 174.49–174.73, N–C = NH.
Some additional NMR techniques were used for further characterization, HSQC (a 2D-NMR method, used to detect 1-bond heteronuclear couplings, 1H to 13C) and HMBC (used to detect 2–3-bond heteronuclear couplings, 1H to 13C). This made a total structural characterization of the imidazoline mixture.
The bidimensional HSQC NMR spectrum was acquired to accurately assess the molecular structure of the fatty imidazoline mixture. In the 2D-NMR HSQC spectra, 1H signals are reported on the abscissa, while 13C ones on the ordinate axis. In Fig. 3c, methyl groups were reported in, at δC 14.1. The CH3 terminal groups generate a single signal due to equivalents carbons, which correspond to two triplets at δH 0.87 and δC 27.77–29.75. The typical signals of CH2 groups of both the main alkyl chains are visible, which corresponding to multiples at δH 0.75–1.03 on the 1H axis, at δC 40.09–41.49 in the typical region of CH2 of the imidazoline ring which corresponding to multiples at δH 3.25–3.40 on the 1H axis.
In the 2D- NMR HMBC spectra (Fig. 3d), 1H signals are reported on the abscissa, while 13C ones on the ordinate axis. For fatty imidazoline mixture C-2 (δ = 174.49–174.73 ppm), C-4 (δ = 39.90–40.28) ppm), C-5 (δ = 41.35–41.6 ppm), have been assigned based on the HMBC correlations C-2/H-6, C-2/H-5, C-2/H4, C-2/-NH, respectively.
Determination of cationic matter
The standardization of the sodium lauryl ether sulfate (SLES) solution was done by using a solution of benzethonium chloride solution
The following equation was used to establish the value about molarity of SLES (T2) = \(\frac{{10 T_{1} }}{{V_{1} }}\) where T1 = molarity of benzethonium chloride solution; V1 = volume of sodium lauryl ether sulfate (SLES) [21].
Molarity of imidazolinium mixture
In a 100 mL graduated cylinder provided with a glass stopper, an amount of 10 mL, 15 mL and 25 mL of standard sodium lauryl ether sulfate solution was versed, chloroform and methylene blue solutions were added and shaken well. An imidazolinium solution was slowly replied, and the mix was shaken easily. The mixture was released to stand until the two layers appeared. Small increments of the imidazolinium solution were added, followed by vigorous shaking until the endpoint is achieved. The endpoint of the titration is achieved when the color has the same intensity in the both layers. Comparisons of the colors of the two layers were made by reflected light using a white sheet of paper as the background. The solution was allowed to remain for one minute before viewing the layers. The reading, at which the color intensity in both phases is the same after viewed under standard conditions of light, was noted. The following equation was utilized to determinate the molarity of the cationic surfactant the Cationic matter value is stated in Table 2 [21].
T2: the SLES molarity; V2: the imidazolinium solution volume
Surface activity
Surface tension measurements
Surface tensiometry furnishes a measure of the interfacial free energy per unit area at the surface between the liquid and the air. This data are imperative to establish the CMC and inspecting the micellization and adsorption properties. As illustrated in Fig. 4, the surface tension of FICM initially decreases with increasing surfactant concentration and then reaches a plateau. The concentration of the inflection point in the curve is taken as the critical micelle concentration (CMC). Also, the temperature effect on surface tension values of FICM is investigated surface tension values and the temperature were inversely proportional due to the increase in molecular thermal agitation and the cohesive action of surfactant molecules with air, resulting in a reduction in surface tension values along the interface curvature [22].
The temperature of the aqueous surfactant solution has two antagonistic effects on the CMC. The first is declining the hydration around the hydrophilic part, which may promote micelle formation, and the second is the disruption of water organization throughout the hydrophobic chain, Therefore, a minimum in CMC is expected to be observed in the variation of CMC with temperature and the CMC–temperature plots appeared as a U-shaped curve as showed in Fig. 5 [22]. Such dependence is analogous to that of conventional ionic surfactant [23]. In general, for ionic surfactants, however, the minima may be outside the experimental range of temperature so that only a decreasing trend is observed.
Adsorption behavior at the air–liquid interface
The effectiveness or surface pressure at the CMC (πCMC) of a surfactant it is their ability to reduce pure water surface tension to that of the surfactant at the CMC [22]. The values of πcmc were obtained by using the following equation:
where γ0 is the surface tension of the solvent at the appropriate temperature and γcmc was that of the surfactant solution at the CMC.
The adsorption efficiency (pC20) is another adsorption parameter, which serves as the aptitude of a surfactant solution to reduce the surface tension of pure water by 20 mN/m [24]. It was defined by Eq. (2).
where C20 is the surfactant concentration (express in mol/l) required to reduce the surface tension of pure solvent by 20 mN/m [25].
Maximum surface excess concentration Γmax (mol/cm2) was calculated according to the Gibbs adsorption equations [24, 26]:
where γ is the surface tension (mN/m). C is the surfactant concentration (mol/l). \(\left( {\partial \gamma /\partial \log C} \right)_{\hbox{max} ,T,P}\) is the maximum slope of the linear part of the plot γ versus log C.
R is the universal gas constant (8.314 J mol K−1) and T represents the absolute temperature.
For ionic surfactants, the factor value n is two [25].
The average area occupied by a surfactant molecule at the air–aqueous solution interface, Amin (nm2), can be assumed from the relation [24, 27]:
where N is Avogadro’s number.
The surface parameters at diverse temperatures were listed in Table 3. These data showed that an increase in temperature involved decreases the effectiveness πcmc and the maximum surface excess (Γmax), and increases minimum surface area at the air/solution interface Amin values.
Conductivity measurements
The electrical conductivity values of the FICM aqueous solutions at different temperatures were determined. As can be seen from Fig. 6, the experimental results match in two straight lines with a significant change of direction at the point of disruption. The breakpoint of the curves can be assigned to CMC. The evaluated CMC at different temperatures were summarized in Table 3. The conductivity-based CMC values are slightly different from those determined by surface tension. It can be observed that with the rise of the temperature, the CMC values decrease.
Foaming property
Figure 7 shows the variation of the height expressed in millimeter (mm) of the foam at time zero at different concentrations, in this figure; we can see that which could be deduced in this study. There is a gradual increase in the height of the foam with increasing concentration up to a certain level above, which at nearly constant height was observed.
Thermodynamics of micellization and adsorption
The standard Gibbs free energy (\(\Delta G_{\text{mic}}^{ \circ }\)), enthalpy (\(\Delta H_{\text{mic}}^{ \circ }\)), and the entropy (\(\Delta S_{\text{mic}}^{ \circ }\)) associated with the micellization process and free energy change of adsorption at the air/solution interface (\(\Delta G_{\text{ads}}^{ \circ }\)), which was calculated at different temperatures using Gibbs Eqs. (5–8) [22] and were described bellows:
where R is the universal gas constant (8.314 J mol K−1), T represents the absolute temperature and Xcmc is the CMC in mole fraction unit.
Values obtained for the thermodynamic parameters at different temperatures are summarized in Table 4. The data showed that the standard free energies of micellization \(\Delta G_{\text{mic}}^{ \circ }\) and adsorption \(\Delta G_{\text{ads}}^{ \circ }\) of the synthesized fatty imidazolinium mixture are all negative. This mentions that the adsorption and the micellization processes occurred spontaneously. Furthermore, the standard free energies of adsorption of the fatty imidazolinium mixture at the interface air/water are more negative than those of the micellization, which denotes that the adsorption process is more advantageous than the micellization [27, 28].The variation of \(\Delta G_{\text{mic}}^{ \circ }\) with temperature is shown in Fig. 8. To better understand the contributions of entropy, as well as enthalpy to the free energy change, \(T\Delta S_{\text{mic}}^{ \circ }\) and \(\Delta H_{\text{mic}}^{ \circ }\), were plotted against temperature. We can also see from this figure that the trend in the change of \(T\Delta S_{\text{mic}}^{ \circ }\) is analogous to \(\Delta H_{\text{mic}}^{ \circ }\) one. It could be noticed afterward, that \(T\Delta S_{\text{mic}}^{ \circ }\) and \(\Delta H_{\text{mic}}^{ \circ }\) gradually decrease with temperature.
Conclusions
The present work covers the synthesis of cationic surfactant mixture based on fatty acids extracted from Sunflower oil through a study of the surface property of synthesis product, measurements of surface property and determination of thermodynamic parameters. Imidazolinium chlorides with various chains and the same polar head constituted the surfactant mixture prepared. The products purchased were characterized by the usual spectroscopic techniques and elemental analysis. The surfactant has proved satisfactory surface properties, while the temperature plays an important role when it’s increased. The γCMC is decreased from 37 to 31 mN/m. While the efficiency decreases from 2.24 to 2.74. The HLB values were assumed for our surfactant and the results achieved indicate that it is O/W emulsifier. The thermodynamic parameters of micelle formation (ΔGmic, ΔHmic, ΔSmic) and adsorption (ΔGads) were determined at different temperatures. The values of the Gibbs free energies of micellization and adsorption are all negatives which confirm that the two processes are spontaneous. Furthermore, the value obtained of ΔGads is lesser than ΔGmic values indicating that adsorption is more favored than micellization. This work has demonstrated that it is possible to obtain surfactant compounds with appropriate surface properties using an available and inexpensive product such as Sunflower oil. The prepared cationic surfactant mixtures exhibit a very interesting surface Synthesized compounds can be used in several industrial application such as we laundry detergent, emulation, corrosion/rust inhibitor and lubricating emulsion
Abbreviations
- CMC:
-
Critical micelle concentration
- Γ max :
-
Maximum surface excess concentration
- pC 20 :
-
Efficiency
- π CMC :
-
Effectiveness
- A min :
-
Minimum area per molecule
- \(\Delta G_{\text{mic}}^{ \circ }\) :
-
Standard Gibbs free energy of micellization
- \(\Delta H_{\text{mic}}^{ \circ }\) :
-
Enthalpy of micellization
- \(\Delta S_{\text{mic}}^{ \circ }\) :
-
The entropy of micellization
- EDA:
-
Ethylenediamine
- FICM:
-
Fatty imidazolinium chloride mixture
- HSQC:
-
Heteronuclear single quantum correlation
- HMBC:
-
Heteronuclear multiple bond connectivity
- log C :
-
The logarithm of the molar concentrations
- KT:
-
Krafft temperature
- HLB:
-
Hydrophilic-lipophilic balance
References
M.T. Garcia et al., Surface activity, self-aggregation and antimicrobial activity of catanionic mixtures of surface active imidazolium- or pyridinium-based ionic liquids and sodium bis(2-ethylhexyl) sulfosuccionate. J. Mol. Liq. 303, 112637 (2020)
X. Zhong et al., Synthesis, surface property and antimicrobial activity of cationic gemini surfactants containing adamantane and amide groups. J. Surfactants Deterg. 17(5), 943–950 (2014)
R. Wang, Y. Li, Y. Li, Interaction between cationic and anionic surfactants: detergency and foaming properties of mixed systems. J. Surfactants Deterg. 17(5), 881–888 (2014)
H. Chen, B. Zhu, H. Liu, Micellar and interfacial behavior of mixed systems containing anionic-nonionic gemini surfactant. IOP Conf. Ser. Mater. Sci. Eng. 729, 012071 (2020)
D. Bajpai, V. Tyagi, Fatty imidazolines: chemistry, synthesis, properties and their industrial applications. J. Oleo Sci. 55(7), 319–329 (2006)
U. Zoller, Handbook of detergents, part E: applications (CRC Press, Boca Raton, 2008)
S.M. Shaban et al., The tail effect of some prepared cationic surfactants on silver nanoparticle preparation and their surface. Thermodyn. Parameters Antimicrob. Act. 22(6), 1445–1460 (2019)
Y. Wang et al., Thermodynamics of self-aggregation of mixed cationic gemini/sodium deoxycholate surfactant systems in aqueous solution. J. Therm. Anal. Calorim. 135, 2903–2913 (2018)
B. Yiğit et al., Imidazolinium chloride salts bearing wing tip groups: synthesis, molecular docking and metabolic enzymes inhibition. J. Mol. Struct. 1179, 709–718 (2018)
A.L. Chong, M. Forsyth, D.R. MacFarlane, Novel imidazolinium ionic liquids and organic salts. Electrochim. Acta 159, 219–226 (2015)
R.G. Bistline, J.W. Hampson, W.M. LinField, Synthesis and properties of fatty imidazolines and their N-(2-aminoethyl) derivatives. J. Am. Oil Chem. Soc. 60(4), 823–828 (1983)
C.M. Phan et al., Micelle and surface tension of double-chain cationic surfactants. ACS Omega 3(9), 10907–10911 (2018)
A. Ali et al., Conductometric and tensiometric studies on the mixed micellar systems of surface-active ionic liquid and cationic surfactants in aqueous medium. J. Mol. Liq. 223, 589–602 (2016)
G.H. Sayed et al., Synthesis, surface, thermodynamic properties of some biodegradable vanillin-modified polyoxyethylene surfactants. J. Surfactants Deterg. 15(6), 735–743 (2012)
N.A. Negm et al., Synthesis, characterization, biodegradation and evaluation of the surface active properties of nonionic surfactants derived from jatropha oil. J. Surfactants Deterg. 16(6), 857–863 (2013)
G. Knothe, Analyzing biodiesel: standards and other methods. J. Am. Oil Chem. Soc. 83(10), 823–833 (2006)
S. Bertouche et al., First approach on moisture determination in food products using alpha-pinene as an alternative solvent for Dean-Stark distillation. Food Chem. 134(1), 602–605 (2012)
M.E. Abdel-Raouf et al., Synthesis and study of the surface properties of alkylnaphthalene and alkylphenanthrene sulfonates. J. Surfactants Deterg. 14(1), 23–30 (2010)
J. Davies, A quantitative kinetic theory of emulsion type. I. Physical chemistry of the emulsifying agent. in Gas/Liquid and Liquid/Liquid Interface. Proceedings of the International Congress of Surface Activity. 1957
R. El-Sayed, H.H. Alotaibi, H.A. Elhady, Synthesis, surface parameters, and biodegradability of water-soluble surfactants for various applications. J. Oleo Sci. 67(5), 551–569 (2018)
D.B. Tripathy, A. Mishra, Convenient synthesis, characterization and surface active properties of novel cationic gemini surfactants with carbonate linkage based on C 12 C 18 sat./unsat. fatty acids. J. Appl. Res. Technol. 15(2), 93–101 (2017)
P. Pillai et al., Synthesis, characterization, surface properties and micellization behaviour of imidazolium-based ionic liquids. J. Surfactants Deterg. 20(6), 1321–1335 (2017)
Z. Lehanine, L. Badache, Thermodynamics of micellization of cationic surfactants based on O-alkyl and O-perfluoro-N, N′-diisopropylisourea: effect of the counter ion nature. J. Chem. Thermodyn. 43(9), 1342–1348 (2011)
L. Berriche et al., Mixed micellization and surface properties of non-ionic/cationic surfactants. J. Dispers. Sci. Technol. 40, 378–389 (2018)
M.J. Rosen, J.T. Kunjappu, Surfactants and interfacial phenomena (Wiley, Hoboken, 2012)
P. Sehgal et al., Interactions between the cationic surfactants bearing different polar head groups: interfacial, conductivity, NMR, and fluorescence studies. J. Dispers. Sci. Technol. 28(8), 1262–1271 (2007)
P. Pillai, A. Mandal, A comprehensive micro scale study of poly-ionic liquid for application in enhanced oil recovery: synthesis, characterization and evaluation of physicochemical properties. J. Mol. Liq. 302, 112553 (2020)
F.I. El-Dossoki, Micellization thermodynamics of some imidazolium ionic liquids in aqueous solutions—conductometric study. J. Solut. Chem. 42(1), 125–135 (2013)
Acknowledgements
The University of Science and Technology Houari Boumediene (U.S.T.H.B) and BOUIRA University (U.A.M.O.B), Algeria are highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gharbi, A., Badache, L., Berriche, L. et al. Synthesis and characterization both micellization and thermodynamic parameter of cationic surfactant mixture derived from vegetable oil. J IRAN CHEM SOC 18, 921–932 (2021). https://doi.org/10.1007/s13738-020-02081-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02081-z