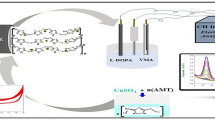
Abstract
In this paper, we report the synthesis and electrocatalytic activity of electrodeposited Fe2O3 nanoparticles modified on a glassy carbon electrode as highly sensitive sensors for determination of catecholamines. Results showed that the Fe2O3 nanoparticles on a glassy carbon electrode exhibit excellent catalytic activity toward catecholamines oxidation, including levodopa, dopamine, and epinephrine, resulting in a marked lowering in the peak potential and considerable improvement of the peak current as compared to the electrochemical activity at the bare glassy carbon electrode. The electrochemical characterizations of catecholamines were performed using cyclic voltammetry, chronoamperometry, and differential pulse voltammetry techniques. The electrocatalytic currents increase linearly with the levodopa, dopamine, and epinephrine concentrations in the ranges of 0.0625–1000, 0.25–1500, and 0.125–1000 µM, respectively, and the detection limits (3σ) were 24 ± 2, 14 ± 2, and 12 ± 2 nM, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today the use of nanomaterials has been increased due to their unique properties, including their remarkable electrical, chemical, mechanical not only for the fundamental scientific interest of the materials, but also for their broad range of applications [1]. Nanoparticles have the significantly advance nanomaterial-based applications which can be made to promote the electron transfer reactions and provide great catalytic surface areas for the modification of electrode surfaces and enhanced electrochemical reactivity [2–4]. In recent years, finding materials containing advantages such as easy preparation, low cost, high stability, and easily separation from solution by an external magnetic field have been the center of attention of researchers [5].
Among various materials, iron oxide nanoparticles have received a lot of attention due to high abundance, low cost, high resistance to corrosion, large surface area-to-volume ratio, and environmentally friendly properties have been more attention [6, 7]. Iron oxide, Fe2O3 (hematite), is the thermodynamic stable crystallographic phase of iron oxide under ambient conditions, which widely used as catalyst, pigment, gas sensor, and electrode materials [8, 9]. Fe2O3 is an attractive material to consider for photo-electrochemical research into solar energy conversion. It shows good stability under operation in aqueous solutions as it resists both dark and photo-corrosion, and its relatively small band gap energy of 2.2 eV enables it to absorb most of the photons of solar spectrum [7]. Many groups have synthesized Fe2O3 nanoparticle through various methods. Goyal et al. [10] used ultrasonic mist chemical vapor deposition method for synthesized Fe2O3 nanopowder. The Fe2O3 nanopowder was used to modify electrode for determination of dopamine. Doping of different metal ions or metal oxides into Fe2O3 will find new application or improve the performance of existing applications. For example, Suresh et al. [11] reported that the Co-doped a-Fe2O3 powders which synthesized by the hydrolysis method exhibit higher sensitivity and better selectivity to ascorbic acid and uric acid than the pure Fe2O3. It is still a challenge to synthesize Fe2O3 nanoparticles via a one-step process. Among various methods that have been employed to synthesize Fe2O3 nanoparticles, considerable attention has been paid to electrochemical methods due to their low cost, high sensitivity, ease of operation, and compatibility with micro-fabrication technology. Electrochemical methods, compared to chemical methods, have shown some important advantages such as being inexpensive, sensitive, selective, and able to generate reproducible results [12].
Levodopa, dopamine, and epinephrine are the important catecholamines neurotransmitter in the mammalian central nervous system and biological body fluids. Many life phenomena are related to the concentration of catecholamines in blood. Therefore, a simple, fast, and sensitive method is necessary for determination of their concentration in both biological fluids and pharmaceutical preparations [13–16]. However, so far several methods have been used for the determination of catecholamines including capillary electrophoresis, spectrophotometry [17], high-performance liquid chromatography [18], and chemiluminescence [19]. Nevertheless, these methods often have diverse disadvantages such as high cost, low selectivity, the use of organic solvents, complex sample preparation procedures, or long analysis time; in contrast, the utilization of electrochemical methods based on a chemically modified gathered much attention of researchers because of high sensitivity, low cost, simplicity, and reproducibility of this approach [20, 21].
In this work, we have developed electrodeposited Fe2O3 nanoparticle as an effective platform for the electrocatalytic oxidation of catecholamines. To the best of our knowledge, no study has been published so far reporting the electroanalytical applications of electrodeposited Fe2O3 nanoparticles as sensors for determination of catecholamines. The experimental results indicate that modified electrode facilitates electron transfer on surface of the electrode, reduces the overpotential, and also offers several advantages such as high repeatability and good stability. The modified electrode with Fe2O3 used for the determination of the levodopa, epinephrine, and dopamine.
Experimental
Apparatus and chemicals
The electrochemical measurements were performed with a potentiostat/galvanostat (SAMA 500, Iran). A conventional three-electrode cell was used. A saturated calomel electrode (SCE), a platinum wire, and a glassy carbon electrode modified by Fe2O3 nanoparticles were used as the reference, counter, and working electrodes, respectively. A Metrohm 781 pH/ion meter was used for pH measurements. The buffer solutions were prepared from orthophosphoric acid and its salts in the pH 7.0. All solutions were freshly prepared with double-distilled water. Analytical grade FeCl3·6H2O and all other reagents were acquired from Merck (Darmstadt, Germany).
Electrodeposition of Fe2O3 nanoparticle on GCE
In fabricating an electrochemical sensor, a GCE surface was initially polished using a slurry alumina on a polishing cloth and then rinsed with water. The modified electrode was prepared as follows: electrochemical deposition of Fe2O3 nanoparticle on GCE was carried out in a conventional three-electrode cell including a mixed aqueous solution of 5 mM FeCl3 + 5 mM NaF + 0.1 M KCl + 1 M H2O2. Electrochemical growth process was done by a potential cycling procedure, at a potential sweep rate of 100 mV s−1, from −0.5 to 0.8 V for a whole of ten cycles [22]. Fluoride was added to decrease the reduction potential of Fe3+ to Fe2+, and the KCl was used as a supporting electrolyte. There are two functions of H2O2: One is to produce OH− during the reduction process so that the pH in the immediate vicinity of the working electrode increases, aiding the electrodeposition of iron hydroxides; the other is to stabilize the dopant precursor in aqueous solution [22, 23].
Results and discussion
Characterization of the Fe2O3 nanoparticles
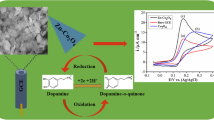
The response of an electrocatalyst is related to its physical morphology of its surface. Figure 1 displays the SEM images of the electrosynthesized iron oxide nanoparticles. It can be seen that the surface of modified electrode is homogeneous and the surface of the substrate is well covered.
Electrocatalytic oxidation of catecholamines at a Fe2O3/GCE
Cyclic voltammetry of catecholamines at a Fe2O3/GCE toward oxidation of LD, DA, and EP in 0.1 M phosphate buffer (Fig. 2) have been investigated. As can be seen, the anodic peak potential for LD, DA, and EP, oxidation at the bare GCE (curves b) are about 225, 164, and 130 mV, respectively, while the corresponding potential at Fe2O3/GCE (curves c) is 100, 118, and 120 mV, respectively. The comparison of the oxidation of catecholamines at Fe2O3/GCE (curve b) and GCE (curve a) shows an enhancement of the anodic peak current (2.9, 3.2, and 3.7 for LD, DA, and EP, respectively), which indicated that the presence of Fe2O3 nanoparticles could enhance the peak currents. Therefore, all the three peak potentials have positive shifts. These shifts in the oxidation peak potentials and the increase in the peak current in the presence of Fe2O3 nanoparticles indicate that the modified electrode plays a catalytic effect on the oxidation of catecholamines. This electrocatalytic effect was attributed to the fine dispersion, nanometer size, large accessible surface per volume, and low resistance, for electron transfer between catecholamines and the modified electrode which results in the improvement on the electrochemical response.
Cyclic voltammetry was used for indicating the effect of potential scan rate on the oxidation of LD, DA, and EP, in 0.1 M phosphate buffer (A, B, and C in Fig. 3, respectively), at the Fe2O3/GCE. As is observed in Fig. 3, the oxidation peak potential shifted to more positive potentials with increasing scan rate, confirming the kinetic limitation in the electrochemical reaction. Also, plots of anodic peak currents (I p) versus the square root (v 1/2) of scan rate were linearly in the range of 5–300 mV s−1. Voltammograms show that the oxidation process of catecholamines at the modified electrode is controlled in a diffusion rather than surface controlled. The slopes of the linear segments in Fig. 3 can be used to extract the kinetic parameters α a (anodic transfer coefficients). The evaluated values are 5.3 × 10−1, 4.3 × 10−1, 5.0 × 10−1 for the LD, DA, and EP, respectively.
CVs of Fe2O3/GCE in 0.1 M phosphate buffer solution (pH 7.0) at various scan rates of A LD, numbers 1–7 correspond to 5, 10, 20, 50, 100, 200, and 300 mV s−1 scan rates, respectively. B DA, numbers 1–9 correspond to 5, 10, 20, 30, 50, 100, 200, 300 and 400 mV s−1 scan rates, respectively. C EP, numbers 1–9 correspond to 5, 10, 20, 30, 40, 50, 100, 200 and 300 mV s−1 scan rates, respectively. Insets A variations of Ip versus the square root (v 1/2) of scan rate. B Ep versus the logarithm current of the scan rate 100 mV s−1/2
Chronoamperometric studies of catecholamines at a Fe2O3/GCE
The oxidation of LD, DA, and EP at Fe2O3/GCE was also studied by chronoamperometry. Chronoamperograms represent the current–time profiles obtained by setting the working electrode potential in 0.5 mM of LD, DA, and EP (A, B, and C in Fig. 4, respectively) at the surface of Fe2O3/GCE (curve a) and bare GCE (curve b). All electrodes reach a steady-state Faradic current response within approximate 20 s. It was observed that the Fe2O3/GCE (curve a) produces a higher steady-state current density in comparison with that of the bare electrode during the whole measurements. This could be attributed to the higher electrochemically active surface area of the Fe2O3/GCE (curve a) which is in agreement with the results of cyclic voltammetry of catecholamine oxidation.
Calibration plot and limit of detection for catecholamines at a Fe2O3/GCE
Differential pulse voltammetry was used to obtain the linear concentration range and detection limit of catecholamines at the Fe2O3/GCE. Figure 5 shows a DPV for determination of LD, DA, and EP (A, B, and C in Fig. 5, respectively), at different concentrations using the Fe2O3/GCE. The voltammograms clearly show that the plot of peak current versus LD concentration consists of two linear segments with different slopes; the linear response was: LD, a slope of 3.67 × 10−1 μA μM−1 for the first linear segment (6.25 × 10−2–20 μM) and a slope of 3.74 × 10−1 μA μM−1 for the second linear segment (20–1000 μM); DA, a slope of 6.46 × 10−1 μA μM−1 for the first linear segment (2.5 × 10−1–10 μM) and a slope of 2.23 × 10−2 μA μM−1 for the second linear segment (10–1500 μM); and EP, a slope of 7.25 × 10−1 μA μM−1 for the first linear segment (1.25 × 10−1–20 μM) and a slope of 3.19 × 10−2 μA μM−1 for the second linear segment (20–1000 μM). The decrease in sensitivity (slope) of the second linear segment is likely due to kinetic limitation [18]. The detection limits (3σ) were 24 ± 2, 14 ± 2, and 12 ± 2 nM for LD, DA, and EP, respectively. The detection limits and linear ranges are comparable with values reported by other research groups for electrocatalytic oxidation of LD, DA, and EP, at the surface of chemically modified electrodes using other mediators (Table 1) [24–32].
DPVs of Fe2O3/GCE in 0.1 M phosphate buffer solution (pH 7.0) containing different concentrations of A LD, numbers 1–16 correspond to 0.0625–1000 μM, B DA, numbers 1–14 correspond to 0.25–1500 μM; C EP, numbers 1–15 correspond to 0.125–1000 μM; insets plot of the peak currents as a function of catecholamine concentration
Repeatability and stability of iron oxide nanoparticles/GCE
The repeatability and stability are one of the important characters of a sensor. CV has been performed to investigation the long-term stability toward oxidation of 0.50 mM of catecholamines, using electrochemical deposition of Fe2O3/GCE. The storage stability of the Fe2O3/GCE was evaluated for a period of one month. When CVs were recorded after the Fe2O3/GCE was stored in atmosphere at room temperature, the peak potential for LD, DA, and EP oxidation was unchanged. The current signals showed <3.7, 3.6, 4.3% decrease relative to the initial response for LD, DA, and EP, respectively, indicating that the Fe2O3/GCE maintained its catalytic activity and could be used for an extended period. The ability to generate a reproducible modified electrode surface was examined using CV data from five separately prepared Fe2O3/GCE. The RSD obtained was lower than 3.3% for various catecholamines, which indicates satisfactory reproducibility in the preparation of electrodes. These results indicate that Fe2O3/GCE has good stability and reproducibility.
Conclusions
The main object of this study was to develop electrodeposited Fe2O3 nanoparticles as an effective platform for the modification of electrode. To the best of our knowledge, no study has been published so far reporting the electroanalytical applications of electrodeposited Fe2O3 nanoparticles as sensors for determination of catecholamines. It is worth noticing that the electrodeposited Fe2O3 nanoparticle is ideally suited for implementation in electrochemical applications. This work puts forward an approach for the fabrication of a novel nanostructured-modified electrode by the use of Fe2O3 nanoparticle. The Fe2O3-modified electrodes not only significantly improve the oxidation currents of catecholamines, but also negatively shift their oxidation potentials. The results show that the determination of catecholamines using electrodeposition method is simpler and more sensitive of other methods.
References
R. Valiev, Nature 419, 887 (2002)
J. Wang, Analyst 130, 421 (2005)
N.G. Sahoo, Y. Pan, L. Li, S.H. Chan, Adv. Mater. 24, 4203 (2012)
N. Ghobadi, F. Dousi, J. Iran. Chem. Soc. 12, 757 (2015)
C.C. Berry, A.S.G. Curtis, J. Phys. D Appl. Phys. 36, R198 (2003)
Y. Zheng, Y. Cheng, Y. Wang, F. Bao, L. Zhou, X. Wei, Y. Zhang, Q. Zheng, J. Phys. Chem. B 110, 3093 (2006)
S. Dehdashtian, M.B. Gholivand, M. Shamsipur, Mater. Sci. Eng. C 58, 53 (2016)
L. Li, Y. Chu, Y. Liu, L. Dong, J. Phys. Chem. C 111, 2123 (2007)
G. Absalan, M. Akhond, A. Bananejad, H. Ershadifar, J. Iran. Chem. Soc. 12, 1293 (2015)
R.N. Goyal, A.K. Pandey, D. Kaur, A. Kumar, J. Nanosci. Nanotechnol. 9, 4692 (2009)
R. Suresh, R. Prabu, A. Vijayaraj, K. Giribabu, A. Stephen, V. Narayanan, Mater. Chem. Phys. 134, 590 (2012)
P.S. Shinde, G.H. Go, W.J. Lee, J. Mater. Chem. 22, 10469 (2012)
M. Roushani, M. Shamsipur, H.R. Rajabi, J. Electroanal. Chem. 712, 19 (2014)
M. Mazloum-Ardakani, A. Khoshroo, Anal. Chim. Acta 798, 25 (2013)
M. Mazloum-Ardakani, A. Khoshroo, Electrochem. Commun. 42, 9 (2014)
A. Afkhami, F. Kafrashi, T. Madrakian, Ionics (Kiel). 21, 2937 (2015)
P. Britz-McKibbin, D.D.Y. Chen, Anal. Chem. 72, 1242 (2000)
L. Jiang, Y. Chen, Y. Luo, Y. Tan, M. Ma, B. Chen, Q. Xie, X. Luo, J. Sep. Sci. 38, 460 (2015)
X. Xu, H. Zhang, H. Shi, C. Ma, B. Cong, W. Kang, Anal. Biochem. 427, 10 (2012)
M. Mazloum-Ardakani, L. Hosseinzadeh, A. Khoshroo, H. Naeimi, M. Moradian, Electroanalysis 26, 275 (2014)
M. Mazloum-Ardakani, A. Khoshroo, L. Hosseinzadeh, Sens. Actuators B Chem. 214, 132 (2015)
A. Kleiman-Shwarsctein, Y.-S. Hu, A.J. Forman, G.D. Stucky, E.W. McFarland, J. Phys. Chem. C 112, 15900 (2008)
R. Schrebler, K. Bello, F. Vera, P. Cury, E. Muñoz, R. del Río, H.G. Meier, R. Córdova, E.A. Dalchiele, Electrochem. Solid-State Lett. 9, C110 (2006)
S.-Y. Yi, J.-H. Lee, H.-G. Hong, J. Appl. Electrochem. 44, 589 (2014)
M. Mazloum-Ardakani, M. Zokaie, A. Khoshroo, Ionics (Kiel). 21, 1741 (2015)
M. Sadiković, B. Nigović, S. Jurić, A. Mornar, J. Electroanal. Chem. 733, 60 (2014)
W. Sun, M. Yang, K. Jiao, Anal. Bioanal. Chem. 389, 1283 (2007)
H. Yang, Y. Li, Y. Liu, Y. Zhang, Y. Zhao, M. Zhao, J. Solid State Electrochem. 19, 145 (2015)
L. Yang, D. Liu, J. Huang, T. You, Sens. Actuators B Chem. 193, 166 (2014)
T. Łuczak, M. Bełtowska-Brzezinska, R. Holze, Electrochim. Acta 123, 135 (2014)
M. Mazloum-Ardakani, S.H. Ahmadi, Z.S. Mahmoudabadi, A. Khoshroo, K.T. Heydar, Ionics (Kiel). 20, 1757 (2014)
M. Taei, M. Jamshidi, J. Solid State Electrochem. 18, 673 (2014)
Acknowledgements
The authors wish to thank the Yazd University Research Council, IUT Research Council and Excellence in Sensors for financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazloum-Ardakani, M., Maleki, M. & Khoshroo, A. High-performance electrochemical sensor based on electrodeposited iron oxide nanoparticle: catecholamine as analytical probe. J IRAN CHEM SOC 14, 1659–1664 (2017). https://doi.org/10.1007/s13738-017-1106-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1106-0