Abstract
Crustaceans are low in fat but rich in omega-3 (n-3) fatty acids (FA). In this study, a mini-scale extraction method was optimized based on the Bligh and Dyer method for the determination of n-3 fatty acids, i.e., alpha-linolenic acid (LNA), eicosapentaenoic (EPA) and docosahexaenoic acid (DHA), in shrimp samples followed by gas chromatography with flame ionization detection. The optimal extraction conditions were determined through a 23 factorial design with a center point. The optimized method extracted comparable amounts of n-3 FA as the original Bligh and Dyer method but was 45 times faster and used 30 times less chloroform and sample and 11 times less total solvent volume. Additionally, the optimized method was successfully applied for the extraction of fatty acid, mainly n-3 fatty acid, from various shrimp species. Thus, the n-3 mini-scale extraction method can be considered a suitable alternative to traditional methods for the extraction of n-3 fatty acid from shrimp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The worldwide annual production of crustaceans has increased in recent years, reaching 6,446,818 tons in 2012, with China being the largest producer [1]. Among the 67,800 crustacean species, shrimp is one of the most consumed, representing 15% of the global seafood trade [2, 3]. Brazil is the largest shrimp producer in South America, particularly white shrimp (Litopenaeus vannamei), pink shrimp (Farfantepenaeus paulensis) and Atlantic seabob shrimp (Xiphopenaeus kroyeri), the latter with the most consumption at 0.6 kg/per capita/year [4, 5]. High shrimp consumption can be linked to its various forms of preparation, such as frozen, fresh, breaded, dried, fried, cooked and roasted, in addition to the health benefits associated with a balanced diet [6].
Shrimp species contain protein, minerals, amino acids, fatty acids, mainly omega-3 (n-3) fatty acids (FA), as alpha-linolenic acid (18:3n-3; ALA), eicosapentaenoic acid (20:5n-3; EPA) and docosahexaenoic acid (22:6n-3; DHA) [7,8,9,10,11,12]. Several benefits are associated with regular n-3 FA consumption, such as a decreased risk of brain disease, cancer, thrombosis, rheumatism, hypertension, heart disease and inflammatory processes [7, 13, 14].
Others important parameters that are linked to food benefits are the n-6/n-3 and PUFA/SFA ratios. These rates help in the control and regulation of growth, bone development, metabolism and reproduction [15, 16]. In addition, according to Simopoulos, the recommended values for the n-6/n-3 and PUFA/SFA ratios are 1-4:1 and a value higher than 0.4, respectively, and it is noted that n-3 FA are the major contributors to the balanced value of these parameters [17].
Various conventional total lipid (TL) extraction methods, e.g., Soxhlet, Folch and Bligh and Dyer, have been used for several food matrices [18,19,20,21,22,23]. The Soxhlet method was the first TL extraction method developed; however, it uses a high temperature, leading to the degradation of some polyunsaturated FA. Also, large proportions of phospholipids are not extracted efficiently, the analysis is time-consuming and specific equipment is required [24, 25]. Folch et al. [19] developed a rapid and simple TL extraction method for fillets poor in lipids (muscle and blood plasma), which does not use a high temperature and extracts both nonpolar and polar lipids. This method was later modified by Bligh and Dyer, decreasing the amount of solvent required [20]. This method, originally developed for fish samples, has become the most applied TL extraction method for several kinds of samples, including milk, fruits, oilseeds and animal fillets [26,27,28,29,30,31].
The Bligh and Dyer extraction method has been widely used for TL extraction from shrimp samples because shrimp species present similar matrix characteristics, i.e., high sample moisture content (around 80%) and low TL content (around 1.5%) [8, 32]. However, this method requires a large amount of sample (a drawback particularly for small samples, such as shrimp), high solvent volume and long analysis time.
Several studies have reported changes in conventional TL extraction methods, including sample amount/preparation, solvent volume/type and time, aiming to develop a cost-efficient, environmentally friendly and efficient TL extraction method [25, 32,33,34,35]. However, to the best of our knowledge, an extraction method for n-3 FA from shrimp fillets has not been developed yet.
Therefore, this work aimed to optimize a miniaturized extraction method for FA, based on Bligh and Dyer method, mainly the n-3 FA (LNA, EPA and DHA) from shrimp samples. For this, the sample amount, chloroform volume and time were optimized using response surface methodology (RSM).
Materials and Methods
Chemical
The standard methyl tricosanoate (23:0) (> 99%) and 189-19 (> 99%) was supplied by Sigma-Aldrich (São Paulo, Brazil). The solvents chloroform (PA) and methanol (PA) were acquired from Labsynth (São Paulo, Brazil) and Anidrol (São Paulo, Brazil), respectively. The carrier (H2) (> 99.95%), auxiliary (N2) (> 99.998%) and detector flame (H2 and synthetic air) (> 99.999%) gases were purchased from White Martins (Maringá, Brazil). The methylation reagents sulfuric acid (PA), isoctano (PA) and sodium chloride (PA) were acquired from Anidrol (São Paulo, Brazil). Ammonium chloride (PA) was acquired from Labsynth (São Paulo, Brazil), and sodium hydroxide (PA) was supplied by Neon (São Paulo, Brazil).
Sample preparation
Seabob shrimp fillets (Xiphopenaeus kroyeri), which were used to optimize the mini-scale Bligh and Dyer method (n-3 mini-scale extraction method), were purchased from a local market in Maringá, PR, Brazil. For method application, quiet freshwater shrimp fillets (Macrobrachium jelskii) were acquired from the Animal Husbandry Program from the State University of Maringa, PR, Brazil. Red shrimp fillets (Pleoticus muelleri) and white shrimp fillets (Litopenaeus schmitti) were obtained from the local market in Maringá, PR, Brazil. The shrimp fillets were macerated using a mortar and pestle and homogenized. Then, all shrimp samples were packed in polyethylene bags in an N2 atmosphere and stored frozen at −18 °C prior to subsequent analysis.
n-3 mini-scale extraction method
The n-3 mini-scale extraction method for the determination of FA from shrimp fillets was optimized using triplicate samples and based on a 23-factorial design with a center point, using Design Expert software, version 7.1.3. Chloroform volume (1.0, 1.5 and 2.0 mL), sample amount (0.1, 0.3 and 0.5 g) and agitation time (2, 4 and 6 min) were optimized at two levels, and a center point (− 1, 0, 1) was used to evaluate the best yield of the n-3 FA sum (LNA, EPA and DHA).
For TL extraction, the respective sample amounts were weighed in Falcon tubes. Then, 2 mL of methanol and half of the total volume of chloroform were added (i.e., 0.5, 0.75 and 1 mL). Then, the mixture was shaken in a Vortex (AP56 Phoenix Model) for half the total agitation time (1, 2 and 3 min). The remaining chloroform was added, and then the tubes were shaken for the remainder of the time. Finally, 4 mL of water was added to the mixture, which was then agitated for 30 s. The Falcon tubes were then centrifuged in a Harrier 18/80R centrifuge at 4529 g for 10 min. The lower fraction, containing the TL, was collected with a microsyringe, the solvent was evaporated using an N2 flow and the samples were stored at −18 °C prior to subsequent analysis.
Bligh and Dyer extraction
The TL was extracted according to Bligh and Dyer [20]. A mixture of the sample (15 g), chloroform (15 mL) and methanol (30 mL) was shaken for 5 min. Then, a further 15 mL of chloroform was added and the tubes were shaken for 2 min, and then 15 mL of distilled water was added and shaken for 5 min. The mixture was filtered and separated in a 250-mL funnel (for 12 h), and then the lower phase was evaporated at 40 °C using a Fisatom 802 evaporator.
FA composition
Fatty acid methyl esters (FAME) were prepared by the methylation of the TL, according to Hartman and Lago [36]. The FAME were separated by a Thermo Scientific gas chromatograph model Trace Ultra, equipped with a flame ionization detector (FID) and a fused silica capillary column CP 7420 Select FAME (100% cyanopropyl-bonded, 100 m × 0.25 mm diameter, 0.25 μm thickness). The flow rates for the carrier (H2), auxiliary (N2) and detector flame (H2 and synthetic air) gases were 1.2, 30, 35 and 350 mL min−1, respectively. The injected volume was 2 μL, and the sample split ratio was 1:40. The injector and detector temperatures were 240 °C. Initially, the column temperature was maintained at 165 °C for 7 min. Then, the column temperature was increased from 165 to 185 °C at 4 °C min−1, maintained at this temperature for 4.67 min and finally increased to 235 °C at 6 °C min−1 and maintained for 5 min.
The quantification of the FA (mg FA per g of TL) was performed using tricosanoic acid methyl ester as an internal standard (23:0) [37]. According to Visentainer, the theoretical FID correction factor values were used to obtain the concentration values [38]. The FA content was calculated using Eq. 1.
where FA is mg fatty acid g−1 TL, AX is the peak area (FA), AIS is the peak area standard (23:0), WIS is the weight (mg) of the standard, WX is the sample weight (g), CFX is the theoretical correction factor and CFAE is the conversion factor necessary to express results as mg of FA rather than as methyl ester.
Precision
The precision of the n-3 mini-scale extraction method was evaluated based on repeatability (intraday) and intermediate precision (interday), which were assessed by analyzing the sum of n-3 FA for five samples on the same day and for five different days, respectively. The results were expressed as relative standard deviation (RSD).
Statistical analysis
Analysis of variance (ANOVA) was used to test the difference between means (stages of development), which were analyzed by Tukey’s test at a 95% (p < 0.05) significance level using Statistica software version 8.0 (StatSoft, USA). All samples were analyzed in four replicates.
Principal and interaction effects were determined by factorial design. The model fit was assessed by ANOVA, using the mathematical model expressed in Eq. 2.
where Y is the expected response (the sum of n-3 mg g−1 TL), X1 is the sample amount, X2 is the chloroform volume, X3 is the agitation time and the other terms refer to the interaction effects.
Results and discussion
n-3 mini-scale extraction method optimization
A miniaturized extraction method (n-3 mini-scale extraction method) was optimized based on the Bligh and Dyer method. The aim was to decrease the time, solvent volume and sample amount, with a focus on an n-3 FA extraction method for shrimp samples. Table 1 shows the factorial design of the n-3 mini-scale extraction method and the yield of the n-3 FA sum.
The highest n-3 sum was obtained using 0.5 g of sample, 1 mL of chloroform and 6 min of agitation time, extracting 98.75 mg g−1 TL. Comparing the results (Table 3) obtained by the original Bligh and Dyer method (78.43 mg g−1 TL), both methods extracted n-3 FA in comparable amounts. However, the chloroform and total solvent volumes, analysis time and sample amount decreased by 30-, 11-, 45- and 30-fold, respectively, using the method optimized in this study compared to the Bligh and Dyer method.
Furthermore, comparing the chromatograms obtained for the shrimp fillets extracted by the n-3 mini-scale extraction method (Fig. 1c) and original Bligh and Dyer method (Fig. 1b), it was observed that both extraction methods presented a similar chromatographic profile without impurities with the same retention time as the target compounds (Fig. 1a).
Additionally, 30% of the FA extracted by the n-3 mini-scale extraction method were n-3 FA. This value was similar to that published by Bragagnolo and Rodrigues-Amaya, who extracted 30% of the n-3 FA sum from X. kroyeri shrimps using the Folch method [39]. Our value is higher than that published by Luzia et al. who extracted 13% of the n-3 FA sum from the same species of shrimp but using the extraction method of Marmer and Maxwell [40, 41].
So, based on several advantages presented by the n-3 mini-scale extraction method, it can be considered a suitable alternative to the traditional methods for n-3 FA extraction from shrimp samples.
ANOVA was used to evaluate whether the factorial design was significant and to assess the contribution of each term. Table 2 shows the ANOVA results of the n-3 FA extraction yield. Analysis of the effects showed that the individual terms (sample amount and agitation time) were significant and only the X2 and X13 terms (chloroform volume and interaction between sample amount and agitation time, respectively) were insignificant. The factors that contributed most to the optimized n-3 FA extraction were the sample amount (47.69%) and the agitation time (13.25%). Equation 3 shows the optimum proposed model.
where Y is the expected response (mg n-3 sum g−1 TL), X1 is the sample amount, X2 is the chloroform volume, X3 is the agitation time and the other terms refer to the interaction effects.
ANOVA results
The value of the regression coefficient (R2 = 0.9962) indicates that 99.62% of the response was explicated by the model, and the adjusted regression coefficient indicates that 99.15% can be explained by the experimental data. The proposed model had a 95% significance level at p < 0.05. The low variation coefficient value (4.91%) indicated good precision and uniformity of the experimental data. The pure error and the lack of fit (p = 0.4798) were insignificant, confirming that this model was suited to the experimental data.
Figure 2a shows the linearity of the actual and predicted data, indicating a good approximation of the points on the regression line and confirming the efficiency of the model fit [42]. Also, the predicted and studentized residuals (Fig. 2b) showed no distinct tendency, supporting error randomness. Therefore, ANOVA indicated that the model was significant. The effects are observed in the response surface models (Fig. 2c).
Precision
According to Huber and Waldbronn, the precision depends on the sample matrix. For a food matrix, a precision value less than 20% is acceptable [43].
Precision was calculated as intra- and interday precision and is expressed as RSD. The resultant values were less than 20%, with RSD for intra and interday of 13.05 and 17.77%, respectively. Based on these results, the n-3 mini-scale extraction method provided reliable n-3 FA extraction from shrimp samples.
Application
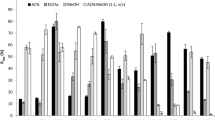
The n-3 mini-scale extraction method was used on three distinct shrimp species, P. muelleri, L. schmitti and M. jelskii. Table 3 shows the individual FA amounts found in the shrimp fillets (including X. kroyeri shrimps), the sum and the rates when the samples were extracted by the n-3 mini-scale extraction method and the Bligh and Dyer method, respectively.
Comparing the n-3 FA sum obtained for all three shrimp species, the n-3 mini-scale extraction method extracted a comparable amount of n-3 FA to the Bligh and Dyer method. However, among the marine species evaluated, P. muelleri was the only species that presented a higher n-3 FA sum than the freshwater shrimp species. All the shrimp species evaluated showed a different n-3 FA composition, e.g. LNA was more abundant in L. schmitti and M. jelskii. On average, LNA was 9.4 and 8.0 times more abundant in M. jelskii than X. kroyeri and P. muelleri,, respectively; EPA was approximately 1.4 times more abundant in P. muelleri and M. jelskii than L. schmitti and X. kroyeri, respectively; and DHA was more abundant in P. muelleri and X. kroyeri. On average, DHA was 3.8 and 3.0 times more abundant in P. muelleri than M. jelskii and L. schmitti, respectively.
Marine aquatic species, such as fish and seafood, may have a higher n-3 FA content than freshwater species as a consequence of their dietary preferences for phytoplankton and microalgae, which are rich in these FA [44,45,46,47]. However, the content and composition of n-3 FA in marine aquatic species can be affected by several other factors, such as ripeness, sex, species, anatomical features and habitat. These factors can influence the metabolic enzymatic activity and efficacy of FA bioconversion [8, 46, 48, 49]. Thus, the different n-3 FA composition between the shrimp species and the similar n-3 FA sum between L. schmitti and X. kroyeri compared to M. jelskii can be explained.
Additionally, the percent of n-3 FA extracted by the n-3 mini-scale extraction method for P. muelleri, L. schmitti and M. jelskii shrimps was the same as those obtained by Jeckel et al. which extracted 29% of the n-3 FA sum from P. muelleri. Similarly, Cabrera et al. and Ramírez et al. extracted 20 and 16% of the n-3 FA sum from L. schmitti and M. jelskii shrimps, respectively, using traditional TL extraction methods [50,51,52].
Although the new method focuses on the n-3 FA extraction, it can also extract efficiently the other fatty acids, as shown in Table 3.
Even the n-3 mini-scale extraction focused on the extraction of n-3 fatty acids, and the fatty acid profile was similar to Balzano et al. and Na et al.'s studies [53, 54].
For saturated FA, the optimized method extracted 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 21:0, 22:0 and 24:0, i.e., four FA more than Balzano et al. and two more than Na et al., being 16:0 and 18:0 the most abundant in all studies [53, 54].
For monounsaturated fatty acids, the n-3 mini-scale method extracted 14:1-7, 15:1n-9, 16:1n-9, 16:1n-7, 16:1n-5, 17:1n-9, 18:1n-9, 18:1n-7, 20:1n-9 and 24:1n-9, being three more FA than Balzano et al.'s study [54]. Furthermore, both works extracted in higher amounts 18:1n-9, followed by 16:1n-7 and 18:1n-7.
For polyunsaturated FA, 18:2n-6, 18:3n-3, 20:4n-6, 20:4n-3, 20:5n-3, 22:5n-6 and 22:6n-3 were extracted, being 22:6n-3 and 20:5n-3 the most abundant.
Comparing all FA amounts, both methods also extracted comparable amounts of most fatty acids, and the FA majorities in all shrimps were 16:0, 18:0, 18:1n-9, 18:2n-6, 20:5n-3 and 22:6n-3; the same result was obtained by Li et al. and Sánchez-Camargo et al. [55, 56].
According to Simopoulos, the n-6/n-3 and AGPI/AGS ratios recommended are 1-4:1 and values higher than 0.4, respectively [17]. Although the X. kroyeri and P. muelleri had more n-3 fatty acids amount than n-6 fatty acids, reflecting the low n-6/n-3 ratio, all shrimps had suitable n-6/n-3 ratio. Finally, the freshwater shrimps had higher AGS content than marine shrimps, and consequently lower PUFA/SFA ratio, but still in the standards.
Conclusions
A faster, more environmentally friendly, miniaturized and effective method for n-3 FA extraction from shrimp samples was developed. A mathematical model describing the best extraction conditions was determined through a 23-factorial design with a center point. The n-3 mini-scale extraction method was 45 times faster, used 30 times less chloroform and sample and 11 times less total solvent. The new method extracted a comparable n-3 FA amount as the Bligh and Dyer method.
The n-3 mini-scale extraction method was used to determine the fatty acid amount of three distinct shrimp species. The results showed that the optimized method was consistent and extracted a comparable n-3 FA amount as the Bligh and Dyer method for all shrimp species evaluated. Thus, the n-3 mini-scale extraction method can be considered a suitable alternative to traditional methods for the extraction of fatty acid, mainly the n-3 FA content, from shrimp.
References
FAO. Food and Agriculture Organization of the United Nations, Fisheries and Aquaculture Department Rome, Italy, 2014. http://www.fao.org/3/a-i3720e.pdf/. Accessed 1 Apr 2020
R.C. Brusca, G.J. Brusca, Invertebrates (Sinauer Associates, Sunderland, 2008)
FAO. Overview: Major Trends and Issues, Fisheries and Aquaculture Department, Rome, Italy, 2013. http://ftp/fao.org/FI/STAT/summary/YB_Overview.pdf/. Accessed 2 Apr 2020
IBAMA. Brazilian Institute of Environment and Renewable Natural Resources. Diagnosis of Shrimp Farming in Ceara State, 2015. dipro/diliq/difape/gerex‐ce. Accessed 2 Apr 2020
I.P. Rocha, Infofish International 4, 36–38 (2015)
G. Mateljan, The world´s healthiest foods (2016). http://www.whfoods.com/genpage.php?tname=foodspice&dbid=107#references. Accessed 2 Apr 2020
K. Tsape, V.J. Sinanoglou, S. Miniadis-Meimaroglou, Food Chem. 122, 292–299 (2010). https://doi.org/10.1016/j.foodchem.2010.02.019
C.M.L. Saiz, G.M.S. Jiménez, M.P. Jatomea, A.B. Hernández, Mar. Drugs 11, 3926–3950 (2013). https://doi.org/10.3390/md11103926
G.M. Lira, K.W.B. Silva, B.C. Figueiredo, N. Bragagnolo, LWT-. Food Sci. Technol. 58, 183–187 (2014). https://doi.org/10.1016/j.lwt.2014.02.057
D. Mika, M. Golebiowski, E. Skorkowski, P. Stepnowski, J. Mar. Biol. Assoc. U. K. 94, 993–1000 (2014). https://doi.org/10.1017/S0025315414000162
T. Muralisankar, P.S. Bhavan, S. Radhakrishnan, C. Seenivasan, N. Manickam, R. Shanthi, J. Basic Appl. Zool. 67, 34–39 (2014). https://doi.org/10.1016/j.jobaz.2014.09.004
J. Walczak, S. Bocian, T. Kowalkowski, T. Trziszka, B. Buszewski, Food Anal. Methods 10, 1264–1272 (2017). https://doi.org/10.1007/s12161-016-0655-7
W.S. Harris, D. Mozaffarian, M. Lefevre, C.D. Toner, J. Colombo, S.C. Cunnane, J.M. Holden, D.M. Klurfeld, M.C. Morris, J. Whelan, J. Nutr. 139, 804S–819S (2009). https://doi.org/10.3945/jn.108.101329
C.H.S. Ruxton, Nutr. Bull. 36, 6–19 (2011). https://doi.org/10.1111/j.1467-3010.2010.01869.x
N.M. Bandarra, P. Rema, I. Batista, P. Pousão-Ferreira, L.M. Valente, S.M. Batista, R.O. Ozório, Eur. J. Lipid Sci. Technol. 113, 1332–1341 (2011). https://doi.org/10.1002/ejlt.201100087
G.M. Berge, P.E. Witten, G. Baeverfjord, A. Vegusdal, S. Wadsworth, B. Ruyter, Aquaculture 296, 299–308 (2009). https://doi.org/10.1016/j.aquaculture.2009.08.029
A.P. Simopoulos, Biomed. Pharmacother. 56, 365–379 (2002). https://doi.org/10.1016/s0753-3322(02)00253-6
Association of Official Analytical Chemists-AOAC. Official methods of analysis of the Association of Official Analytical Chemists 1995 (method 920.39,C), Arlington
J. Folch, M. Lees, S. Stanley, J. Biol. Chem. 226, 497–509 (1957)
E.G. Bligh, W.J. Dyer, Can. J Biochem. Physiol. 37, 911–917 (1959). https://doi.org/10.1139/o59-099
R. Intarasirisawat, S. Benjakul, W. Visessanguan, J. Wu, LWT Food Sci. Technol. 58, 280–286 (2014). https://doi.org/10.1016/j.lwt.2014.02.036
R.K. Bastos, E.P. Frigo, H.J. Alves, J. Dieter, S.N.M. Souza, A.A.F. Silva, V. Kothe, Ind. Crops Prod. 74, 642–647 (2015). https://doi.org/10.1016/j.indcrop.2015.05.040
I.M. Rivas-Serna, R. Polakowski, G.K. Shoemaker, V.C. Mazurak, M.T. Clandinin, J. Food Compos. Anal. 44, 45–55 (2015). https://doi.org/10.1016/j.jfca.2015.06.006
J.L.L. García, M.D.L. Castro, J. Chromatogr. A 1034, 237–242 (2004). https://doi.org/10.1016/j.chroma.2004.02.020
S. Lino, B. Solé, V. Matos, R. Pereira, J.R. Xavier, R.S. Santos, A. Colaço, Arquipelago Life and Marine Sciences 31, 9–14 (2013)
S.J. Iverson, S.L.C. Lang, M.H. Cooper, Lipids 36, 1283–1287 (2001). https://doi.org/10.1007/s11745-001-0843-0
S.A. Adler, A.V. Dahl, S.K. Jensen, E. Thuen, A.M. Gustavsson, H. Steinshamn, Livest. Sci. 154, 93–102 (2012). https://doi.org/10.1016/j.livsci.2013.03.013
T.S. Ferrão, D.F. Ferreira, D.W. Flores, G. Bernardi, D. Link, J.B. Barin, R. Wagner, Food Res. Int. 54, 57–62 (2013). https://doi.org/10.1016/j.foodres.2013.06.002
J.M. Shin, Y.O. Hwang, O.J. Tu, H.B. Jo, J.H. Kim, Y.Z. Chae, K.H. Rhu, S.K. Park, Food Chem. 13, 703–709 (2013). https://doi.org/10.1016/j.foodchem.2012.08.033
M.M. Sawada, L.L. Venâncio, T.A. Toda, C.E.C. Rodrigues, Food Res. Int. 62, 662–670 (2014). https://doi.org/10.1016/j.foodres.2014.04.039
F.T. Macagnan, L.R. Santos, B.S. Roberto, F.A. Moura, M. Bizzani, L.P. Silva, Bioactive Carbohydr. Diet. Fibre 6, 1–6 (2015). https://doi.org/10.1016/j.bcdf.2015.04.001
M. Bulbul, M.K. Kader, M.A. Ambak, M.S. Hossain, M. Ishikawa, S. Koshio, Aquaculture 438, 98–104 (2015). https://doi.org/10.1016/j.aquaculture.2015.01.007
D.S. Ivanove, R.R. Colovic, J.D. Levic, S.A. Sredanovic, Eur. J. Lipid Sci. Technol. 114, 807–815 (2012). https://doi.org/10.1002/ejlt.201100347
L. Xiao, S.A. Mjos, B.O. Haugsgjerd, J. Food Compos. Anal. 25, 198–207 (2012). https://doi.org/10.1016/j.jfca.2011.08.003
R.F. Castanha, L.A.S. Morais, A.P. Mariano, R.T.R. Monteiro, Braz. Arch. Boil. Technol. 56, 629–636 (2013). https://doi.org/10.1590/S1516-89132013000400013
L. Hartman, R.C.A. Lago, Lab. Pract. 22, 475–476 (1973)
J.D. Joseph, R.G. Ackman, J. Assoc. Off. Anal. Chem. Int. 75, 488–506 (1992). https://doi.org/10.1093/jaoac/75.3.488
J.V. Visentainer, Quím. Nova 35, 274–279 (2012). https://doi.org/10.1590/S0100-40422012000200008
N. Bragagnolo, D.B. Rodriguez-Amaya, J. Food Compos. Anal. 14, 359–369 (2001). https://doi.org/10.1006/jfca.2000.0981
L.A. Luzia, G.R. Sampaio, C.M.N. Castellucci, E.A.F.S. Torres, Food Chem. 83, 93–97 (2003). https://doi.org/10.1016/S0308-8146(03)00054-2
W.N. Marmer, R.J. Maxwell, Lipids 16, 365–371 (1981). https://doi.org/10.1007/BF02534964
B.B. Neto, I.S. Scarminio, R.E. Bruns, Como Fazer Experimentos: Pesquisa e Desenvolvimento na Ciência e na Indústria, 3rd edn. (UNICAMP, Campinas, 2007), p. 480
L. Huber, LC/GC Int. 11, 96–105 (1998)
E. Boselli, D. Pacetti, P. Lucci, N.G. Frega, J. Agric. Food Chem. 60, 3234–3245 (2012). https://doi.org/10.1021/jf205159a
R.S. Hellberg, C.A.M. Dewitt, M.T. Morrissey, Compr. Rev. Food Sci. Food Saf. 11, 490–517 (2012). https://doi.org/10.1111/j.1541-4337.2012.00200.x
D.R. Tocher, Aquaculture 449, 94–107 (2015). https://doi.org/10.1016/j.aquaculture.2015.01.010
R. Larsen, K.E. Eilertsen, E.O. Elvevoll, Biotechnol. Adv. 29, 508–518 (2011). https://doi.org/10.1016/j.biotechadv.2011.05.017
D.R. Tocher, Rev. Fish. Sci. 11, 107–184 (2003). https://doi.org/10.1080/713610925
F.L. Santos, V.B. Azeredo, A.S.A. Martins, Food Sci. Technol. 27, 851–855 (2007). https://doi.org/10.1590/S0101-20612007000400027
W.H. Jeckel, J.E.A. Moreno, V.J. Moreno, Comp. Biochem. Physiol. 98, 261–266 (1991). https://doi.org/10.1016/0305-0491(91)90176-E
T. Cabrera, G. Cabrera, J. Rosas, A. Velásquez, M. Silva, Arch. Latinoam. Nutr. 55, 194–200 (2005)
E. Ramírez, A. Silva, M. Guevara, M. Nunez, R. Bauza, B. Arredondo-Vega, Zootec. Trop. 28, 65–72 (2010)
W. Na, H. He, X. Dong, B. Tan, Q. Yang, S. Chi, S. Zhang, H. Liu, Y. Yang, Aquac. Rep. 17, 100321 (2020). https://doi.org/10.1016/j.aqrep.2020.100321
M. Balzano, D. Pacetti, P. Lucci, D. Fiorini, N.G. Frega, J. Food Compos. Anal. 59, 88 (2017). https://doi.org/10.1016/j.jfca.2017.01.013
G. Li, A.J. Sinclair, D. Li, J. Agric. Food Chem. 59, 1871–1881 (2011). https://doi.org/10.1021/jf104154q
A.P. Sánchez-Camargo, M.A.A. Meireles, A.L.K. Ferreira, E. Saito, F.A. Cabral, J. Supercrit. Fluids 61, 71–77 (2012). https://doi.org/10.1016/j.supflu.2011.09.017
Acknowledgments
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brazil (CNPq) and Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná for the financial support and scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
dos Santos, V.J., Nicácio, A.E., Suzuki, R.M. et al. Determination of n-3 fatty acids in shrimp using a mini-scale extraction method and GC-FID analysis. J IRAN CHEM SOC 18, 375–383 (2021). https://doi.org/10.1007/s13738-020-02033-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02033-7