Abstract
A novel 1,1-cyclobutanedicarboxylato-8-hydroxyquinolinatopalladate(II), Na[Pd(8-QO)(cbdca)] (8-QO = 8-hydroxyquinoline and cbdca = 1,1-cyclobutanedicarboxylate) has been designed and synthesized. The structure of the complex has been characterized by elemental analysis, molar conductance, FT-IR, UV–Vis, 13C NMR, 1H NMR spectroscopy. The cytotoxic activities of the complex have been tested against human foreskin fibroblast normal cell line, HFFF2 and two human breast cancer cell lines, T47D and MCF-7 by MTT assay techniques. The binding properties of the antitumor complex with calf thymus DNA (ctDNA) have been investigated under physiological condition in Tris–HCl buffer solution at pH 7.0 by absorption spectroscopy, fluorescence titration spectra, EB displacement and gel chromatography studies. All these results demonstrate that the water soluble complex can cooperatively bind to ctDNA at low concentrations through a static quenching procedure. Thermodynamic parameters were calculated according to Van’t Hoff equation which indicated that hydrogen bond and van der Waals force play predominant roles in the binding process. Finally, the results of molecular docking calculations clarify the binding mode which is in good accordance with experimental results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that deoxyribonucleic acid (DNA) as the carrier of genetic information in organisms plays an important role in a broad range of vital biological processes, such as gene expression, gene transcription, mutagenesis, and carcinogenesis. The study on the interaction between small molecules and DNA has been the focus of some recent research in the scope of life science, chemistry, clinical medicine and genetics [1, 2]. A large number of evidences indicate the mechanism of action of anticancer agents binds through distinctive binding modes to the DNA of cancer infected cell in such a way that the cell cannot replicate further. This inhibition of replication finally leads to the death of the infected cell [3]. The molecular forces contributing to biomolecules interactions with small molecules substrates may involve hydrogen bond, van der Waals force, hydrophobic interaction force, and electrostatic force [1, 4, 5]. Therefore, an understanding that how these small molecules bind to DNA will potentially be useful in the design of such new compounds, which can recognize specific sites or conformations of DNA [6, 7]. The pharmacological efficacy of metal complexes depends on the nature of the metal ions and the ligands. It is declared in the literature that synthesized from same ligands with different metal ions possesses different biological properties [2, 8].
Transition metal complexes are currently being used to bind and react at specific sequences of DNA in a search for novel chemotherapeutics and probing DNA, and for the development of highly sensitive diagnostic agents [9, 10]. It is well known that metal ions present in the complexes are not only accelerating the drug action but also increase the effectiveness of the organic ligands [10, 11]. Extensible research efforts have been made to develop novel metal-based antitumor complexes with the aim of improving effectiveness and reducing the severe side effects of the current clinical platinum chemotherapeutic agents such as cisplatin and its analogues. Special attention has been paid to platinum complexes with different structural features from those of already used drugs and complexes of the other platinum-group metals like ruthenium, rhodium, palladium and iridium [12,13,14]. On the basis of the structural and thermodynamic analogy between platinum(II) and palladium(II) complexes, there is also much interest in the study of palladium(II) derivatives as potential anticancer drugs [15, 16]. Herein, to investigate the DNA-binding properties of anionic complexes, the synthesis, characterization, cytotoxicity and DNA interaction as well as computational studies of palladium(II) complex having 8-hydroxyquinoline and cyclobutanedicarboxylate ligands have been reported. To the best of our knowledge, DNA-binding studies of anionic Pd(II) complex have not been demonstrated to date. The interaction between water soluble palladium(II) complex and ctDNA has been investigated by spectroscopic and molecular docking techniques. We believe that the results gained from this study will be valuable for the improvement of new therapeutic reagents.
Experimental
Materials and methods
Palladium(II) chloride anhydrous and sodium chloride were obtained from Fluka (Switzerland). 8-hydroxyquinoline, 1,1-cyclobutanedicarboxylic acid, sodium bicarbonate, silver nitrate, Sephadex G-25 and tris(hydroxymethyl) amino methane hydrochloride (Tris–HCl buffer) were obtained from Merck (Germany). Calf thymus DNA (ctDNA) and ethidium bromide (EB) were obtained from Sigma Chemical Co. (USA) and used as received. The stock solutions of Pd(II) complex (1 mM) were made in Tris–HCl buffer by gentle stirring and heating at 308 K. The stock solution of ctDNA (4 mg mL−1) was prepared by dissolving of ctDNA in 20 mM NaCl–20 mM Tris–HCl buffer at pH 7.0 overnight and was stored at 277 K for about a week. The ctDNA concentration per nucleotide was determined by absorption spectroscopy using the molar absorption coefficient (6600 M−1 cm−1) at 260 nm [17, 18]. Solutions of ctDNA gave a ratio of UV–Vis absorbance of 1.8–1.9:1 at 260 and 280 nm, indicating that the ctDNA was sufficiently free of protein [17]. The cell lines for the cytotoxicity studies were obtained from the Cell Bank of Pasteur Institute in Tehran (Iran). Solvents were used of reagent grade and purified before being used by the standard methods. Double-distilled water was used as solvent. All experiments were repeated multiple times to obtain consistent results.
The melting point of the compound was determined on a Unimelt capillary melting point apparatus and reported uncorrected. A conductivity measurement of the complex was taken on a Systronics conductivity bridge 305, using a conductivity cell of cell constant 1.0. Infrared spectrum (4000–400 cm−1) was determined with KBr disks on a JASCO-460 plus FT-IR spectrophotometer. UV–Vis spectrum was recorded on a PerkinElmer lambda-25 recording spectrophotometer. 1H NMR spectrum was measured on a Bruker DRX-500 Avance spectrometer at 500 MHz, using TMS as the internal reference in DMSO-d6. Fluorescence intensity changes were studied by using a Hitachi spectrofluorimeter, MPF-4 model, equipped with a thermostatically controlled cuvette compartment. A 1.0 cm quartz cuvette was used for the following spectroscopic measurements.
Synthesis of sodium 1,1-cyclobutanedicarboxylato-8-hydroxyquinolinatopalladate(II) Na[Pd(8-QO)(cbdca)]
To a solution of PdCl2 (0.177 g, 1 mM) in distilled water (20 mL) was added NaCl (0.118 g, 2 mM) and the reaction mixture was stirred at 333–343 K until a light brown solution was formed. In another one, 8-QOH (0.145 g, 1 mM) in distilled water and ethanol mixture (1 mL, 1:1 v/v) was added NaHCO3 (0.084 g, 1 mM) and the solution stirred at 313 K until a light yellow solution was formed. A light yellow solution of 8-QONa (1 mM) was added drop-wise to a light brown solution of Na2PdCl4 (1 mM) in distilled water (100 mL) under stirring at home temperature. and the color of the mixture changed to deep green. Then the mixture was stirred for 0.5 h at 313 K. AgNO3 (0.340 g, 2 mM) was added to the solution of Na[Pd(8-QO)Cl2]) and stirred in the dark at 55 °C for 6 h and then overnight at room temperature. The AgCl precipitate was filtered off through Whatman 42 filter paper. To clear yellow-orange solution containing [(8-QO)Pd(H2O)2]NO3 (1 mM), which formed, was slowly added a solution of (CH2)4(COONa)2 (0.144 g, 1 mM). (CH2)4(COONa)2 was prepared by adding of NaHCO3 (0.170 g, 2 mM) to (CH2)4(COOH)2 (0.144 g, 1 mM). The yellow mixture was stirred for 3 h at 313–323 K. The volume of the solution was reduced to 25 mL and then cooled down to filter. The solvent was removed by evaporation at 308–313 K and was dried. The residue was dissolved in acetonitrile-methanol (1:1 v/v, 10 mL) and then evaporated at 308–313 K to complete dryness. Recrystallization was carried out by stirring the crude product in mixture of methanol–acetonitrile (1:1 v/v, 10 mL) followed by filtering the undissolved particles. Diffusion of ether into the precipitate for 3 days gave a yellow powder. The powder was isolated by filtration, washed with ether and dried at 313 K. The preparation steps of Sodium 1,1-cyclobutanedicarboxylato-8-hydroxyquinolinatopalladate(II) are shown in Scheme 1. Yield: 0.278 g (67%) and decomposes at 481–483 K. Anal. Calc. for NaC15H14NO5Pd (454): C, 39.65; H, 2.64; N, 6.17%. Found: C, 39.68; H, 2.60; N, 6.21%.
Cytotoxicity measurements
Cell proliferation was evaluated by using a system based on the tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT] that is reduced by living cells to yield a soluble formazan product which can be assayed colorimetrically [10, 19]. The MTT assay is dependent on the cleavage and conversion of the soluble yellowish MTT to the insoluble purple formazan by active mitochondrial dehydrogenase of living cells. The cell lines, HFFF2, MCF-7 and T47D, were maintained in a RPMI medium supplemented with 10% heat-inactivated fetal calf serum and 2 mM l-glutamine, streptomycin and penicillin (5 μg mL−1), at 310 K under a 5% CO2/95% air atmosphere. Harvested cells were seeded into 96-well plates (2 × 104 cell/mL) with varying concentrations of the sterilized complex (0–250 μM) and incubated for 48 h. At the end of the 4 h incubation period, 25 μL of MTT solution (5 mg mL−1 in PBS) was added to each well containing fresh culture media. The insoluble produced formazan was then dissolved in solution containing 10% SDS and 50% DMF (under dark condition for 2 h at 310 K), and optical density (OD) was read against reagent blank with a multi-well scanning spectrophotometer (ELISA reader, Model Expert 96, Asys Hitchech, Austria) at a wavelength of 570 nm. Absorbance is read as a function of concentration of converted dye. The OD value of study groups was divided by the OD value of untreated control and presented as a percentage of the control (as 100%). Results were analyzed for statistical significance using a two-tailed Student’s t test. Changes were considered significant at p < 0.05. In this experiment, the clear stock solution (2 mM, in deionized water) was sterilized by filtering through sterilizing membrane (0.1 nm) and then varying concentrations of the sterilized complex (0–1000 μM) were added to harvested cells.
UV–Vis spectra
The UV spectra have been recorded at 260 nm and at 640 nm to eliminate the interference of turbidity for a constant ctDNA concentration (14 μM) with increasing complex concentrations from 27 to 437 μM and 27–428 μM at 300 and 310 K, respectively. Thus, addition of metal complex was continued until no further changes in the absorption readings were observed. Also, to obtain the maximum ΔA (ΔAmax), change in the absorbance when all binding sites on ctDNA were occupied by metal complex, the successive fixed amount of the complex solution (200 μM) was added to ctDNA solution (62–98 and 51–123 μM at 300 and 310 K, respectively). In addition, to affirm quantitatively the affinity of the complex bound to ctDNA, a fixed amount of ctDNA (77.25 μM) was titrated with varying concentrations of the complex (90–210 and 75–215 μM at 300 and 310 K, respectively).
Fluorescence spectra
Fluorescence spectroscopy is a method to study the interaction of small molecules with ctDNA and is a source of information for biochemical systems. Fluorescence titration experiments were performed by maintaining the ctDNA and EB concentrations as constant (60 and 2 μM, respectively) with increasing amounts of Pd(II) complex (0–133 μM).
The mode of binding was characterized by scatchard plots. For obtaining this plots, The Pd(II) complex solution was incubated with ctDNA at room temperature for 4 h. The appropriate amount of EB was added before an additional incubation at 298 K for 2 h before the fluorescence spectral measurement. Saturation curves of fluorescence intensity for [Pd(8-QO)(cbdca)]−-DNA system at different rf (the ratio of the concentration of metal complex to DNA concentration) values (3.33, 9.33 and 14.67) were obtained in the presence of increased concentrations of EB (2, 4, …, 20 µM). The emission spectra were recorded at 293, 300 and 310 K in the emission wavelength range of 540–700 nm with an excitation wavelength at 471 nm. The widths of both the excitation slit and emission slit were set at 5.0 nm. The fluorescence spectra of the Pd(II) complex at the highest denaturant concentrations at 471 nm excitation wavelength have been checked, and the emission intensities of these compounds were negligible.
Gel filtration
The Pd(II) complex (425.6 µM) was incubated with calf thymus DNA (187.5 µM) for 4 h at 300 K in Tris–HCl buffer (pH 7.0). It was then passed through a Sephadex G-25 column equilibrated with the same buffer. The elusion of the column fraction of 2.0 mL was monitored at 373 and 260 nm for DNA-Pd(II) complex.
Computational studies
The geometry of the Pd(II) complex optimized by using B3LYP method with 6-311G* basis set for H, C, O, and N atoms and LANL2DZ basis set for Pd atom, utilizing Gaussian 03 program. To ensure this optimized geometry is a real local minimum, vibrational frequency was calculated analytically at the same level of theory. Also, 1H NMR calculation was performed; at first the optimized molecular geometry of complex was obtained in DMSO by applying CPCM method. Then, 1H chemical shifts for complex were calculated at B3LYP/6–311+G(d,p) levels in solvent by using GIAO method. Finally, HOMO and LUMO energy values and energy gap for this complex were calculated to explain the chemical hardness (η) and chemical potential (μ) [20].
Molecular docking
The crystal structure of DNA (encoded 453D) was obtained from the Brookhaven Protein Data Bank (http://www.pdb.org), and optimized structure of complex was selected for the docking study. Therefore, AutoDock 4.2 using Lamarckian genetic algorithm together with the AutoDock Tools was employed and blind docking was down [21, 22]. In the docking analysis, the binding site was centered on the DNA and a grid box was made with 65 × 45 × 59 points and a 0.452 Å grid spacing in which almost the entire macromolecules were involved [23]. The binding position and bound conformation of the DNA with Pd complex, and the rough estimate of their interactions were examined with the AutoDock results [23, 24].
Results and discussion
Characterization of Pd(II) complex
The palladium(II) complex has been synthesized and reported in the experimental section. The complex is soluble in water and stable to air and moisture without any kind of decomposition also after several months. The complex has been characterized by elemental analysis, molar conductivity measurements and 1H NMR, 13C NMR, FT-IR and UV–Visible spectroscopic techniques. All the results are in good agreement with the proposed structure of the complex. The conductivity measurement of 10−4 M of the complex was 140 Ω−1 mol−1 cm2. It shows that the molar conductance was within the range of 118–131 cm2 O−1 mol−1 meaning that this complex was in conformity for 1:1 electrolytic natures [25]. Solid-state FT-IR spectroscopy of the complex shows four main characteristic bands at 1112, 1332 and 1612 and 1466 cm−1 assigned to ν(C–O), ν(N–C) and ν(C=C) aromatic modes, respectively. The observed ν(COO−) at 1659, cm−1 and ν(C–H)aliphatic at 2943 cm−1 in comparison with free ligand suggests the coordination of 1,1-cyclobutanecarboxylate to palladium center through its oxygen [26, 27]. The complex shows bands within the range 585–595 and 470–490 cm−1 may be assignable to the ν(M–N) and ν(M–O), respectively [28, 29]. Two absorption bands with varied intensity were observed in both of the UV and visible region. The band at 373 nm (ε = 0.13) is assigned to metal to π* orbital of 8-hydroxyquinoline charge transfer. Besides, a band was also observed at 258 nm (ε = 0.72), corresponding to the internal π→π* transitions of 8-hydroxyquinoline ligand [30]. The later bands generally show overlapping, and therefore it is not possible to assign them properly. The 1H NMR spectrum of the complex in DMSO-d6 exhibited signal at the doublet observed at 6.69, 6.95, 7.97 and 8.42 ppm; the triplet at 7.33 ppm and a quartet at 7.51 ppm are assigned to H-1, H-3, H-4, H-6, H-5 and H-2 protons, respectively. The signal due to the H-a and H-b,b’ protons appears the triplet at 3.43 and the multiplet at 2.74 ppm, respectively (Scheme 2a). The 13C NMR signals due to –C1H, –C8H, –C9, –C6H, –C5, –C3H, –C7H, –C4H and –C2H of 8-Q ligand in DMSO appear at 168.53, 145.36, 144.63, 139.25, 130.78, 130.59, 121.79, 113.43 and 111.63 ppm, respectively. Also, the 13C NMR spectra of cbdca ligand occur at 177.86, 177.60, 57.06, 31.37, 29.35 and 16.89 ppm due to C10 = O, C11 = O, C12 cyclic, C13cyclic, C15 cyclic and C14 cyclic, respectively (Scheme 2b).
Geometry optimization and energies of the complexes
Before computing the frequencies and electronic properties, analyze the molecular structure of the complex is required. Thus, the complex structure was optimized at DFT level of theory shown in Fig. 1a and some geometrical parameters are presented in Table 1.
The validity of the applied method was checked by X-ray diffraction data of similar complex [31], and very good agreement between the computed structure and X-ray diffraction data was observed. Furthermore, Fig. 1b represents very excellent correlation between calculated 1H NMR chemical shifts of Pd(II) complex with the corresponding experimental data in DMSO-d6 solvent.
As well known the frontier molecular orbitals, HOMO and LUMO, have main role in the reactivity, electric and optic properties of compounds [22, 23]. Thus, the total energy for different orbital transitions, energy gap between HOMO and LUMO (∆E), electron affinity (A), ionization potential (I), electronegativity (χ), chemical hardness (η) and softness (S) have been calculated as follows (see Table 2):
Also three-dimensional plot of the HOMO and LUMO of Pd(II) complex is shown in Fig. 2. It is demonstrated the HOMO and LUMO are localized on approximately the 8-hydroxyquinoline ligand (Fig. 2 and Table 2).
Cytotoxic activity
Cytotoxicity of the Pd(II) complex was tested against normal human cell line, HFFF2 and cancer cell lines, MCF-7 and T47D measured by MTT assay. As shown in Fig. 3, the number of growing cells was significantly reduced after 48 h in the presence of various concentrations of the Pd(II) complex. Moreover, cell viability percentage of normal human cell line is more than cancer cell lines. Therefore, this antitumor agent is less toxic to normal cells. This result is also confirmed by the concentration of Pd(II) complex that is required for 50% inhibition of cell lines (Cc50) value of the Pd(II) complex for normal human cell line, HFFF2 and cancer cell lines, MCF-7 and T47D that is 690, 610 and 530 μM, respectively. In addition, Cc50 value is comparable with those of antitumor cationic Pd(II) complexes reported earlier [32,33,34] and the Pd(II) complex is more effective in inhibiting MCF-7 cell lines than T47D cell lines. From the observed results, it is concluded that the complex has antitumor activity.
Absorption spectroscopic studies
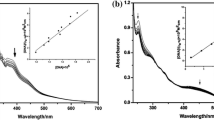
UV–Vis absorption spectroscopy is one of the most useful techniques in DNA-binding studies [4, 35]. The results of UV–Vis spectrum analysis of ctDNA in the presence of complex at 300 and 310 K are shown in Fig. 4. The concentration of the metal complex at midpoint of transition, [L]1/2, according to the denaturation plots is 0.300 mM at 300 K and 0.276 mM at 310 K. These values were mentioned that the complex can bind to ctDNA at low concentrations comparable with cationic complexes [36, 37]. Thus, if the complex will be used as antitumor agents, low doses will be needed, which may have fewer side effects.
The values of unfolding equilibrium constant, K, and unfolding free energy, ∆G°, of ctDNA in the presence of Pd(II) complex have been calculated using Fig. 4 and Pace method [38]. In this method, Pace had assumed two-state mechanism, nature and denature, and then calculated unfolding free energy of ctDNA by using Eqs. 2 and 3:
where A obs is absorbance readings in transition region, A N and AD are absorbance readings of nature and denatured conformation of ctDNA, respectively. A straight line is observed when the values of ΔG° are plotted against the concentrations of the metal complex in the transition region at 300 K and at 310 K (Fig. 5a). The equation for these lines can be written as follow [39]:
Here the values of \( \Delta G_{{ ( {\text{H}}_{ 2} {\text{O)}}}}{^\circ } \) for each curve are measured from the intercept on ordinate of the plots and it is conformational stability of ctDNA in the absence of metal complex. The values of ∆G° (Table 3) are decreased by increasing the temperature because of decreasing in ctDNA stability [40]. The metal complex ability to interaction with ctDNA, m, was measured from the slope of each curve in Fig. 5a. The values of m for the palladium complex are much higher than those of Pd(II) complexes reported earlier [36, 37] which indicate the higher ability of the complex to interact with ctDNA. Another important thermodynamic parameter found is the molar enthalpy of ctDNA denaturation in absence of metal complex, \( \Delta H_{{ ( {\text{H}}_{ 2} {\text{O)}}}}{^\circ } \). The molar enthalpy of ctDNA denaturation in the presence of metal complexes, ∆H°conformation, in the range of 300 and 310 K was calculated using Gibbs–Helmholtz equation [41].
Upon plotting the values of these enthalpies versus the concentrations of metal complex, straight lines were obtained as shown in Fig. 5b. Intrapolation of this line (intercept on ordinate, i.e., absence of metal complex) gives the values of \( \Delta H_{{ ( {\text{H}}_{ 2} {\text{O)}}}}{^\circ } \) (Table 3). Descending the enthalpy change with increasing concentrations of this complex indicated that the stability of ctDNA had been decreased. In addition, it can be concluded that the interaction of Pd(II) complex with ctDNA is exothermic [42].
Generally, Gibbs free energy and enthalpy decrease with increasing concentration of Pd(II) complex. Thus, Pd(II) complex binding to ctDNA is spontaneous reaction and in presence of this complex enthalpy is favorable.
For calculating binding parameters, change in absorbance, ∆A, was calculated by subtracting the absorbance reading of mixed solutions of the metal complex with various concentrations of ctDNA, from absorbance reading of free metal complex. The maximum ∆A (∆A max) of the metal complex totally bound to ctDNA was determined by intrapolation of a plot of reciprocal of ∆A s against the reciprocal of ctDNA concentration (i.e., intercept on ordinate) (Fig. 6a). The values of ∆A max are given in Table 4. Titration of fixed amount of ctDNA with varying amount of the metal complex was obtained the concentration of the metal complex bound to ctDNA, [L]b, using the Eq. 6 [43].
where [L]t is the total concentration of metal complex. The concentration of the free metal complex, [L]f, and the ratio of the concentration of bound metal complex to the total ctDNA concentration, v, can be calculated from Eqs. 7 and 8, respectively.
The Scatchard plots were obtained separately at 300 and 310 K by plotting v/[L]f versus v (Fig. 6b) [44]. These plots are curvilinear concave downwards, suggesting cooperative binding because of the type of cooperatively can be verified from the nature of the plot (concave downward curve indicates cooperative, concave upward anti-cooperative and straight line non-cooperative). To obtain the binding parameters, the above experimental data (v and [L]f) were substituted in Eq. (9), i.e., the Hill equation [45].
This equation contain the following unknown parameters n, K and g where n is the Hill coefficient (n = 1 indicates non-cooperative, n > 1 is cooperative and n < 1 shows anti-cooperative binding of ctDNA with metal complex), g is the number of binding sites per 1000 nucleotides of ctDNA, and K is apparent binding constant. Using Eureka software, the theoretical values of these parameters were deduced (Table 4). The values of n indicated that the Pd(II) complex binds to ctDNA cooperatively; thus, the binding at one site increases the affinity of binding at the other sites and has a series of two binding site [46]. The apparent binding constants of the Pd(II) complex were obtained and are higher than other metal complexes such as [Ru(bpy)2(maip)](ClO4)2 2.3 × 103 M−1 and C6H24N4O6CuSn2Cl6 4.75 × 104 M−1 [47, 48]. It was apparent from Table 4 that the maximum errors between experimental and theoretical values of v are low.
a Changes in the absorbance of fixed amount of the metal complex in the interaction with varying amount of DNA at 300 and 310 K. b Scatchard plots for binding of Na[Pd(8-QO)(cbdca)] to DNA (experimental (dots) and theoretical (lines)). c Molar enthalpy of binding in the interaction between DNA and Na[Pd(8-QO)(cbdca)] versus free concentrations of complex at pH 7.0 and 300 K
Finding the area under versus ln[L]f plot and using Wyman-Jons equation (Eq. 10) [49], we can calculate the K app at 300 and 310 K for each particular v.
Then the binding enthalpy (∆H°) process can be observed using the Van’t Hoff equation [50].
Plots of the values of ∆H° versus the values of [L]f are shown in Fig. 6c for Na[Pd(8-QO)(cbdca)] complex at 300 K. Observed deviation in the plot may be due to binding of metal complex to macromolecule or might indicate ctDNA denaturation. Similar observations can be seen in the literature where Pd(II) and Pt(II) complexes have been interacted with ctDNA [37, 51].
Fluorescence measurements
Fluorescence is a very useful method to verify the binding mode of complexes to DNA. Ethidium bromide (EB) is frequently used as a sensitive probe, since its fluorescence intensity greatly enhanced in the presence of DNA, due to its strong intercalation between adjacent base pairs. The addition of a second molecule could quench this enhanced fluorescence. Hence, competitive ethidium bromide (EB) binding studies were undertaken to gain support for the extent of binding of palladium(II) complexes with ctDNA. Figure 7 illustrates the fluorescence emission spectra of DNA-EB system with increasing concentrations of the Pd(II) complex. It was apparent that the significant reduction in the fluorescence intensity was occurred. This result suggested that the complex could bind to ctDNA. Also, the experiment revealed that addition of the Pd(II) complex to DNA bound EB does not cause releasing EB molecules, while the emission intensity decreases steadily. In case of EB release, a weak intensity spectrum due to free EB near the base line should appear which has not been observed in our cases (see Fig. 7). This implies that the Pd(II) complex and EB bind with DNA independently and binding of this complex affects the binding of the EB by slight slip out of EB from hydrophobic to hydrophilic regions diminuendo in emission intensities. Thus, this experiment supports the groove binding expectation [52].
Quenching of fluorescence emission of a macromolecule induced by a quencher can be classified either as dynamic or static, or it can be due to a combined mechanism. In static quenching, the quencher forms a non-fluorescent complex with the fluorophore of the macromolecule, whereas in dynamic quenching the quencher binds to the fluorophore during the lifetime of the excited state, which induces charge transfer from the fluorophore to the quencher, consequently causing the fluorophore to return to the ground state without emitting a photon. Both static quenching and dynamic quenching require molecular contact between the fluorophore and the quencher [53]. For the dynamic quenching, the mechanism is usually described by the classical Stern–Volmer equation [54]:
F 0 and F are the fluorescence intensities of ctDNA in the absence and in the presence of Pd(II) complex, respectively. [L]t is the total concentration of quencher (Pd(II) complex, [L]), k q is the DNA–EB complex quenching rate constant, τ 0 is the average lifetime of fluorophore in the absence of Pd(II) complex with value of 10−9–10−7 s [55], and K SV is the Stern–Volmer dynamic quenching constant. The K SV value is obtained from the slope of F 0/F versus [L]t linear plot (inset in Fig. 7). The values of K SV and K are listed in Table 5.
For static quenching, the binding constant (K b) and number of binding (n) were estimated by the following equation [56]:
According to the equation above, the binding parameters can be obtained from the slope of log (F 0 − F)/F against log[L]t at 293, 300 and 310 K (Fig. 8 and Table 5). The number of binding sites for this complex is in good agreement with the above absorption spectral results. The results showed that the values of K b were greater than K SV, and the values of k q were much larger than the maximum diffusion collision quenching rate constant of various quenchers with the biopolymer (2.0 × 1010 Ms−1), suggesting that the fluorescence quenching interaction was static quenching [57, 58]. These results were similar to that reported about the interaction of other cationic metal complexes to ctDNA [6, 59, 60].
The fluorescence titration method was used to determinate the mode of binding between Pd(II) complex and ctDNA and analyzed according to Scatchard equation [6].
where r is the moles of ethidium bromide bound per mole of ctDNA, [L]f is the molar concentration of the free ethidium bromide, n is number of binding sites, and K a is the apparent binding constant. The apparent binding constant at three different concentrations for Pd(II)-DNA-EB interaction is the slope obtained from the respective Scatchard plots. The number of binding sites has been calculated from the ratio of the intercept to the slope obtained from the plot of r/C f versus r. The fluorescence Scatchard plots obtained for binding of EB to ctDNA in absence (♦) and presence (○, Δ, ◊) of various concentrations of Pd(II) complex are shown in Fig. 9 and the data are summarized in Table 6. As can be seen from the plots, a decrease in slope and the intercept resulted upon the addition of the metal complex concentration indicating the hydrogen binding of the complex with the ctDNA. Thus, the results obtained from Scatchard plots validate those obtained from emission spectral studies.
Thermodynamic parameters of DNA binding
Four classes of interactions that can play a role in the non-covalent binding of drug molecules to biomolecules are hydrogen bonds, van der Waals forces, electrostatic interactions, and hydrophobic bond interactions [61]. The signs and magnitudes of thermodynamic parameters of binding reaction are the main evidence for confirming the binding mode. If the enthalpy change (ΔH) does not vary significantly over the temperature range studied, then its value and that of entropy change (ΔS) can be determined using the modified Van’t Hoff equation [62]:
The ln K versus 1/T plots gave a straight line according to the above equation. The enthalpy and entropy have been obtained from the slope (− ΔH/R) and intercept (ΔS/R) of the Van’t Hoff plot (Fig. 10) and are presented in Table 5. The values of Gibbs free energy change (ΔG) were calculated from Eq. 3 at 293, 300 and 310 K.
The negative values of ΔG revealed that the binding process was spontaneous. Also, both enthalpy and entropy changes are negative, suggesting that the Pd(II) complex was hold in DNA grooves via hydrogen and Van der Waals binding [63].
ctDNA-binding modes
The influence of the complex on the ctDNA was monitored by gel chromatography (Fig. 11), and the results suggested that the ctDNA has been separated from the metal complex and the binding mode is non-covalent as the fluorescence method had been shown. Therefore, hydrogen binding and van der Waals force play major role in the binding process [64].
Molecular docking of Pd(II) complex with DNA
In order to deepen understanding of binding mode and binding events of the Pd(II) complex, molecular docking calculations have been performed with DNA duplex of sequence d (CGCGAATTCGCG)2. The docking results are presented as binding free energy [65] and are presented in Table 7. According to Table 7, the binding free energy value dominated by the vdW_hb_desolv energy is negative which indicates this binding is spontaneous and the Pd(II) complex has a good binding affinity to DNA. The best energy ranked results of the Na[Pd(8-QO)(cbdca)] with DNA are illustrated in Fig. 12. As viewed from the figure, Na[Pd(8-QO)(cbdca)] is situated within the DNA major groove in the region of GC–CG residue. The negative binding energy value (ΔG binding) revealed that the binding process was spontaneous and this is in very well agreement with the experimental DNA-binding results. As well as, docking results demonstrated that the most interactions between Pd(II) complex and ctDNA are hydrogen bonds and van der Waals forces which confirmed the experimental results. Therefore, we can conclude that there is a reciprocal complement between spectroscopic techniques and molecular docking modeling [66].
Conclusion
In conclusion, a new anionic water soluble palladium(II) complex derived from the 8-hydroxyquinoline and 1,1-cyclobutanedicarboxylic acid has been synthesized and thoroughly characterized. In vitro cytotoxicity results showed that the complex was a potential antitumor agent. The binding mechanism, binding sites, and binding mode between the palladium complex and ctDNA have been investigated employing different spectroscopic techniques. The experimental results indicated that the complex can cooperatively binds to ctDNA at low concentration. Fluorescence titration spectra revealed the presence of static quenching mechanism in the interaction of Pd(II) complex to ctDNA. The thermodynamic data, negative values of ΔH, ΔS and ΔG, indicate that the hydrogen bond and van der Waals force played major roles in the interaction. Generally, this anionic palladium(II)complex in comparison with the cationic metal complexes showed comparable interaction with ctDNA and gave an idea about design of novel, powerful agents for probing and targeting nucleic acids.
Change history
29 March 2018
Unfortunately, in the original version of the article the family name of the fifth author is wrong. The correct name is displayed above.
References
G. Zhang, Y. Ma, Food Chem. 141, 41 (2013)
A. Kathiravan, R. Renganathan, Polyhedron 28, 1374 (2009)
S. Sobha, R. Mahalakshmi, N. Raman, Spectrochim. Acta A 92, 175 (2012)
P. Zhu, G. Zhang, Y. Ma, Y. Zhang, H. Miao, Y. Wu, Spectrochim. Acta A 112, 7 (2013)
N. Akbay, Z. Seferoğlu, E. Gök, J. Fluoresc. 19, 1045 (2009)
H. Wu, J. Yuan, Y. Bai, G. Pan, H. Wang, X. Shu, J. Photochem. Photobiol. B 107, 65 (2012)
B.M. Zeglis, V.C. Pierre, J.K. Barton, Chem. Commun. 44, 4565 (2007)
A. Colak, Ü. Terzi, M. Col, Ş.A. Karaoglu, S. Karaböcek, A. Küçükdumlu, F.A. Ayaz, Eur. J. Med. Chem. 45, 5169 (2010)
L.N. Ji, X.-H. Zou, J.-G. Liu, Coord. Chem. Rev. 216, 513 (2001)
D.S. Raja, N.S. Bhuvanesh, K. Natarajan, Inorg. Chim. Acta 385, 81 (2012)
Z.A. Siddiqi, M. Khalid, S. Kumar, M. Shahid, S. Noor, Eur. J. Med. Chem. 45, 264 (2010)
Z.D. Matović, E. Mrkalić, G. Bogdanović, V. Kojić, A. Meetsma, R. Jelić, J. Inorg. Biochem. 121, 134 (2013)
J. Stierstorfer, K.R. Tarantik, T.M. Klapötke, Eur. J. Inorg. Chem. 15, 5775 (2009)
M.A. Jakupec, M. Galanski, V.B. Arion, C.G. Hartinger, B.K. Keppler, Dalton Trans. 2, 183 (2008)
K.S. Prasad, L.S. Kumar, S. Chandan, R.N. Kumar, H.D. Revanasiddappa, Spectrochim. Acta A 107, 108 (2013)
E.J. Gao, M.-C. Zhu, Y. Huang, L. Liu, H.Y. Liu, F.C. Liu, S. Ma, C.-Y. Shi, Eur. J. Med. Chem. 45, 1034 (2010)
J. Marmur, J. Mol. Biol. 3, 208 (1961)
M. Reichmann, S. Rice, C. Thomas, P. Doty, J. Am. Chem. Soc. 76, 3047 (1954)
E. Gao, M. Zhu, H. Yin, L. Liu, Q. Wu, Y. Sun, J. Inorg. Biochem. 102, 1958 (2008)
M. Arivazhagan, S. Jeyavijayan, Spectrochim. Acta A 79, 376 (2011)
U. Gowthaman, M. Jayakanthan, D. Sundar, BMC Bioinform. 9, 1 (2008)
A. Heydari, H. Mansouri-Torshizi, RSC Adv. 6, 96121 (2016)
Z. Asadi, M. Asadi, M.R. Shorkaei, J. Iran. Chem. Soc. 13, 429 (2016)
M. Eslami Moghadam, M. Saidifar, A. Divsalar, H. Mansouri-Torshizi, A.A. Saboury, H. Farhangian, M. Ghadamgahi, J. Biomol. Struct. Dyn. 34(1), 206 (2016)
W.J. Geary, Coord. Chem. Rev. 7, 81 (1971)
V.X. Jin, J.D. Ranford, Inorg. Chim. Acta 304, 38 (2000)
D.L. Pavia, G.M. Lampman, G.S. Kriz, J.A. Vyvyan, Introduction to Spectroscopy (Cengage Learning, Boston, 2008)
P. Štarha, Z. Trávníček, I. Popa, Inorg. Biochem. 103(7), 978 (2009)
K. Nakamoto, Applications in Coordination, Organometallic, and Bioinorganic Chemistry (Wiley, Hoboken, 1997)
M. Saeidfar, H. Masouri-Torshizi, G.R. Behbehani, A. Divsalar, A. Saboury, Bull. Korean Chem. Soc. 30, 1951 (2009)
Z. Sorinezami, H. Mansouri-Torshizi, J. Mater. Sci. Mater. E 27, 1558 (2016)
H. Mansouri-Torshizi, S. Shahraki, Z.S. Nezami, A. Ghahghaei, S. Najmedini, A. Divsalar, H. Ghaemi, A.-A. Saboury, Complex Met. 1, 23 (2014)
S. Shahraki, H. Mansouri-Torshizi, Z.S. Nezami, A. Ghahghaei, F. Yaghoubi, A. Divsalar, A.A. Saboury, F.H. Shirazi, Iran. J. Pharm. Res. 13, 1279 (2014)
F. Shiri, S. Shahraki, S. Baneshi, M. Nejati-Yazdinejad, M.H. Majd, RSC Adv. 6, 106516 (2016)
J. Luo, Z. Ma, H. Liang, J. Chen, Z. Zeng, Spectrochim. Acta A 90, 202 (2012)
S. Shahraki, F. Shiri, H. Mansouri-Torshizi, J. Shahraki, J. Iran. Chem. Soc. 13, 723 (2016)
S. Shahraki, H. Mansouri-Torshizi, M. Sadeghi, A. Divsalar, A.-A. Saboury, Biomacromol. J. 1, 242 (2015)
R.F. Greene, C.N. Pace, Biol. Chem. 249, 5388 (1974)
A. King, B. Nicholson, Biochem. J. 114, 679 (1969)
S.Z. Bathaie, A. Bolhasani, R. Hoshyar, B. Ranjbar, F. Sabouni, A.A. Moosavi-Movahedi, DNA Cell Biol. 26, 533 (2007)
G. Barrow, Physical Chemistry, chap. 7 (MC Graw-Hill, New York, 1988)
A. Divsalar, A.A. Saboury, L. Ahadi, E. Zemanatiyar, H. Mansouri-Torshizi, BMB Rep. 43, 766 (2010)
M. Islami-Moghaddam, H. Mansouri-Torshizi, A. Divsalar, A. Saboury, J. Iran. Chem. Soc. 6, 552 (2009)
G. Scatchard, Ann. N. Y. Acad. Sci. 51, 660 (1949)
A.V. Hill, J. Physiol. (Lond.) 40, 4 (1910)
H. Mansouri-Torshizi, M. Saeidifar, A. Divsalar, A.A. Saboury, Spectrochim. Acta A 77, 312 (2010)
Y.J. Liu, C.H. Zeng, H.L. Huang, L.X. He, F.H. Wu, Eur. J. Med. Chem. 45, 564 (2010)
F. Arjmand, F. Sayeed, J. Mol. Struct. 965, 14 (2010)
H. Mansoori-Torshizi, M. Islami-Moghaddam, A.A. Saboury, Acta Biochim. Biophys. Sin. 35, 886 (2003)
C. Dong, S. Ma, Y. Liu, Spectrochim. Acta A 103, 179 (2013)
H. Mansouri-Torshizi, M. Eslami-Moghadam, A. Divsalar, A.-A. Saboury, Acta Chim. Slov. 58, 811 (2011)
M. Khorasani-Motlagh, M. Noroozifar, S. Mirkazehi-Rigi, Spectrochim. Acta A 75(2), 598 (2010)
T. Zohoorian-Abootorabi, H. Sanee, H. Iranfar, M.R. Saberi, J. Chamani, Spectrochim. Acta A 88, 177 (2012)
S. Tabassum, W.M. Al-Asbahy, M. Afzal, F. Arjmand, J. Photochem. Photobiol. B 114, 132 (2012)
S. Bi, H. Zhang, C. Qiao, Y. Sun, C. Liu, Spectrochim. Acta A 69, 123 (2008)
M.-F. Zhang, Z.-Q. Xu, Y.-S. Ge, F.-L. Jiang, Y. Liu, J. Photochem. Photobiol. B 108, 34 (2012)
W.R. Ware, J. Phys. Chem. 66, 455 (1962)
Y. Sun, Z. Ji, X. Liang, G. Li, S. Yang, S. Wei, Y. Zhao, X. Hu, J. Fan, Spectrochim. Acta A 87, 171 (2012)
G. Zhang, X. Hu, P. Fu, J. Photochem. Photobiol. B 108, 53 (2012)
F. Xue, C.-Z. Xie, Y.W. Zhang, Z. Qiao, X. Qiao, J.-Y. Xu, S.P. Yan, J. Inorg. Biochem. 115, 78 (2012)
S. Kashanian, M.M. Khodaei, H. Roshanfekr, N. Shahabadi, G. Mansouri, Spectrochim. Acta A 86, 351 (2012)
J. Zhu, L. Wu, Q. Zhang, X. Chen, X. Liu, Spectrochim. Acta A 95, 252 (2012)
P.D. Ross, S. Subramanian, Biochemistry 20, 3096 (1981)
H. Mansuri-Torshizi, T. Srivastava, H. Parekh, M. Chitnis, J. Inorg. Biochem. 45, 135 (1992)
L. Zhu, K. Zheng, Y.-T. Li, Z.-Y. Wu, C.-W. Yan, J. Photochem. Photobiol. B 155, 86 (2016)
M. Sakthi, A. Ramu, J. Mol. Struct. 1149, 727 (2017)
Acknowledgements
We are grateful for the financial support of the University of Zabol, Materials and Energy Research Center, University of Sistan and Baluchestan and Cancer Research center, Shahid Beheshti University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahraki, S., Heidari, A., Mirzaei, H.R. et al. Synthesis, characterization, cytotoxicity, DNA binding and computational studies of an anionic palladium(II) complex derived from 8-hydroxyquinoline and 1,1-cyclobutanedicarboxylate. J IRAN CHEM SOC 15, 697–709 (2018). https://doi.org/10.1007/s13738-017-1269-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1269-8