Abstract
A simple and ultrasensitive sensor based on carbon paste electrode (CPE) modified by multiwall carbon nanotube (MWCNT) was developed for the electrochemical determination of anticancer drug dacarbazine (Dac). The electrochemical behavior of Dac was studied in 0.1 M phosphate buffer solution (PBS) using cyclic voltammetry (CV) while differential pulse voltammetry (DPV) was used for quantification. Compared with the unmodified carbon paste electrode, the modified electrode facilitates the electron transfer of Dac, since it notably increases the oxidation peak current of Dac. The electronic transfer property of the MWCNT carbon paste electrode was characterized by electrochemical impedance spectroscopy (EIS). Also, morphology of the electrode was characterized by scanning electron microscopy (SEM). Under optimized conditions, the modified electrode exhibited a linear response over the concentration ranging from 0.4 to 40 nM and 40–2500 nM Dac, with a detection limit of 0.12 nM. The proposed sensor exhibited a high sensitivity, good stability and was successfully applied for Dac determination in pharmaceutical sample, with good percentage recovery. In addition, to evaluate precision and accuracy of DPV method, high-performance liquid chromatography (HPLC) method was used.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dacarbazine (scheme 1) is an anticancer drug which is used for the treatment of Hodgkin’s disease, malignant melanoma, childhood solid tumors and soft tissue sarcoma [1]. In recent years, Dac has arisen as important chemotherapeutic agent, in particular for the treatment of malignant melanoma [2]. Dac causes methylation of the DNA bases and these DNA lesions lead to cell death, mainly via apoptosis [3]. It is the only US Food and Drug Administration (FDA)-approved chemotherapeutic agent for treating wild-type melanoma, a skin cancer that accounts for the vast majority of skin cancer deaths. It is the most active single agent currently used for treating metastatic melanoma [4]. Also, Dac is supplied as a sterile lyophilized powder that can be reconstituted for intravenous injection. Dacarbazine can also be dispensed orally, but this way of administration is limited because of its incomplete absorption in the body [5]. Like other chemotherapeutical agents, Dac also causes side effect by killing normal dividing cells [4]. The widespread use of Dac and the need for clinical and pharmaceutical studies require fast and sensitive analytical techniques to determine this drug in a variety of biological fluids and in the environment [6]. Stability and compatibility assays of pharmaceutical formulations of Dac by LC-UV [7,8,9,10] and LC–MS [11] have been described. Both of these methods have also been used for the quantification of Dac and its degradation products in urine and plasma [12,13,14]. Also, HILIC-MS/MS method with a two-step extraction process is applied for the analysis of Dac and its terminal metabolite (5-amino-4-imidazole-carboxamide) in human plasma [14].
Carbon nanotubes (CNTs) exhibit excellent mechanical, electrical and electrocatalytic properties [15]. In the past decade, massive amount of works have been done on both multiwall carbon nanotube (MWCNT)- and single-walled carbon nanotube (SWCNT)-based electrochemical sensors and biosensors [16]. Carbon nanotubes (CNTs) are considered as an important group of nanostructures with attractive electronic, chemical and mechanical properties [17, 18]. The compatibility and electrochemical applications of CNTs to immobilize a variety of species on their external and internal surfaces have been reported [19,20,21,22,23]. Multiwall carbon nano tubes (MWCNTs) have attracted considerable attention in electrochemical field because of their unique physical and chemical properties [24, 25]. MWCNTs are often used in the fabrication of sensors to promote the electron transfer reactions of various molecules and increase the available electroactive surface area of various electroactive substances [26].
Remarkable research and development activities aimed at the realization of biosensors for the measurement of chemical and biological compounds have been witnessed in the recent years [27]. In addition, characteristics such as higher selectivity, better sensitivity, higher speed, repeatability, cost and time made electrochemical methods more suitable for measurement of some drugs [28]. Due to advantages such as non-toxic, low background current, wide applicable potential range, rapid renewability and easy fabrication, modified carbon paste electrode (MCPE) has attracted considerable attention in recent years [29, 30].
Therefore in the present work, multiwall carbon nanotube (MWCNT) was used for construction of a simple and ultrasensitive electrochemical sensor for determination of Dac. There are only a few electrochemical studies on behavior of anticancer drug Dac. The electrochemistry of Dac has been studied by using dropping mercury electrode, hanging mercury drop electrode and glassy carbon electrode [31]. In the other research, the electrochemical behavior of the antitumor agent Dac and its major metabolite, 5-aminoimidazole-4-carboxamide (AIC), at carbon paste electrodes has been studied [32]. The electrochemical properties and the DNA/DNA base binding behavior of Dac drug at GCE [33, 34] and also the interaction of Dac with ss dsDNA at SPCE have been studied [35]. In the other works, electrochemical reduction of the dacarbazine–Cu2+ complex has been investigated using cyclic voltammetry and square wave voltammetry at a hanging mercury drop electrode [6]. Also, binding mode and thermodynamic studies on the interaction of dacarbazine and dacarbazine–Cu(II) complex with single- and double-stranded DNA has been performed [2].
In the present work, a differential pulse voltammetric method for the determination of anticancer drug Dac by using a MWCNT-modified carbon paste electrode with desirable electrochemical characteristics has been described. The proposed sensor has the best detection limit and linear range in comparison with the former reports for electrochemical determination of Dac. This method is convenient and useful because of its simplicity, low cost and low detection limit. Finally, the analytical performance of this modified electrode in the quantification of Dac in pharmaceutical sample has been evaluated.
Experimental
Apparatus and chemicals
Cyclic voltammetric (CV) and differential pulse voltammetric (DPV) measurements were performed using an Autolab PGSTAT302 N (Metrohm Autolab BV, Netherlands) with an FRA32 M impedance analysis module. The working electrode was an unmodified or modified carbon paste electrode. The auxiliary and reference electrodes were a platinum wire and saturated calomel electrode (SCE), respectively. A Metrohm 710 pH meter was used for pH measurements. The surface morphology of modified electrodes was characterized with a scanning electron microscope (SEM) (Philips XL 30). All measurements were carried out at ambient temperature (25 ± 1 °C). Dac and multiwall carbon nanotube (MWCNT) were of analytical grade from Sigma-Aldrich. The buffer solutions were prepared from orthophosphoric acid and its salts in the pH range of 2.0–12.0. All other reagents were of analytical grade, and solutions were freshly prepared with double distilled water.
Preparation of modified electrode
The unmodified and modified carbon paste were prepared by thoroughly mixing analytical grade graphite and paraffin oil in the absence and presence of modifier and then each paste was mixed in a mortar for at least 10 min to become homogeneous. Each paste was packed into one end of a metallic spacer which after inserting in a holder was used as a working electrode (see Fig. 1). The surface of each electrode was polished using a butter paper to produce reproducible working surface and then was used for determination of Dac by voltammetric techniques. The modified carbon paste was prepared by mixing different percentages of graphite powder, paraffin oil and modifier. The best results were obtained at 60:30:10% of graphite, paraffin oil and modifier.
Analytical procedure
The modified electrode was first activated in a 10 ml volume of 0.1 M phosphate buffer by potential scanning from − 0.25 to 1 V versus SCE at a scan rate of 100 mV s−1, until a low and steady background was obtained. DP voltammetric experiments were conducted under the instrumental conditions of 50 mV pulse amplitude, 40 ms pulse width and 60 mVs−1 scan rate. For recording the cyclic voltammograms, the potential was scanned from the 0 to 1.2 V using the scan rate of 100 mVs−1.
Results and discussion
Characterization of the modified electrode
Electrochemical impedance spectroscopy (EIS) technique is an effective method for probing the features of surface modified electrodes. In the impedance spectrum, the semicircle diameter of impedance equals the electron transfer resistance (Ret). Figure 2 shows the typical Nyquist plots of bare CPE (curve a with Ret = 134.0 Ω) and MWCNT/CPE (curve b with Ret = 24.4 Ω), in 0.1 M KCl solution containing 5 mM [Fe(CN)6]3−/4− as an electrochemical redox marker. MWCNT/CPE shows a small semicircle, at the high-frequency region, in the comparison with the bare CPE. These observations can be attributed to the presented MWCNT (with good conductivity and large surface area) in the modified electrode. MWCNT could effectively increase the rate electron transfer between electrode surface and [Fe(CN)6]3−/4− and decrease interface electron transfer resistance.
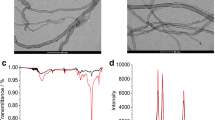
Figure 3 displays a typical morphology of CPE (A) and MWCNT/CPE (B). It can be seen that on the surface of CPE (Fig. 3a), the layer of irregular flakes of graphite powder was present and isolated from each other. After multiwall carbon nanotube added to the carbon paste matrix, it can be seen that multiwall carbon nanotubes were dispersed on the electrode with special three-dimensional structure (Fig. 3b). It is noticeable that scales in Fig. 3a and b are 30 and 1 µm, respectively.
Electrochemical behavior of Dac at MWCNT/CPE
The voltammograms of the phosphate buffer solution (pH 2) in the absence (curves a and b) and presence (curves c and d) of 2 μM Dac at the surface of the bare CPE and MWCNT/CPE were recorded, and the results are shown in Fig. 4. The oxidation of Dac at the bare carbon paste electrode produced a weak anodic peak (curve c), while its peak current increased considerably when the bare electrode was modified with MWCNT. The presence of MWCNT facilitates the electron transfer at MWCNT/CPE, and so the sensitivity of oxidation of Dac increases at the surface of modified electrode. Also the peak potential of oxidation of Dac at the surface of modified electrode shifted toward less positive potentials compared with unmodified electrode. This observation is a result of simpler oxidation of Dac at the surface of modified electrode in the comparison of bare electrode.
Determination of electrochemical active surface area
In order to measure the electrochemically active surface area of the modified electrode, the chronoamperogram of 0.1 mM potassium ferrocyanide as a redox probe was recorded. In chronoamperometric studies, the current, i, for the electrochemical reaction (at a mass-transfer-limited rate) of ferrocyanide which diffuses to an electrode surface is described by Cottrell equation [36]:
where A is the electrochemical active area, D is the diffusion coefficient, C* is the bulk concentration of ferrocyanide and the other parameters have their typical meanings. Under diffusion control, a plot of i versus t−1/2 will be linear and from the slope, the value of A can be obtained, since the precise value of the diffusion coefficient of ferrocyanide is well known (6.20 × 10−6 cm2 s−1). The electrochemically active area of MWCNT/CPE was 0.09 cm2.
To understand the reaction mechanism, the effect of scan rate (ѵ) on the peak currents (Ip) of Dac at the modified electrode was investigated. Therefore, the cyclic voltammograms of 2 μM Dac at different scan rates including 25, 50, 100, 200, 250 and 500 mVs−1 were investigated at the MWCNT/CPE (Fig. 5). As shown in inset 5A, a linear relationship (Ip(µA) = 0.2536 ѵ (mVs−1) + 8.13) was obtained between the peak current and the scan rate in the range of 25–100 mVs−1, which revealed that the oxidation of Dac was an adsorption-controlled process in the low scan rates. But in the high scan rates (200–500 mVs−1), a linear relationship (Ip(µA) = 7.8079 ѵ1/2 (mVs−1) − 54.707) was obtained between the peak current and the scan rate, which revealed that the oxidation of Dac was a diffusion-controlled process in the high scan rates (see inset 5B). Only an oxidation peak was observed even at low scan rates, suggesting that the electrode reaction of Dac under these conditions was totally irreversible.
Cyclic voltammograms of 0.1 M phosphate buffer (pH 2) containing 2 µM Dac at different scan rates. The letters of a–f correspond to 25, 50, 100, 200, 250 and 500 mVs−1, respectively. Inset a: variation of the Ip with ʋ (for low scan rates) and inset b: variation of the Ip with ʋ1/2 (for high scan rates)
As it is shown by increasing the scan rate, the peak potential is shifted to more positive potential. Because of the irreversible electrode process of the oxidation reaction of Dac, the following Laviron’s equation [37] was used to estimate αn and ks values:
where α is the electron transfer coefficient, ks is the standard rate constant of the surface reaction, v is the scan rate, n is the electron transfer numbers and E° is the formal potential. ks and αn values can be concluded from the intercept and slope of the linear plot of Ep with respect to ln v, if the E° is known. The E° value at MWCNT/CPE can be deduced from the intercept of Ep versus v plot on the ordinate by extrapolating the line to v = 0 (E° = 0.95). By finding E°, and graphical representations of Ep versus ln v for Dac in the presence of MWCNT, the αn = 1 and ks = 2164 s−1 values were obtained from the slope and intercept, respectively.
On the other hand, for a totally irreversible electrode reaction process, α was assumed as 0.5. On the basis of the above discussion, the n was calculated to be 2 which indicated that two electrons were involved in the oxidation process of Dac at the surface of modified electrode.
On the other hand, the surface concentration of electroactive species (Г) was estimated using the slope of the linear plot of current density versus ʋ according to the following Eq. [38] and found to be 6.56 × 10−5 mol/cm2 (Fig. 9b).
The electrochemical behavior of Dac is pH dependent. Therefore, the effect of pH on the electrochemical behavior of 2 μM Dac in the pH range of 2.0–12.0 was investigated and the results are shown in Fig. 6a. In addition, for a better resolution, the cyclic voltammograms in the pH ranging from 2.0 to 9.0 are given in a distinct figure (Fig. 6b). As can be seen in these figures, the maximum current at the MWCNT-modified CPE was obtained in pH 2.0, and thus, this pH was chosen as the optimum pH for electrooxidation of Dac at the surface of MWCNT/CPE.
a: Effect of pH on cyclic voltammograms of 0.1 M phosphate buffer solution containing 2 µM Dac at 100 mVs−1 (at the higher range of PH: 2–12, a to g are pHs; 2, 4, 6, 7.4, 9, 11 and 12, respectively). Inset: Variation of the peak potential with pH. b: Like a, but at the lower range of PH: 2–9, a to g are pHs; 2, 4, 6, 7.4 and 9, respectively
Also the shift in the anodic peak potential at various pHs revealed that the oxidation of Dac is pH dependent. The oxidation of Dac occurs in one step for electrolytes with pH < 6, whereas two oxidation peaks can be observed in solutions with higher pH. The same behavior has been reported previously for dacarbazine’s analog compound, temozolomide (TMZ) [39]. Both Dac and TMZ exert their antitumour activity through the linear triazene, 5-(3-methyltriazen-1-yl)-imidazo-4-carboximide.
The mechanism of electrooxidation of Dac has been reported by other researchers previously (see scheme 2) [32].
Optimization of the amount of modifier in the electrode
Primarily, experiments show that the amount of MWCNT modifier in carbon paste electrode influences the anodic peak current of Dac. Therefore, five electrodes containing different percents of MWCNT (0, 5, 10, 15 and 20%) were prepared and examined for detection of 12 nM Dac under identical conditions. The maximum peak current was obtained when the amounts of the graphite powder, paraffin oil and MWCNT in the paste were 60:30:10% (w/w) (Fig. 7).
Analytical performance
Differential pulse voltammetry (DPV) (with pulse amplitude of 50 mV, pulse width of 40 ms and scan rate of 60 mVs−1) was used to measure Dac. The DPV signals obtained for tested different concentrations of Dac as well as the plot of peak current versus concentration of Dac is shown in Fig. 8. Two linear relationships are obtained over Dac concentrations ranging from 0.4 to 40 nM (Fig. 8, inset A) and 40–2500 nM, respectively (Fig. 8, inset B). The linear regression equations are:
where IP is the peak current and CDac is the concentration of Dac.
The limits of detection (LOD) and quantitation (LOQ) were calculated using the relation ks/m [40], where k = 3 for LOD and 10 for LOQ, s representing the standard deviation of the peaks current of the blank (n = 10) and m representing the slope of the first plot of peak current versus concentration of Dac. Both LOD and LOQ values were found to be 0.12 and 0.4 nM, respectively, which indicated the sensitivity of the proposed method.
This proposed electrode has the highest sensitivity for the direct electrochemical determination of Dac in comparison with the earlier studies. The results of this research show sharp and well-known peaks for anticancer drug Dac. In addition, in comparison with the electrochemical sensors which are based on the interaction of Dac with DNA, the proposed sensor has a simple strategy and fast response (there is no incubation of electrode in analyte solution as a time consuming process).
Reproducibility, repeatability and the long-term stability of the modified carbon paste electrode were investigated. Reproducibility of proposed electrode was investigated by using DPV. For this purpose, the voltammograms of the phosphate buffer solution (pH 2) containing 2 μM Dac at the surface of MWCNT/CPE were recorded. The obtained results of five replicate measurements show a standard deviation less than ± 0.96 for DPV currents. In repeatability investigations, the recovery of analyzed target (phosphate buffer solution (pH 2) containing 2 μM Dac) in 10 replicate measurements was calculated to be above 96%, and the relative standard deviation (RSD) was lower than 3.6%. For the investigation of electrode stability, the voltammograms of the phosphate buffer solution (pH 2) containing of 1.5 μM Dac at the surface of proposed electrode after 1 month were recorded and were compared with the primary data.
The obtained results showed that the proposed electrode retained 95% of its initial activity after 1 month, demonstrating its stability. These results indicate the acceptable properties for the proposed electrode.
On the other hand, the oxidation of Dac at the surface of MWCNT including scan rate effect and differential pulse voltammetry (DPV) was performed in biological pH (pH 7.4). As can be seen in Fig. 9, in this pH two oxidation peaks were observed for 2 μM Dac at the MWCNT/CPE at the scan rates of higher than 50 mV. A plot of peak height (Ip) against scan rate (v), in the range of 25–500 mVs−1, was constructed and it found to be linear with regression equation of Ip(µA) = 0.0963 v (mVs−1) + 9.6124 (R = 0.9997) indicating that the process is adsorption controlled.
Also, differential pulse voltammetry (DPV) was used to measure Dac in pH 7.4. The DPV signals obtained for tested different concentrations of Dac as well as the plot of peak current versus concentration of Dac are shown in Fig. 10. Two linear relationships are obtained over Dac concentrations ranging from 5 to 30 nM (Fig. 10, inset A) and 30–1500 nM, respectively (Fig. 10, inset B). The linear regression equations are:
Both LOD and LOQ values were found to be 1.5 and 5 nM, respectively.
Interference studies
For investigation of analytical application of the proposed method, the effects of some common interferences on the determination of 2 μM Dac were examined. The tolerance limit was defined as the maximum concentration of the substances that caused an error of less than 5% in Dac determination. The observations showed that when a 500-fold uric acid, dopamine and ascorbic acid were present in the synthetic mixture, the average recovery in the determination of Dac was 105%.
Analytical application
In order to demonstrate the application of the proposed method for determination of Dac in pharmaceutical preparations, it was used for voltammetric determination of Dac in the dacarbazine medac (200 mg vial). Each vial of dacarbazine medac 200 mg contains 200 mg dacarbazine (as dacarbazine citrate, manufactured by Medac GmbH). There was no need for any precipitation, evaporation or extraction steps prior to the DPV assay. The Dac content in Dac vial was determined by the standard addition method in order to prevent any matrix effects. The results for the analysis of Dac vial using the voltammetric method are summarized in Table 1. The results in Table 1 show that the recoveries of the spiked samples are acceptable. Thus, the modified electrode can be efficiently used for determination of Dac in pharmaceutical preparations. In addition, to evaluate precision and accuracy of DPV method, high-performance liquid chromatography (HPLC) method was used. The results obtained by the proposed method and also HPLC are summarized in Table 2. The consistency between the results was checked by the F-test method, and the calculated F-values for all samples are also included in Table 2. Considering the above-mentioned possible sources of error, all of the F-values given in Table 2 clearly indicate that there is no statistically significant difference between the values obtained by two methods. In all cases, the calculated F-values are lower than the F-value of 6.39 expected at a P = 0.05. Thus, the obtained results confirm the reliability of the proposed sensor.
Conclusions
This work reports the fabrication of an ultrasensitive electrochemical sensor for determination of anticancer drug dacarbazine based on multiwall carbon nanotube-modified carbon paste electrode. The oxidation peak at the bare carbon paste electrode is weak while the response is considerably improved at the multiwall carbon nanotube-modified carbon paste electrode. Also the peak potential shifts to less positive values. These observations indicate the easier electron transfer and simpler oxidation of Dac at the surface of modified electrode in the comparison of bare electrode. The proposed sensor has been shown to be promising for determination of Dac with many desirable properties including simple fabrication procedure, high stability, good reproducibility and repeatability, good sensitivity and fast response time. The most important advantages of the designed sensor in comparison with the other Dac electrochemical sensors by using the different electrodes (carbon pate electrode [32], hanging mercury drop electrode [6, 31] and screen-printed carbon electrode [35]) that have been reported previously, are low detection limit and wide linear range (see Table 3). Also, Dac was determined by the standard addition method in dacarbazine medac and the obtained values were in good agreement with high-performance liquid chromatography method. These results confirm the reliability of the proposed sensor. We hope that the proposed method leads to improving the fabrication of some new electrochemical sensors for determination of different species.
Abbreviations
- Dac:
-
Dacarbazine
- MWCNT:
-
Multiwall carbon nanotube
- CPE:
-
Carbon paste electrode
- MCPE:
-
Modified carbon paste electrode
- MWCNT/CPE:
-
Multiwall carbon nanotube carbon paste electrode
- CV:
-
Cyclic voltammetry
- DPV:
-
Differential pulse voltammetry
- HPLC:
-
High-performance liquid chromatography
- SEM:
-
Scanning electron microscopy
- EIS:
-
Electrochemical impedance spectroscopy
- SCE:
-
Saturated calomel electrode
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantitation
- RSD:
-
Relative standard deviation
References
S. Bahrpeyma, B. Hemmateenejad, K. Javidnia, Photo-degradation study of dacarbazine by spectrophotometric–chemometrics and HPLC methods. J. Iran. Chem. Soc. 13(2), 221–229 (2016)
Y. Temerk, H. Ibrahim, Binding mode and thermodynamic studies on the interaction of the anticancer drug dacarbazine and dacarbazine–Cu (II) complex with single and double stranded DNA. J. Pharm. Biomed. Anal. 95, 26–33 (2014)
N. Wald, Y. Le Corre, L. Martin, V. Mathieu, E. Goormaghtigh, Infrared spectra of primary melanomas can predict response to chemotherapy: the example of dacarbazine. Biochim. et Biophys. Acta (BBA)-Mol. Basis of Dis. 1862(2), 174–181 (2016)
M. Almousallam, C. Moia, H. Zhu, Development of nanostructured lipid carrier for dacarbazine delivery. Int. Nano Lett. 5(4), 241–248 (2015)
M. Maafi, L.Y. Lee, Determination of Dacarbazine Φ-Order Photokinetics, Quantum Yields, and Potential for Actinometry. J. Pharm. Sci. 104(10), 3501–3509 (2015)
Y.M. Temerk, M.M. Kamal, M.S. Ibrahim, H.S. Ibrahim, W. Schuhmann, Electrochemical behaviour of the anticancer Dacarbazine-Cu2 + complex and its analytical applications. Electroanalysis 23(7), 1638–1644 (2011)
C. Beitz, T. Bertsch, D. Hannak, W. Schrammel, C. Einberger, M. Wehling, Compatibility of plastics with cytotoxic drug solutions—comparison of polyethylene with other container materials. Int. J. Pharm. 185(1), 113–121 (1999)
J.A. Benvenuto, R. Anderson, K. Kerkof, R. Smith, T.L. Loo, Stability and compatibility of antitumor agents in glass and plastic containers. Am. J. Health Syst. Pharm. 38(12), 1914–1918 (1981)
G. Lunn, E. Sansone, Reductive destruction of dacarbazine, procarbazine hydrochloride, isoniazid, and iproniazid. Am. J. Health Syst. Pharm. 44(11), 2519–2524 (1987)
J. Stewart, F. Warren, D. King, T. Venkateshwaran, G. Ponder, J. Fox, Stability of ondansetron hydrochloride, doxorubicin hydrochloride, and dacarbazine or vincristine sulfate in elastomeric portable infusion devices and polyvinyl chloride bags. Am. J. Health Syst. Pharm. 54(8), 915–920 (1997)
B.V. Shetty, R.L. Schowen, M. Slavik, C.M. Riley, Degradation of dacarbazine in aqueous solution. J. Pharm. Biomed. Anal. 10(9), 675–683 (1992)
D. Fiore, A. Jackson, M. Didolkar, V. Dandu, Simultaneous determination of dacarbazine, its photolytic degradation product, 2-azahypoxanthine, and the metabolite 5-aminoimidazole-4-carboxamide in plasma and urine by high-pressure liquid chromatography. Antimicrob. Agents Chemother. 27(6), 977–979 (1985)
S.L. Safgren, J.M. Reid, R. Rios, M.M. Ames, Validated high-performance liquid chromatographic assay for simultaneous determination of dacarbazine and the plasma metabolites 5-(3-hydroxymethyl-3-methyl-1-triazeno) imidazole-4-carboxamide and 5-(3-methyl-1-triazeno) imidazole-4-carboxamide. J. Chromatogr. B Biomed. Sci. Appl. 754(1), 91–96 (2001)
Y. Liu, W. Zhang, Y. Yang, Validated hydrophilic interaction LC–MS/MS method for simultaneous quantification of dacarbazine and 5-amino-4-imidazole-carboxamide in human plasma. Talanta 77(1), 412–421 (2008)
J. Wang, Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis 17(1), 7–14 (2005)
C.B. Jacobs, M.J. Peairs, B.J. Venton, Review: carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 662(2), 105–127 (2010)
C.N.R. Rao, B. Satishkumar, A. Govindaraj, M. Nath, Nanotubes. ChemPhysChem 2(2), 78–105 (2001)
R.H. Baughman, A.A. Zakhidov, W.A. de Heer, Carbon nanotubes–the route toward applications. Science 297(5582), 787–792 (2002)
R.J. Chen, Y. Zhang, D. Wang, H. Dai, Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 123(16), 3838–3839 (2001)
A. Salimi, A. Noorbakhsh, S. Soltanian, Electroless deposition of thionin onto glassy carbon electrode modified with single wall and multiwall carbon nanotubes: improvement of the electrochemical reversibility and stability. Electroanalysis 18(7), 703–711 (2006)
A. Salimi, H. MamKhezri, R. Hallaj, S. Zandi, Modification of glassy carbon electrode with multi-walled carbon nanotubes and iron (III)-porphyrin film: application to chlorate, bromate and iodate detection. Electrochim. Acta 52(20), 6097–6105 (2007)
H. Teymourian, A. Salimi, R. Hallaj, Electrocatalytic oxidation of NADH at electrogenerated NAD + oxidation product immobilized onto multiwalled carbon nanotubes/ionic liquid nanocomposite: application to ethanol biosensing. Talanta 90, 91–98 (2012)
H. Teymourian, A. Salimi, R. Hallaj, Low potential detection of NADH based on Fe3O4 nanoparticles/multiwalled carbon nanotubes composite: fabrication of integrated dehydrogenase-based lactate biosensor. Biosens. Bioelectron. 33(1), 60–68 (2012)
P. Wang, H. Wu, Z. Dai, X. Zou, Simultaneous detection of guanine, adenine, thymine and cytosine at choline monolayer supported multiwalled carbon nanotubes film. Biosens. Bioelectron. 26(7), 3339–3345 (2011)
A.P. Periasamy, Y.-J. Chang, S.-M. Chen, Amperometric glucose sensor based on glucose oxidase immobilized on gelatin-multiwalled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 80(2), 114–120 (2011)
P. Lu, Y.-L. Hsieh, Multiwalled carbon nanotube (MWCNT) reinforced cellulose fibers by electrospinning. ACS Appl. Mater. Interfaces 2(8), 2413–2420 (2010)
K.V. Gobi, H. Tanaka, Y. Shoyama, N. Miura, Continuous flow immunosensor for highly selective and real-time detection of sub-ppb levels of 2-hydroxybiphenyl by using surface plasmon resonance imaging. Biosens. Bioelectron. 20(2), 350–357 (2004)
M.D. Tezerjani, A. Benvidi, A.D. Firouzabadi, M. Mazloum-Ardakani, A. Akbari, Epinephrine electrochemical sensor based on a carbon paste electrode modified with hydroquinone derivative and graphene oxide nano-sheets: simultaneous determination of epinephrine, acetaminophen and dopamine. Measurement 101, 183–189 (2017)
I. Švancara, K. Vytřas, J. Barek, J. Zima, Carbon paste electrodes in modern electroanalysis. Crit. Rev. Anal. Chem. 31(4), 311–345 (2001)
K. Vytřas, I. Švancara, R. Metelka, Carbon paste electrodes in electroanalytical chemistry. J. Serb. Chem. Soc. 74(10), 1021–1033 (2009)
A.M. Ordieres, A.C. Garcia, P.T. Blanco, W.F. Smyth, An electroanalytical study of the anticancer drug dacarbazine. Anal. Chim. Acta 202, 141–149 (1987)
J.R.B. Rodriguez, A.C. Garcia, A.J.M. Ordieres, P.T. Blanco, Electrochemical oxidation of dacarbazine and its major metabolite (AIC) on carbon electrodes. Electroanalysis 1(6), 529–534 (1989)
M. Song, R. Zhang, X. Wang, Nano-titanium dioxide enhanced biosensing of the interaction of dacarbazine with DNA and DNA bases. Mater. Lett. 60(17), 2143–2147 (2006)
Q. Shen, X. Wang, D. Fu, The amplification effect of functionalized gold nanoparticles on the binding of anticancer drug dacarbazine to DNA and DNA bases. Appl. Surf. Sci. 255(2), 577–580 (2008)
A.-E. Radi, A. Eissa, H.M. Nassef, Voltammetric and spectroscopic studies on the binding of the antitumor drug dacarbazine with DNA. J. Electroanal. Chem. 717, 24–28 (2014)
A.J. Bard, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications (Wiley, New York, 2001)
E. Laviron, Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 52(3), 355–393 (1974)
M. Sharp, M. Petersson, K. Edström, Preliminary determinations of electron transfer kinetics involving ferrocene covalently attached to a platinum surface. J. Electroanal. Chem. Interfacial Electrochem. 95(1), 123–130 (1979)
M. Ghalkhani, I.P. Fernandes, S.C.B. Oliveira, S. Shahrokhian, A.M. Oliveira-Brett, Electrochemical redox behaviour of temozolomide using a glassy carbon electrode. Electroanalysis 22(22), 2633–2640 (2010)
t.N.F. The United States Pharmacopoeia, USP 24, NF 19, USP, Convention Inc., MD, 2000, 2151
Acknowledgements
The authors gratefully acknowledge the support of this work by the Shohadaye Hoveizeh University Research for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dehdashtian, S., Behbahanian, N. & Taherzadeh, K.M. An ultrasensitive electrochemical sensor for direct determination of anticancer drug dacarbazine based on multiwall carbon nanotube-modified carbon paste electrode and application in pharmaceutical sample. J IRAN CHEM SOC 15, 931–941 (2018). https://doi.org/10.1007/s13738-018-1291-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1291-5