Abstract
In this study, a new Cr(III)-imprinted polymer (Cr(III)-IIP) is prepared from CrCl3·6H2O, methacrylic acid functional monomer, ethyleneglycoldimethacrylate cross-linking agent, 2,2ʹ-azobisisobutyronitrile radical initiator and 2,2-(azanediylbis (ethane-2,1-diyl))bis(isoindoline-1,3-dione) ligand. To obtain the maximum adsorption capacity, the optimum condition was studied through pH, type and concentration of eluent, IIP weight, sample volume as well as the adsorption and desorption times. The Cr(III) ion content was determined via flame atomic absorption spectrometer. In optimum conditions, the adsorption capacity of the IIP for Cr(III) was obtained to be 74.65 mg g−1, using 50 mg of IIP and the initial pH solution of 3.0. Both the adsorption and desorption times for quantitative analyses of Cr(III) ions were 15 and 5 min; respectively. After elution of the adsorbed ions by 3 mL of 4 mol L−1 HNO3 aqueous solution, the established IIP-based SPE procedure provides a reasonable pre-concentration factor of 100. The IIP-based pre-concentration method provides a low detection limit of 1.7 µg L−1 with good repeatability (RSD = 3.22%). Reusability studies confirmed that synthesis IIP is reusable and recoverable up to six cycles. According to the selectivity experiments, it was concluded that the prepared sorbent possesses more affinity toward Cr(III) ions than other ions such as Al3+, Pb2+, Cu2+, Mn2+, Fe2+, Zn2+, and Ni2+ ions. To evaluate the potential applicability of the proposed separation method, the pre-concentration and determination of trace amounts of Cr(III) were performed successfully in food samples with complex matrices, a bestial sample (i.e. cow liver) and an herbal product (i.e., broccoli) as real samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the contaminant impacts of various pollutants for the environment, development of the separation methods for selective and sensitive determination of these species in the environmental samples, especially water samples, is important [1,2,3]. Among the various types of environmental pollutants, chromium is a hard metal with high boiling point, and high polished, significant resistance at the deterioration and rust measures. Since Cr(II) is a very strong reducing agent, it is not found in biological systems [4]. Cr(III) ion is toxic to most of the biotic organisms as well as biotic organisms, higher than 0.05 ppm concentration of Cr(III) lead to severe detrimental and lethal effect on the prevailing flora fauna [5], and it act as carcinogenic agent in animals and causes severe irritation problems in humans [6]. Chromium (III) possesses toxic effect on humans, animals and plants. The exposure of living organisms to Cr(III) compounds can lead to damage of skin, respiratory tract, kidneys and increases risk of lung cancer. The toxicity of chromate ions is connected to its strong oxidative potential and the possibility of free diffusion across the cell membranes [7]. The maximum allowed limits for contaminants in treated wastewater are enforced in both developed and many developing countries [8]. Chromium (III) is an essential micronutrient playing an important role in the metabolism of glucose as a component of glucose tolerance factor (GTF) as well as in the metabolism of some lipids, such as cholesterol [7]. The determination of chromium species is not possible directly by instrumental methods including flame and/or graphite furnace atomic absorption spectrometry. To solve this problem generally, separation/pre-concentration procedures including liquid–liquid extraction, ion-exchange, electroanalytical techniques, membrane filtration, cloud point extraction, solid phase extraction [9,10,11] and ion-imprinted polymer [12, 13] are applied. During the last years, ion-imprinted polymers (IIPs) have received much attention by various groups due to some benefits such as high selectivity, reusability, chemical stability and adsorption capacity [14, 15]. The high selectivity of IIPs can be explained by the polymer memory effect towards interaction with a specific ligand, coordination geometry, coordination number, charge and size of the metal ion [16, 17].
This work aimed to develop a separation method based on IIP materials as selective sorbent towards target ion for solid-phase extraction of Cr(III) ions from aqueous solution. After synthesis and characterization of the IIP particles, the pre-concentration percentage of chromium ions from aqueous media was studied as a function of experimental parameters using the selective polymeric materials. After optimization, the porous Cr(III)-IIP was functioned as efficient sorbent for separation of Cr(III) ions prior to its determined by flame atomic absorption spectrometer (FAAS), as a good technique for analysis of trace amounts of metal ions.
Experimental
Materials
Methacrylic acid (MAA), ethyleneglycoldimethacrylate (EGDMA), and 2,2′-azobisisobutyro nitrile (AIBN) were purchased from Aldrich (Milwaukee, WI, USA). Ethanol by reagent grade from Merck chemical company was used as a solvent. 2,2-(azanediylbis (ethane-2,1-diyl))bis(isoindoline-1,3-dione) and CrCl3·6H2O and nitrate or chloride salts of other cations was provided from Merck chemical company.
Apparatus
An Analytic Jena model nova 400 flame atomic absorption spectrometer was used for measurement of chromium ions and other metallic cations from solution. A 780 digital pH meter (Metrohm), equipped with a combined Ag/AgCl glass electrode was used for pH setting. Scanning microscopy was carried out using SEM Philips, XL30 instrument. The FTIR spectra of the synthesized material were recorded using an infrared spectrometer (Bruker FT-IR vertex 70) by KBr pellets in the range of 400–4000 cm−1. X-ray diffraction patterns were collected using Cu-Kα radiation on a Philips-PW 17C diffractometer.
Preparation of Cr3+ ion-imprinted polymer (Cr(III)-IIP)
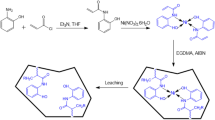
The Cr(III)-IIP were prepared by precipitation polymerization technique similar to the literature, with some modifications [18]. For this purpose, 1 mmol of 2,2-(azanediylbis(ethane-2,1-diyl))bis(isoindoline-1,3-dione) and 1 mmol of CrCl3·6H2O were dissolved in 40 mL of ethanol as porogen solvent, and stirred by magnetic stirrer for 1 h. Then 4.7 mL MAA, 0.4 mmol AIBN and 30 mmol EGDMA were added to the mixture. The polymerization mixture was purged for 10 min with N2 gas to remove the molecular oxygen and stirred in the oil bath at 60 °C under a nitrogen atmosphere for 24 h. After polymerization, the obtained polymer was washed with ethanol and deionized water to remove unreacted material. Imprinted ion was leached with 3 × 50 mL nitric acid by continuous stirring for 18 h and was kept in an oven to dry. The synthetic producer of unleached and leached Cr(III)-IIP is shown in the Scheme 1. The non-imprinted polymer (NIP) was prepared in the same way without chromium ions.
Extraction procedure
The extraction procedure of Cr(III) was performed at optimum pH (3.0). The pH of sample solution was adjusted by drop-wise addition of 0.10 M solution of sodium hydroxide or hydrochloric acid. 50 mg of dried polymer was added to 25 mL of aqueous solution containing 1.0 µg mL−1 of Cr(III), and stirred for 15 min. After that, the mixture was centrifuged (3000 rpm, 10 min) and the supernatant was removed. The Cr(III) ions pre-concentrated onto sorbent were eluted by 3 mL solution of nitric acid with 4 M concentration and stirred for 5 min. The resulting solution was centrifuged and the Cr(III) contents of the solution were determined by FAAS. The extraction percentage of Cr(III) ions could be obtained by the following equation:
Ci and Cf are the concentrations of Cr(III) ion before and after extraction in the solution.
Adsorption capacity (Q) can be calculated from following equation:
where m indicates the mass of sorbent (mg); instead of the “Ci − Cf” the concentration of chromium ions in desorption solution that were determined by FAAS can used. The distribution ratio (mL g−1) of Cr(III)was determined and defined by Eq. (3),v (L) and m (g) are initial solution volume and mass of IIP material, respectively. Ci (mg L−1) and Cf (mg L−1) displayed the initial and equilibrium concentration of Cr(III).
Following Eqs. (4 and 5) were used to calculate the selectivity coefficient \(({K_{\frac{{{\text{C}}{{\text{r}}^{3+}}}}{{{{\text{M}}^{n+}}}}}})\) and relative selectivity coefficient (K′) of Cr(III) ions.
\(K_{{\text{d}}}^{{{\text{C}}{{\text{r}}^{3+}}}}\)and \(K_{{\text{d}}}^{{{{\text{M}}^{n+}}}}\) are the distribution ratios of chromium and potentially interfering ions, respectively.
Real sample preparation
1 g of cow liver was transferred to a furnace for 6 h at 650 °C. The residue was cooled and treated with 10 mL concentrated nitric acid, and then after the mixture was heated at 220 °C for 1 h. After cooling, 5 mL hydrogen peroxide was added to the mixture. The final residue was diluted using deionized water and the pH of solution adjusted to 3. The broccoli as a one type of vegetables was chosen for analysis, which was collected from the local supermarket. Sample was air dried followed by drying for 2 h and then powdered. 1 g of the powder placed in the furnace for 3 h at 300 °C. After cooling the residue and treated with 10 mL of concentrated nitric acid, the mixture was heated at 150 °C for 1 h, and then 5 mL of hydrogen peroxide was added to the dried mixture. Then the resulting material was diluted with deionized water and the pH of solution adjusted to 3.
Results and discussion
Characterization studies
The XRD patterns of the unleached (A), leached (B) and NIP (C) ion-imprinted polymer are shown in Fig. 1. The similarity between XRD patterns confirmed that the three samples have identical structures and shows the structure stability of as-prepared IIP against leaching process under acidic conditions. Meanwhile, the slight difference between the XRD patterns of unleached and leached IIP samples is due to the presence of Cr ions in the polymer backbone [19].
The surface morphology of the leached and unleached polymers was studied by SEM (Fig. 2). SEM images show spherical shape and narrow distribution of the particles in both leached and unleached IIP. From the SEM images, the particles of both samples have an irregularity in shape; however, after washing process, a significant decrease in the average particle size from 788 nm in unleached polymer to 331 nm in leached polymer with an improvement in their distributions can be observed.
FT-IR spectra of unleached and leached Cr(III)-IIP particles are shown in Fig. 3. As seen, a similarity in the spectra patterns is obvious, which may be due to the similar backbone of the polymer samples. Moreover, it can be due to that the polymeric network was remained stable after leaching process using strong acidic solution. Meanwhile, the chemical stability of the ligand in the polymer matrix may be concluded [20, 21]. However, the observed frequency shift in the IR spectra was assigned to the removal of imprinted metal ion from the polymer [22]. The different frequencies for N–H band were observed in leached and unleached IIP. The frequency of N–H band in unleached IIP appears at 3562 cm−1 and was shifted to 3645 cm−1 in leached IIP. This is assigned to the fact that chromium ions coordinated with non-bonding electron pairs of nitrogen atoms in the incorporated ligand.
Sorption/desorption studies
Among the factors that affected on metal adsorptive processes by IIPs, pH was found to be most essential parameter that is a logarithmic quantity. To study the pH effect on the extraction efficiency, pH of 25 mL sample solution containing 1 µg mL−1 of chromium ion was adjusted in the range of 2–10 with drop-wise addition of sodium hydroxide or hydrochloric acid. The adsorption of Cr(III) ions on the prepared IIP particles increased up to pH 3, then decreased continuously from pH 3 to 10 (Fig. 4). Based on obtained data, it could be said that the quantitative adsorption of Cr(III) ions on the IIP particles maybe occurred at pH 3, thus this pH was chosen as optimum. At pH < 3, protonation of N-atoms in the used ligand may limit the strong interaction and complexation between Cr(III) ions and the donor atoms in the cavity, thus the adsorption efficiency was decreased. Beside, a significant comparison between the hydrogen ions and chromium ions for occupying the active sites at high acidic media is not unexpected. On the other hand, by considering the very small solubility product constant of Cr(OH)3 (Ksp = 7 × 10−31), hydrolysis of Cr(III) ions with the high charge density in neutral as well as alkaline media is not unexpected [23]. Therefore, the free concentration of Cr(III) ions was decreased and causes a decrease in its adsorption efficiency. From the above discussion, a possible mechanism based on strong complexation between Cr(III) ions and the incorporated ligand beside the good fitness of the imprinted metal ion to the created cavity may be proposed for efficient sorption of Cr(III) at pH 3. Furthermore, the effectiveness of Cr(III) retention on the control polymer was examined through the similar approach which provide the maximum extraction percentage < 60% at the optimum pH value.
The type, volume and concentration of the eluent solution are the important factors to have a successful elution process for desorption of the adsorbed analytes on the sorbent surface. For this, various aqueous solution of HNO3, H2SO4 and HCl were prepared in the concentration and volume range of 0.5–4.0 mol L−1, and 1.0–10.0 mL, respectively. After loading step, the particles were eluted by the solutions in the separate experiments and the release amount of Cr(III) was determined by FAAS method. As obtained, the highest extraction was attained after using 3 mL of 4 mol L−1 HNO3 aqueous solution as eluent solution. The required eluent with harsh condition may be attributed to the high stability constant of the formed complex between the template ion and the imprinted cavities in the polymeric network. The results are presented in Table 1.
The adsorption efficiency was studied as a function of the different amount of sorbent, as an important factor in the adsorption process. For this, 25 mL of aqueous solution containing 1.0 µg mL−1 Cr(III) ions which adjusted at pH 3 were prepared in the presence of different weight of IIP particles (10–120 mg), and then the extraction of Cr(III) ions was calculated after the sorption step. As it can be obvious (Table 1), increasing the IIP weight from 10 to 50 mg causes the adhesion of the quantitative recovery of Cr(III). However, no significant changes were observed in the Cr(III) recovery by continuous increasing IIP weight up to 120 mg. So, 50 mg of IIP particles was applied for further investigations (Table 2).
To study the effect of both adsorption and desorption time on the Cr(III) recovery, a wide stirring time (from 2 to 30 min) was applied at optimum conditions. As it can be observed from Fig. 5, the maximum recovery was observed after applying 15 and 5 min stirring for adsorption and desorption processes, respectively. The acceptable adsorption and desorption of Cr(III) ions can be attributed to affinity of as-prepared nano-scaled IIP sorbent with high surface area and affinity toward Cr(III) ions [15] (Figs. 6, 7, 8).
To determine the possible maximum aqueous sample volume as well as the pre-concentration factor, 50 mg of IIP in different sample volumes from 25 to 600 mL containing the same concentrations of Cr(III) ions were prepared at optimum conditions, and then the recovery percentages were calculated. The results showed that the dilution was not significant effects on Cr(III) recovery factor for the used sample volumes up to 300 mL; however, at higher sample volumes, the recovery of Cr(III) ions was decreased, significantly. Thus, an acceptable pre-concentration factor of 100 can be achieved for separation of Cr(III) ions from aqueous solutions using the present synthetic IIP particles.
Reusability of the prepared IIP particles was studied in the consecutive sorption experiments. To examine this factor, 50 mg of IIP particles was applied for different adsorption /desorption cycles. By considering the quantitative extraction > 95%, Cr(III)-IIP particles are reusable for five cycles without decreasing in adsorption–desorption activity. From the sixth to tenth cycles, the recovery factor decreased continuously and reached less than 80%.
The maximum adsorption capacity is defined as the highest amount of metal ions adsorbed per gram of the sorbent particles. To obtain and compare the adsorption capacity for IIP or NIP particles, 50 mg of each sorbent was added to 25 mL solutions containing 1.0–70.0 µg mL−1 of Cr(III) ions. The adsorption value increased with the adhesion of Cr(III) concentration from up to 50.0 µg mL−1, and then the level-off was achieved at the concentration range from 50.0 to 70.0 µg mL−1 (Fig. 5). From Eq. (2), the results indicated that the adsorption capacity of IIP (74.65 mg g−1) particles are greater than NIP particles (51.5 mg g−1).
The linear range, precision and detection limit
To determine chromium ions, the calibration curve was established under optimal conditions and the analytical data of the present method are given in Table 1. As it can be seen, a linearity behavior was achieved for Cr(III) ions over the concentration range of 0.01–18.0 µg mL−1, with an excellent correlation coefficient of 0.9995. The detection limit was calculated from 3Sb/m formula to be 1.7 µg L−1; where m is the slope of the calibration curve and Sb is standard deviation of five replicate blank signals. The relative standard deviation was found to be 3.22% for five separate experiments.
Selectivity studies
The selective performance of the proposed IIP for Cr(III) adsorption was investigated in the presence of the other interference ions. In IIP particles, the cavities created after removal of the template are supplementary to the imprinted ion in size and coordination geometries. Therefore, the selectivity coefficient of Cr(III) ions was evaluated by batch experiments and extraction of Cr(III) ions from a solution containing Pb2+, Al3+, Cu2+, Fe2+ Ni2+ and Zn2+ ions, and results are reported in Table 3. Polymer material (50.0 mg) was added to 25 mL aqueous solutions containing 1.00 μg mL−1 Cr3+/Mn+. Based on the obtained data, the following facts could be suggested:
-
1.
Regarding the non-imprinting material, the relative selectivity coefficients indicated adsorption affinity as well as the imprinting material selectivity of for the template.
-
2.
Regarding the potentially interfering ions, the selectivity coefficients of NIP for Cr(III) are very low.
-
3.
Regarding the potentially interfering ions, the selectivity coefficients of Cr(III)-IIP are excellent for Cr(III) ions.
Real sample analysis
As a synthetic solid-phase extractor, the applicability of the prepared IIP sorbent was tested for pre-concentration of trace levels of Cr(III) ions in aqueous solution of liver and broccoli samples. Through conducting the standard sample preparation methods, the analytical samples of the real samples were obtained and their Cr(III) ion content was determined based on the standard addition method (Table 4). It was found that the present IIP-based solid-phase extraction method can be applied for quantitative extraction and successful determination of chromium ions in various complex real samples with acceptable precision and accuracy, even in the presence of various diverse ions.
Conclusion
Based on the ion-imprinted polymer, the solid-phase extraction can be considered as a suitable technique for the metal ions’ pre-concentration. The acceptable adsorption/desorption kinetic, high adsorption capacity, reasonable selectivity, simple synthesis and the prepared sorbent stability make a lot of attention to ion-imprinted polymers. In this study, Cr(III)-IIP is prepared successfully and is applied for pre-concentration and determination of Cr(III) ions in some food samples in Table 5; the recent synthesized Cr-IIP which reported for separation and pre-concentration of Cr(III) ions are listed [12, 13, 24,25,26,27,28,29,30] which are extracted from various real samples based on different ligands and detection using different techniques. By comparing the sorbent characteristics, it can be concluded that as-prepared Cr-IIP nanoparticles can be categorized as an efficient and favorable sorbent with high adsorption capacity for separation and Cr(III) ion pre-concentration with acceptable precision and selectivity.
References
S.G. Hosseini, S.M. Pourmortazavi, Kh. Gholivand, Desalination 245, 298 (2009)
M. Shamsipur, M.M. Zahedi, S.M. Pourmortazavi, I. Kohsari, Anal. Methods 5, 496 (2013)
M. Shamsipur, M.M. Zahedi, S.M. Pourmortazavi, Anal. Sci. 29, 1055 (2013)
C. Gabriel, C.P. Raptopoulou, C. Drouza, N. Lalioti, A. Salifoglou, Polyhedron 28, 3209 (2009)
A. Assadi, M.H. Dehghani, N. Rastkari, S. Nasseri, A.H. Mahvi,Environ. Prot. Eng. 38, 5 (2014)
M.H. Dehghani, B. Heibati, A. Asadi, I. Tyagi, S. Agarwal, V.K. Gupta, J. Ind. Eng. Chem. 33, 197 (2016)
J. Kotaś, Z. Stasicka Environ. Pollut. 107, 263 (2000)
E.J. Yurkow, J. Hong, S. Min, S. Wang, Environ. Pollut. 117, 1 (2002)
C.G. Bruhn, L. Villablanca, V.H. Campos, S. Basualto, J. Tapia JCCHEMS 42, 83 (1997)
M.A. Eid, E.A. Al-Ashkara, K.A. Eid, E.H.A. Nashy, E.H. Borai, J. Am. Leather Chem. Assoc. 97, 451 (2002)
M. Mohammadhosseini, M.S. Tehrani, M.R. Ganjali, J. Chin. Chem. Soc. 53, 549 (2006)
B. Leśniewska, B. Godlewska-Żyłkiewicz, A.Z. Wilczewska, Microchem. J. 105, 88 (2012)
N. Zhang, J.S. Suleiman, M. He, B. Hu, Talanta 75, 536 (2008)
M. Shamsipur, H.R. Rajabi, S.M. Pourmortazavi, M. Roushani, Spectrochim. Acta Part A 117, 24 (2014)
M. Shamsipur, H.R. Rajabi, M.H. Beyzavi, H. Sharghi, Microchim. Acta 180, 791 (2013)
H.R. Rajabi, M. Shamsipur, M.M. Zahedi, M. Roushani, Chem. Eng. J. 259, 330 (2015)
M. Roushani, S. Abbasi, H. Khani, R. Sahraei, Food Chem. 173, 266 (2015)
M. Roushani, Z. Saedi, F. Hamdi, B.Z. Dizajdizi, J. Electroanal. Chem. 804, 1 (2017)
M. Roushani, T.M. Beygi, Z. Saedi, Spectrochim. Acta Part A 153, 637 (2016)
H.R. Rajabi, A. Zarezadeh, Gh. Karimipour, RSC Adv. 7, 14923 (2017)
Q. Liang, J. Geng, H. Luo, W. Fang, Y. Yin, J. Mol. Liq. 248, 767 (2017)
H.R. Rajabi, F. Fereidonipour, New J. Chem. 41, 8828 (2017)
R. Huang, X. Ma, X. Li, L. Guo, X. Xie, M. Zhang, J. Li, J. Colloid Interface Sci. 514, 544 (2018)
E. Birlik, A. Ersoz, E. Acıkkalp, A. Denizli, R. Say, J. Hazard. Mater. 140, 110 (2007)
Q. He, X. Chang, H. Zheng, N. Jiang, X. Wang, Int. J. Environ. Anal. Chem. 88, 373 (2008)
B. Lesniewska, I. Jakubowska, E. Zambrzycka, B. Godlewska-ZyLkiewicz, Turk. J. Chem. 40, 933 (2016)
L. Trzonkowska, B. Leśniewska, B. Godlewska-Żyłkiewicz, React. Funct. Polym. 117, 131 (2017)
L. Trzonkowska, B. Leśniewska, B. Godlewska-Żyłkiewicz, Anal. Methods 7, 1517 (2015)
F. An, B. Gao, Desalination 249, 1390 (2009)
Y. Liu, X. Meng, J. Han, Z. Liu, M. Meng, Y. Wang, R. Chen, S. Tian, J. Sep. Sci. 36, 3949 (2013)
Acknowledgements
The authors thank the Ilam University Research Council for financial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Roushani, M., Saedi, Z., Hamdi, F. et al. Application of ion-imprinted polymer synthesized by precipitation polymerization as an efficient and selective sorbent for separation and pre-concentration of chromium ions from some real samples. J IRAN CHEM SOC 15, 2241–2249 (2018). https://doi.org/10.1007/s13738-018-1413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1413-0