Abstract
The design, synthesis and characterization of a series of 1-alkyl-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium halide derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors for the treatment of Alzheimer’s disease were reported. The strategy for this synthesis was based on aldol condensation reaction between 4-pyridinecarboxaldehyde with 5,6-dimethoxy-1-indanone in the presence of NaOH in EtOH-H2O solution as the first step and N-alkylation reaction of the produced 5,6-dimethoxy-2-[(pyridin-4-yl)methylene]-1-indanone with various alkyl halides (R–X) in the second step. This reaction was carried out under reflux temperature in polar aprotic solvents such as acetone or acetonitrile. 1H and 13C NMR and FTIR spectroscopy along with CHN analysis were used to confirm the structure of our synthesized compounds. Biological activities including acetylcholinesterase and butyrylcholinesterase (BuChE) inhibitions for synthesized compounds were tested using the Ellman method. The results were compared with donepezil and galantamine, and among the synthesized compounds, the highest inhibitory activity with BuChE IC50 = 0.55 μM was observed.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, which seems to be directly related to age [1]. The etiology of AD has not been fully understood yet; but there are several reasons such as β-amyloid (Aβ) deposits, τ-protein aggregation, oxidative stress and low levels of acetylcholine (Ach) have been considered as the main causes of this disease [2, 3]. Acetylcholine (ACh), the main neurotransmitter of the cholinergic pathway, plays an important role in cognition impairment (e.g., Alzheimer), and reducing the activity of this substance is one of the main processes involved in AD development. Serine hydrolase enzymes include that acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) are responsible for the hydrolysis of acetylcholine (ACh) [4]. AChE inhibition has been known as a strategy to the AD treatment through the ACh-level enhancement for years.
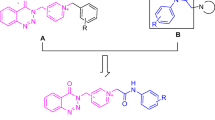
According to several reports, various derivatives of 1-indanone moiety have important role in biological activities such as antiproliferative activity, acetylcholinesterase inhibition in donepezil (Aricept) for the treatment of Alzheimer’s disease, and etc. [5,6,7,8]. Tacrine, donepezil, rivastigmine and galantamine include the acetylcholine esterase inhibitors (AChE) for the treatment of Alzheimer’s disease [8,9,10,11,12]. Donepezil hydrochloride is the second drug approved by the US FDA for the treatment of AD [13, 14]. At first glance, donepezil is divided into four parts that are shown in Fig. 1; part 1: 1-indanone moiety, part 2: linkage moiety, part 3: piperidine moiety and part 4: benzyl moiety. Choosing the best moiety to replace with other analogous to improve the inhibition activity is one of the most challenging debates among the scientists [15].
According to different results that were mentioned in researches on compounds having high acetylcholinesterase inhibitions, 5,6-dimethoxy-1-indanone was chosen as part 1, carbon with double-bond form as linker, pyridine as part 3 and pyridinium cation form of various alkyls as part 4 for increasing solubility in water of acetylcholinesterase and butyrylcholinesterase inhibitors.
Organic heterocyclic salts such as pyridinium, quinolinium and N-methylimidazolium salts are an important class of new compounds, which were used as nonlinear optics, ionic liquids, key intermediate in organic reactions and biological applications. It is obvious that the compounds in salt form were better dissolved in aqueous media; therefore, most drugs are in the form of salt [16,17,18,19,20,21]. In this context, a series of 1-alkyl-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium halide derivatives were prepared from 5,6-dimethoxy-2-[(pyridin-4-yl)methylene]-1-indanone and various alkyl halides. Biological activities including acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibitions for synthesized compounds were assayed. The results were compared with donepezil and galantamine.
Experimental
Materials and instruments
All materials used in synthesis were purchased from Merck and Sigma-Aldrich. All reactions were carried out under argon atmosphere. Column chromatography was performed using SiO2 (60 Å, 230–400 mesh, particle size 0.040–0.063 mm) at 25 °C. Melting points were determined on a MEL-TEMP model 1202D and are uncorrected. FTIR spectra were recorded on a Bruker Tensor 27 spectrometer as KBr disks. The 1H NMR and 13C NMR spectra were recorded on a Bruker Spectrospin Avance 400 and 100 MHz spectrometer, respectively, used solvents was CDCl3 and DMSO-d6. 13C NMR spectra were determined on the same instrument at 100 MHz. All chemical shifts were reported as δ (ppm), and coupling constants (J) were given in Hz. The elemental analyses were carried out with an Elementor Vario EL. III instrument.
Synthesis
Synthesis of 5,6-dimethoxy-2-[(pyridin-4-yl)methylene]-1-indanone (3)
To a solution of 1 mmol of 4-pyridinecarboxaldehyde and 1 mmol of 5,6-dimethoxy-1-indanone in 10 ml EtOH, aqueous solution of NaOH (10%) was added dropwise. The reaction mixture was stirred overnight at room temperature. The obtained solid was filtered and recrystallized from EtOH to give 3 as an off-white solid [22]; yield 67%; mp: 118–120 °C; FTIR (KBr) ν 3008, 2937, 1691, 1600, 1465, 1315, 1268, 1029 cm−1; 1H NMR (400 MHz, CDCl3): 3.82 (3H, s, OCH3), 3.91 (3H, s, OCH3), 4.03 (2H, s, CH2), 7.17 (1H, s, Ar), 7.22 (1H, s, Ar), 7.42 (1H, s, =CH), 7.67 (2H, d, 3JH–H = 5.36, pyridine-H), 8.64 (2H, d, 3JH–H = 5.46, pyridine-H).
General procedure for the synthesis of compounds (5a–o)
5,6-Dimethoxy-2-[(pyridin-4-yl)methylene)-1-indanone (0.35 mmol) was dissolved in 4 cc acetone (for synthesis of 5a) or acetonitrile (for synthesis of 5b–o) under reflux temperature, and then 1.05 mmol of appropriate alkyl halides was added. The reaction mixture was stirred for 48 h under reflux condition. The precipitate was filtered and washed with appropriate solvent. The obtained solid was dried under reduced pressure to afford related compounds.
1-Methyl-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium iodide (5a)
From 0.14 g of methyliodide, 0.11 g (0.8 mmol) of cloudy white solid was obtained in 80% yield; mp: 222–224 °C; FTIR (KBr) ν 3008, 2934, 1685, 1640, 1462, 1317, 1273, 1026 cm−1; 1H NMR (400 MHz, DMSO): δ 3.84 (3H, s, OCH3), 3.92 (3H, s, OCH3), 4.17 (2H, s, CH2), 4.28 (3H, s, CH3), 7.12 (1H, s, Ar–H), 7.28 (1H, s, Ar–H), 7.55 (1H, s, =CH), 8.18 (2H, d, 3JH–H = 5.90, pyridine-H), 9.15 (2H, d, 3JH–H = 5.70, pyridine-H); 13CNMR (100 MHz, DMSO): 32.24, 55.46, 56.23, 61.24, 123.07, 128.44, 129.31, 138.02, 138.55, 139.12, 145.62, 146.24, 150.01, 155.62, 156.16, 192.04; Anal. Calc. for C18H18INO3: C, 51.08; H, 4.29; N, 3.31%. Found: C, 51.11; H, 4.27; N, 3.32%.
1-Benzyl-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium chloride (5b)
From 0.13 g of benzylchloride, 0.12 g (0.31 mmol) of white solid was obtained in 90% yield; mp: 226–228 °C; FTIR (KBr) ν 3008, 2833, 1683, 1600, 1462, 1317, 1272,1071 cm−1; 1H NMR (400 MHz, DMSO): δ 3.92 (3H, s, OCH3), 3.85 (3H, s, OCH3), 4.18 (2H, s, CH2), 5.76 (2H, s, CH2), 7.18 (1H, s, Ar–H),7.27 (1H, s, Ar–H), 6.47–7.58 (3H, m, Ar–H, =CH), 7.61–7.72 (3H, m, Ar–H), 8.15 (2H, d, 3JH–H = 6.20, pyridine-H), 9.13 (2H, d, 3JH–H = 6.20, pyridine-H); 13C NMR (100 MHz, DMSO): 191.20, 157.18, 151.71, 150.12, 147.02, 146.30, 145.11,134.18, 130.02, 129.71, 129.70, 129.21, 128.52, 125.25, 108.31, 105.20, 63.12, 56.71, 55.92, 31.19; Anal. Calc. for C24H22ClNO3: C, 70.67; H, 5.44; N, 3.43%. Found: C, 70.63; H, 5.45; N, 3.42%.
1-(4-Bromobenzyl)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium bromide (5c)
From 0.26 g of 4-bromobenzylbromide, 0.12 g (0.23 mmol) of white solid was obtained in 66% yield; mp: 228–229 °C; FTIR (KBr) ν 3048, 2834, 1682, 1600, 1464, 1320, 1272, 1020, 803, 533 cm−1; 1H NMR (400 MHz, DMSO): δ 3.84 (3H, s, OCH3), 3.91 (3H, s, OCH3), 4.13 (2H, s, CH2), 5.70 (2H, s, CH2), 7.18 (1H, s, Ar–H), 7.29 (1H, s, Ar–H), 7.56–7.61 (3H, m, Ar–H, =CH), 7.66–7.72 (2H, d, 3JH–H = 8.37, Ar–H), 8.39 (2H, d, 3JH–H = 6.50, pyridine-H), 9.21 (2H, d, 3JH–H = 6.50, pyridine-H); 13C NMR (100 MHz, DMSO): 190.99, 156.81, 152.03, 150.21, 146.99, 146.31, 145.20, 134.32, 132.60, 131.61, 130.01, 128.61, 124.96, 123.42, 108.41, 105.30, 62.41, 57.17, 56.31, 32.01; Anal. Calc. for C24H21Br2NO3: C, 54.26; H, 3.98; N, 2.64%. Found: C, 54.29; H, 3.97; N, 2.63%.
1-(3-Bromobenzyl)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium bromide (5d)
From 0.26 g of 3-bromobenzylbromide, 0.11 g (0.22 mmol) of white solid was obtained in 63% yield; mp: 232–234 °C; FTIR (KBr) ν 3004, 2939, 1690, 1600, 1467, 1312, 1268, 1038, 699, 785, 845 cm−1; 1H NMR (400 MHz, DMSO): δ 3.69 (3H, s, OCH3), 3.91 (2H, s, OCH3), 4.16 (2H, s, CH2), 5.78 (2H, s, CH2),7.19 (1H, s, Ar–H), 7.26 (1H, s, Ar–H), 7.44 (1H, m, Ar–H), 7.58 (1H, s, =CH), 7.68–7.59 (2H, m, Ar–H), 7.89 (1H, s, Ar–H), 8.42 (2H, d, 3JH–H = 6.16, pyridine-H), 9.16 (2H, d, 3JH–H = 6.14, pyridine-H); 13C NMR (100 MHz, DMSO): 191.18, 156.81, 151.78, 150.20, 146.90, 46.31, 144.98, 137.21, 132.80, 132.22, 132.01, 129.80, 128.35, 128.50, 124.93, 122.71, 108.40, 105.32, 61.99, 56.77, 56.30, 32.08; Anal. Calc. for C24H21Br2NO3: C, 54.26; H, 3.98; N, 2.64%. Found: C, 54.28; H, 3.97; N, 2.65%.
1-(2-Bromobenzyl)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium bromide (5e)
From 0.26 g of 2-bromobenzylbromide, 0.12 g (0.23 mmol) of off-white solid was obtained in 66% yield; mp: 212–214 °C; FTIR (KBr) ν 2932, 1689, 1590, 1464, 1309, 1027, 847, 752, 539 cm−1; 1HNMR (400 MHz, DMSO): δ 3.76 (3H, s, OCH3), 3.92 (3H, s, OCH3), 4.18 (2H, s, CH2), 5.86 (2H, s, CH2), 7.20 (1H, s, Ar), 7.28 (1H, s, Ar), 7.37 (1H, dd, 3JH–H = 7.6 Hz, Ar), 7.44 (1H, m, Ar), 7.51 (1H, s, =CH), 7.60 (1H, s, Ar), 7.79 (1H, dd, 3JH–H = 7.9, Hz, Ar), 8.48 (2H, d, 3JH–H = 6.55, pyridine-H), 9.15 (2H, d, 3JH–H = 6.55, pyridine-H); 13C NMR (100 MHz, DMSO): 191.33, 156.11, 152.30, 150.17, 147.22, 146.30, 145.71, 133.90, 133.68,131.92, 131.60, 129.79, 129.22, 128.50, 125.31, 123.76, 108.40, 105.28, 63.30, 56.70, 56.28, 31.90; Anal. Calc. For C24H21Br2NO3: C, 54.26; H, 3.98; N, 2.64%. Found: C, 54.23; H, 3.97; N, 2.63%.
1-(2-Chlorobenzyl)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium chloride (5f)
From 0.17 g of 1-chloro-2-(chloromethyl)benzene, 0.09 g (0.21 mmol) of white solid was obtained in 60% yield; mp: 222–224 °C; FTIR (KBr) ν 3004, 2944, 1691, 1600, 1640, 1464, 1317, 1272, 1024, 757, 855, 525 cm−1; 1H NMR (400 MHz, DMSO): δ 3.76 (3H, s, OCH3), 3.92 (3H, s, OCH3), 4.17 (2H, s, CH2), 5.92 (2H, s, CH2), 7.36 (1H, s, Ar), 7.45 (1H, s, Ar), 7.52 (1H, dd, 3JH–H = 7.5 Hz, Ar), 7.58 (1H, m, Ar), 7.49 (1H, s, =CH), 7.63 (1H, s, Ar), 7.80 (1H, dd, 3JH–H = 7.5, Hz, Ar), 8.79 (2H, d, 3JH–H = 6.50, pyridine-H), 9.14 (2H, d, 3JH–H = 6.50, pyridine-H); 13C NMR (100 MHz, DMSO): 191.36, 156.11, 152.30, 150.17, 147.22, 146.30, 145.71, 133.90, 133.67,131.92, 138.60, 129.81, 129.23, 128.50, 125.33, 123.76, 108.40, 105.27, 63.32, 55.93, 56.25, 31.89; Anal. Calc. for C24H21Cl2NO3: C, 65.17; H, 4.79; N, 3.17%. Found: C, 65.21; H, 4.80; N, 3.16%.
1-Hexyl-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium bromide (5g)
From 0.17 g of 1-bromohexane, 0.08 g (0.19 mmol) of white solid was obtained in 54% yield; mp: 228–231 °C; FTIR (KBr) ν 2931, 1686, 1642, 1591, 1466, 1312, 1271, 1121, 845 cm−1; 1H NMR (400 MHz, DMSO): δ 0.80 (3H, s, CH3), 1.05–1.15 (6H, m, CH2), 1.91–1.94 (2H, m, CH2), 3.73 (3H, s, OCH3), 3.84 (3H, s, OCH3), 4.11 (2H, m, CH2), 4.93 (2H, t, CH2), 7.40 (1H, s, Ar–H), 7.43 (1H, s, =CH), 7.56 (1H, s, Ar–H), 8.75 (2H, d, 3JH–H = 6.71, pyridine-H), 9.09 (2H, d, 3JH–H = 6.71, pyridine-H); 13C NMR (100 MHz, DMSO): 14.25, 22.73, 27.21, 31.52, 32.28, 52.06, 55.47, 56.26, 60.96, 122.93, 128.42, 129.33, 137.99, 138.56, 139.14, 145.61, 146.22, 150.13, 155.54, 156.14, 191.84; Anal. Calc. for C23H28BrNO3: C, 61.89; H, 6.32; N, 3.14%. Found: C, 61.87; H, 6.31; N, 3.15%.
1-(2-Propenyl)-4-[(5,6-dimethoxy-1-indaanone-2-yl)methylene]pyridinium chloride (5h)
From 0.08 g of 3-chloroprop-1-ene, 0.07 g (0.26 mmol) of white solid was obtained in 74% yield; mp: 225–230 °C; FTIR (KBr) ν 2937, 1688, 1505, 1316, 1269, 1028, 830 cm−1; 1H NMR (400 MHz, DMSO): δ 3.75 (3H, s, OCH3), 3.94 (3H, s, OCH3), 4.15 (2H, s, CH2), 5.28 (2H, m, CH2), 5.24–5.31 (2H, m, =CH2), 6.12 (1H, m, =CH), 7.35 (1H, s, Ar–H), 7.42 (1H, s, =CH), 7.60 (1H, s, Ar–H), 8.70 (2H, d, 3JH–H = 5.25, pyridine-H), 9.12 (2H, d, 3JH–H = 5.48, pyridine-H); 13C NMR (100 MHz, DMSO): 52.06, 55.47, 56.32, 58.28, 117.2, 122.94, 128.41, 129.32, 132.90, 138.01, 138.56, 139.14, 145.56, 146.25, 151.05, 155.56, 156.12, 192.03; Anal. Calc. for C20H20ClNO3: C, 67.13; H, 5.63; N, 3.91%. Found: C, 67.15; H, 5.62; N, 3.90%.
1-[2-(Pyrrolidin-1-yl)ethyl]-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium bromide (5i)
From 0.18 g of 1-(2-bromoethyl)pyrrolidine, 0.08 g (0.17 mmol) of white solid was obtained in 49% yield; mp: 230–236 °C; FTIR (KBr) ν 2929, 2861, 1687, 1463, 1316, 1268, 1031, 870 cm−1; 1H NMR (400 MHz, DMSO): δ 1.83–1.90 (4H, m, CH2), 2.46–2.59 (6H, m, CH2–N), 3.74 (3H, s, CH3), 3.96 (3H, s, OCH3), 4.16 (2H, m, CH2), 5.03 (2H, t, CH2), 7.28 (1H, s, Ar–H), 7.45 (1H, s, =CH), 7.63 (1H, s, Ar–H), 8.92 (2H, d, 3JH–H = 6.00, pyridine-H), 9.08 (2H, d, 3JH–H = 5.93, pyridine-H); 13C NMR (100 MHz, DMSO): 23.67, 32.41, 48.94, 55.24, 56.16, 56.24, 57.05, 115.6, 120.71, 133.58, 138.5, 139.33, 139.82, 142.50, 144.51, 147.32, 150.16, 155.52, 191.64; Anal. Calc. for C23H27BrN2O3: C, 60.14; H, 5.92; N, 6.10%. Found: C, 60.15; H, 5.93; N, 6.09%.
1-(2-Hydroxyethyl)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium chloride (5j)
From 0.08 g of 2-chloroethanol, 0.07 g (0.19 mmol) of white solid was obtained in 54% yield; mp: 229–232 °C; FTIR (KBr) ν 3400, 2935, 1692, 1591, 1463, 1316, 1032, 853 cm−1; 1H NMR (400 MHz, DMSO): δ 3.63–3.66 (2H, m, CH2-OH), 3.75 (3H, s, OCH3), 3.92 (3H, s, OCH3), 4.17 (2H, s, CH2), 4.69 (2H, t, CH2), 7.34 (1H, s, Ar–H), 7.38 (1H, s, =CH), 7.63 (1H, s, Ar–H), 8.46 (2H, d, 3JH–H = 6.55, pyridine-H), 9.14 (2H, d, 3JH–H = 6.56, pyridine-H), –OH not detected; 13C NMR (100 MHz, DMSO): 32.45, 55.19, 55.26, 56.24, 60.52, 116.06, 120.72, 133.61, 138.51, 139.32, 139.79, 142.51, 145.01, 147.33, 150.17, 155.44, 191.82; Anal. Calc. for C19H20ClNO4: C, 63.07; H, 5.57; N, 3.87%. Found: C, 63.09; H, 5.58; N, 3.86%.
1-(Acetic acid)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium chloride (5k)
From 0.10 g of 2-chloroacetic acid, 0.09 g (0.25 mmol) of white solid was obtained in 71% yield; mp: 231–236 °C; FTIR (KBr) ν 3426, 2943, 1688, 1503, 1314, 1272, 1029, 848 cm−1; 1H NMR (400 MHz, DMSO): δ 3.76 (3H, s, OCH3), 3.94 (3H, s, OCH3), 4.15 (4H, s, CH2), 4.98 (1H, m, CH2), 7.35 (1H, d, Ar–H), 7.42 (1H, s, =CH), 7.66 (1H, s, Ar–H), 8.12 (2H, d, 3JH–H = 5.70, pyridine-H), 9.18 (2H, d, 3JH–H = 5.68, pyridine-H), –OH not detected; 13C NMR (100 MHz, DMSO): 33.06, 56.18, 56.33, 58.86, 115.6, 120.71, 133.58, 138.50, 138.96, 139.31, 142.50, 144.51, 147.32, 150.16, 155.52, 178.99, 192.15; Anal. Calc. for C19H18ClNO5: C, 60.73; H, 4.38; N, 3.73%. Found: C, 60.75; H, 4.37; N, 3.74%.
1-(Propionic acid)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium chloride (5l)
From 0.11 g of 3-chloropropanoic acid, 0.11 g (0.28 mmol) of white solid was obtained in 80% yield; mp: 241–243 °C; FTIR (KBr) ν 3532, 3052, 2836, 1689, 1730, 1641, 1568, 1466, 1317, 1271, 1025, 846 cm−1; 1H NMR (400 MHz, DMSO): δ 3.02 (2H, m, CH2 –COOH), 3.75 (3H, s, OCH3), 3.94 (3H, s, OCH3), 4.16 (2H, s, CH2), 4.72 (2H, m, –CH2–N), 7.33 (1H, s, Ar–H), 7.41 (1H, s, =CH), 7.65 (1H, s, Ar–H), 8.13 (2H, d, 3JH–H = 6.10, pyridine-H), 9.11 (2H, d, 3JH–H = 6.00, pyridine-H), –OH not detected; 13C NMR (100 MHz, DMSO): 32.95, 34.92, 48.32, 56.14, 56.25, 115.6, 120.71, 133.56, 138.52, 138.84, 139.29, 142.51, 144.53, 147.32, 151.01, 155.52, 176.91, 192.18; Anal. Calc. for C20H20ClNO5: C, 61.62; H, 5.17; N, 3.59%. Found: C, 61.64; H, 5.18; N, 3.60%.
1-(Butyric acid)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium chloride (5m)
From 0.13 g of 4-chlorobutanoic acid, 0.11 g (0.30 mmol) of white solid was obtained in 86% yield; mp: 232–236 °C; FTIR (KBr) ν 3392, 2937, 1691, 1593, 1499, 1315, 1263, 1026, 823 cm−1; 1H NMR (400 MHz, DMSO): δ 2.98 (2H, t, CH2 –COOH), 2.36 (2H, m, CH2), 3.75 (3H, s, OCH3), 3.95 (3H, s, OCH3), 4.17 (2H, s, CH2), 4.68 (2H, m, –CH2–N), 7.34 (1H, s, Ar–H), 7.40 (1H, s, =CH), 7.68 (1H, s, Ar–H), 8.12 (2H, d, 3JH–H = 6.10, pyridine-H), 9.11 (2H, d, 3JH–H = 6.00, pyridine-H), –OH not detected; 13C NMR (100 MHz, DMSO): 24.86, 32.54, 34.92, 48.32, 53.21, 56.14, 56.25, 115.6, 120.71, 133.55, 137.94, 138.81, 138.97, 142.51, 144.53, 147.32, 151.05, 155.52, 178.43, 192.18; Anal. Calc. for C21H22ClNO5: C, 62.46; H, 5.49; N, 3.47%. Found: C, 62.44; H, 5.50; N, 3.46%.
1-(4-Oxopentyl)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium chloride (5n)
From 0.12 g of 5-chloropentan-2-one, 0.07 g (0.19 mmol) of white solid was obtained in 54% yield; mp: 228–234 °C; FTIR (KBr) ν 2834, 1689, 1593, 1316, 1265, 1026, 824 cm−1; 1H NMR (400 MHz, DMSO): δ 2.10 (3H, s, CH3), 2.15 (2H, t, CH2), 2.40 (2H, t, CH2-CO), 3.75 (3H, s, OCH3), 3.94 (3H, s,OCH3), 4.18 (2H, s, CH2), 4.68 (2H, s, CH2–N), 7.35 (1H, s, Ar–H), 7.43 (1H, s, =CH), 7.68 (1H, s, Ar–H), 8.16 (2H, d, 3JH–H = 5.38, pyridine-H), 9.13 (2H, d, 3JH–H = 5.36, pyridine-H); 13C NMR (100 MHz, DMSO): 22.93, 29.81, 32.26, 42.71, 53.31, 55.61, 56.25, 122.93, 128.42, 129.33, 138.01, 138.54, 139.17, 145.62, 146.22, 150.13, 155.54, 156.14, 192.13, 207.71; Anal. Calc. for C22H24ClNO4: C, 65.75; H, 6.02; N, 3.49%. Found: C, 65.77; H, 6.01; N, 3.48%.
1-[(5-Hydroxy-4H-pyran-4-one-2-yl)methyl]-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene] pyridinium chloride (5o)
From 0.17 g of 5-(chloromethyl)-2-hydroxy-4H-pyran-4-one, 0.11 g (0.26 mmol) of light brown solid was obtained in 74% yield; mp: 254–256 °C; FTIR (KBr) ν 3395, 3051, 2835, 1688, 1644, 1596, 1467, 1317, 1272, 1052, 855 cm−1; 1H NMR (400 MHz, DMSO): δ 3.75 (3H, s, OCH3), 3.95 (3H, s,OCH3), 4.16 (2H, s, CH2), 4.40 (2H, s, CH2–N), 6.68 (1H, s, =CH2 pyran), 6.82 (1H, s =CH2 pyran), 7.34 (1H, s, Ar–H), 7.44 (1H, s, =CH), 7.67 (1H, s, Ar–H), 8.15(2H, d, 3JH–H = 5.46, pyridine-H), 9.13 (2H, d, 3JH–H = 5.42, pyridine-H), –OH not detected; 13C NMR (100 MHz, DMSO): 32.44, 55.46, 56.18, 64.41, 113.31, 122.91, 128.01, 128.42, 129.33, 137.99, 138.56, 139.12, 145.63, 146.28, 150.13, 155.54, 156.14, 163.54, 172.71, 181.32, 191.91; Anal. Calc. for C23H20ClNO6: C, 62.52; H, 4.56; N, 3.17%. Found: C, 62.54; H, 4.55; N, 3.18%.
Biochemical studies: cholinesterase inhibitory activities
Cholinesterase activity of the new synthesized compounds was measured using the Ellman method [23], and acetylthiocholine or butyrylthiocoline was used as substrate for AChE or BuChE, respectively. The 50% inhibitory concentration was measured and expressed as IC50. Human erythrocytes AChE (hAChE) and human plasmatic BuChE (hBuChE) were produced from fresh blood [24]. 5,5′-Dithiobis (2-nitrobenzoic acid) (Ellman’s reagent, DTNB), phosphate buffer (PB), acetylthiocholine (ATC) and butylthiocholine (BTC) were purchased from Sigma-Aldrich, Praque. Quartz cuvettes were used for measuring purposes. All of the synthesized compounds were solved in DMSO. The Na2HPO4 buffers (0.1 M) with pH = 7 and 8 were used to prepare 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB, 3.5 mM) and acetylthiocholine (ATC, 7 mM) or butylthiocholine (BTC, 7 mM) solutions, respectively. All tests were carried out in 0.1 M of Na2HPO4 buffer, pH = 8. The assay medium (1 mL) consists of 550 μL of phosphate buffer (pH 8), 150 μL of DTNB, and 150 μL of substrate, 150 μL of inhibitor (10−4 to 10−10 M) in test cuvettes and 700 μL of phosphate buffer (pH 8), 150 μL of DTNB, and 150 μL of inhibitor solutin (10−4 to 10−10 M) in control cuvettes were incubation for 5 min in 37 °C. The reaction was initiated by an immediate addition of 50 μL of enzyme. The activity was determined by measuring the increase in absorbance at 412 nm at 5-min interval using a spectrophotometer Helios Zeta (Thermospectronic, Cambridge, UK). Each experiment was carried out twice. BuChE study was conducted in a similar situation that is described above. Nonlinear and linear regressions were used to estimate the drug concentration inducing 50% inhibition of the AChE or BuChE activity.
Results and discussion
Chemistry
4-Pyridinecarboxaldehyde (1) is one of the well-known aromatic aldehydes used in organic synthesis. Synthesis of 5,6-dimethoxy-2-[(pyridin-4-yl)methylene)-1-indanone (3) was reported in the literature as an aldol condensation reaction between 5,6-dimethoxy-1-indanone (2) and 4-pyridinecarboxaldehyde (1) under alkaline or acidic conditions [22]. This compound was prepared in the presence of NaOH 10% in ethanol. In continuation, the 1-alkyl-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium halide derivatives (4a-p) were synthesized via N-alkylation reaction of 5,6-dimethoxy-2-[(pyridin-4-yl)methylene]-1-indanone (3) with various alkyl halides (R–X). This reaction was carried out under reflux temperature in acetone or acetonitrile as solvent (Scheme 1).
According to the results previously reported, polar aprotic solvents such as acetone, acetonitrile and DMSO are the suitable solvents for this type of reactions [6]. According to the results obtained from experiments in the case of iodide alkyl halides, the reaction was completed in mild temperature. Acetone was chosen as solvent, because the iodide is good leaving groups. In the case of chloride and bromide alkyl halides, higher temperature was required for completion of reaction and acetonitrile was used as solvent for these compounds.
With the precursor 3 in hand, the focus was on the optimization of reaction condition for compound 5b as representative of the final products. As can be seen in Table 1, the N-alkylation reaction was surveyed in the variety of indanone:alkylhalide ratio, time and temperature. Among different times, the optimal results were obtained in 48 h and for reflux temperature (80 °C for CH3CN). By increasing the indanone:alkylhalide ratio until 1:3, the yield was increased up to 90%, but there was no change in yield afterward.
All molecular structures of the synthesized compounds were confirmed by 1H NMR, 13C NMR and FTIR spectroscopy and elemental analysis.
Inhibition of human AChE and BuChE
The extent of inhibition was expressed as the chemical concentration at which 50% of enzyme activity was inhibited (IC50). The IC50 values of compounds 3 and 5a–o were determined against human erythrocyte AChE and human plasmatic butyrylcholinesterase using the method of Ellman et al. [23]. The IC50 values and selectivity index (SI) of synthesized pyridinium halide derivatives and the control compounds, donepezil and galantamine are summarized in Table 2. These synthesized derivatives demonstrated inhibitory activity against both hAChE and hBuChE with IC50 values ranging from micro-molar to submicromolar concentrations. The IC50 values of the synthesized compounds suggest strong BuChE inhibition in comparison with AChE for these derivatives. Among these compounds, the highest inhibition of hAChE was recorded for 5g (IC50: 4.6 μM, weaker than donepezil and galantamine). The most potent inhibitor of hBuChE is 5f which showed IC50: 0.55 μM, while this value is approximately 26.18 times higher than donepezil and 57.45 times higher than galantamine; but in the case of hAChE, this compound showed IC50: 22.1 μM that is weaker than donepezil and galantamine. It is notable that the compounds with chlorine and bromine atoms substitution on phenyl ring show better inhibition compared with the others. Summarized results in Table 1 indicated that bromine atom in ortho position shows a better inhibition for hAChE (compound 5e); meanwhile, a better inhibition of hBuChE was obtained when the bromine set in meta position (compound 5d).
Conclusion
In summary, a series of 1-alkyl-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium halide were designed and synthesized with acceptable yield using N-alkylation reaction of 5,6-dimethoxy-2-[(pyridin-4-yl)methylene)1-indanone. Anti-AChE and anti-BuChE effects of all synthesized compounds were tested, 1-hexyl-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium bromide (5g) displayed excellent inhibition of hAChE, and the most potent inhibitor of hBuChE was 1-(2-chlorobenzyl)-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium chloride (5f).
References
N. Chitranshi, S. Gupta, P.K. Tripathi, P.K. Seth, Med. Chem. Res. 22, 2328 (2013)
M.A. Ali, M.S. Yar, M.Z. Hasan, M.J. Ahsan, S. Pandian, Bioorganic Med. Chem. Lett. 19, 5075 (2009)
F.C. Meng, F. Mao, W.J. Shan, F. Qin, L. Huang, X.S. Li, Bioorganic Med. Chem. Lett. 22, 4462 (2012)
A. Saxena, W. Sun, C. Luo, T.M. Myers, I. Koplovitz, D.E. Lenz et al., J. Mol. Neurosci. 30(1–2), 145 (2006)
M.G. Cardozo, T. Kawai, Y. Iimura, H. Sugimoto, Y. Yamanishi, A.J. Hopfinger, J. Med. Chem. 35, 590 (1992)
L. Peauger, R. Azzouz, V. Gembus, M.L. Tintas, J. Sopková-de Oliveira Santos, P. Bohn, C. Papamicaël, V. Levacher, J. Med. Chem. 60(13), 5909 (2017)
H. Sugimoto, Y. Iimura, Y. Yamanishi, K. Yamatsu, Bioorganic Med. Chem. Lett. 2(8), 871 (1992)
S. Hamulakova, L. Janovec, M. Hrabinova, K. Spilovska, J. Korabecny, P. Kristian, K. Kuca, J. Imrich, J. Med. Chem. 57(16), 7073 (2014)
P. Camps, X. Formosa, C. Galdeano, T. Gómez, D. Muñoz-Torrero, L. Ramírez, E. Viayna, E. Gómez, N. Isambert, R. Lavilla, A. Badia, Chem. Biol. Interact. 187(1–3), 411 (2010)
K.C. Miles, C.C. Le, J.P. Stambuli, Chem. Eur. J. 20(36), 11336 (2014)
J.K. Clark, P. Cowley, A.W. Muir, R. Palin, E. Pow, A.B. Prosser, R. Taylor, M.G. Zhang, Bioorganic Med. Chem. Lett. 12(18), 2565 (2002)
H. Sugimoto, Y. Yamanishi, Y. Iimura, Y. Kawakami, Curr. Med. Chem. 7, 303 (2000)
M.H. Tabert, X. Liu, R.L. Doty, M. Serby, D. Zamora, G.H. Pelton, K. Marder, M.W. Albers, Y. Stern, D.P. Devanand, Ann. Neurol. 58, 155 (2005)
M. Pohanka, Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 155, 3 (2011)
B. Lantaño, J.M. Aguirre, E.V. Drago, M. Bollini, D.J. de la Faba, J.D. Mufato, Synth. Commun. 47(23), 2202 (2017)
A. Shaabani, A. Sarvary, S. Keshipour, A.H. Rezayan, R. Ghadari, Tetrahedron 66(10), 1911 (2010)
M.H. Chahma, C. Combellas, A. Thiebault, Synthesis 2004(04), 517 (2004)
R.P. Singh, R.D. Verma, D.T. Meshri, J.N. Shreeve, Angew. Chem. 45(22), 3584 (2006)
A.S. Kiselyov, Tetrahedron Lett. 46(26), 4487 (2005)
S. Keshipour, S. Shaabani, A. Shaabani, Tetrahedron Lett. 53(52), 7085 (2012)
S. Keshipour, A. Shaabani, M. Pedarpour, A. Sarvary, Res. Chem. Intermediat. 40(1), 149 (2014)
J.S. Lan, T. Zhang, Y. Liu, J. Yang, S.S. Xie, J. Liu, Y. Ding, Eur. J. Med. Chem. 133, 184 (2017)
G.L. Ellman, K.D. Courtney, V. Andres Jr., R.M. Fesrtherstone, Biochem. Pharmacol. 7, 88 (1961)
G. Karimi, M. Iranshahi, F. Hosseinalizadeh, B. Riahi, A. Sahebkar, Pharmacology 1, 566 (2010)
Acknowledgements
This project was financially supported by a grant from Deputy for Research and Technology of Iranian Ministry of Health and Medical Education. The authors would like to acknowledge the financial support from Tabriz University of medical sciences and the University of Tabriz.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Farrokhi, H., Mozaffarnia, S., Rahimpour, K. et al. Synthesis, characterization and investigation of AChE and BuChE inhibitory activity of 1-alkyl-4-[(5,6-dimethoxy-1-indanone-2-yl)methylene]pyridinium halide derivatives. J IRAN CHEM SOC 17, 593–600 (2020). https://doi.org/10.1007/s13738-019-01804-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01804-1