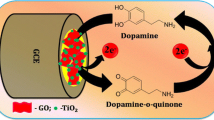
Abstract
A non-enzymatic dopamine electrochemical sensing probe was developed. A hexagonal shape zinc-doped cobalt oxide (Zn-Co2O4) nanostructure was prepared by a facile hydrothermal approach. The combination of Zn, which has an abundance of electrons, and Co3O4 exhibited a synergistically electron-rich nanocomposite. The crystallinity of the nanostructure was investigated using X-ray diffraction. A scanning electron microscope (SEM) was used to examine the surface morphology, revealing hexagonal nanoparticles with an average particle size of 400 nm. High-resolution transmission electron microscopy (HR-TEM) was used to confirm the nanostructure of the doped material. The nanostructure’s bonding and functional groups were verified using Fourier transform infrared spectroscopy (FTIR). The electrochemical characterization was conducted by using electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and amperometry. The resistivity of the electrode was confirmed through EIS and showed that the bare glassy carbon electrode (GCE) exhibited higher charge transfer resistance as compared to modified Zn-Co2O4/GCE. The sensing probe was developed by modifying the surface of GCE with Zn-Co2O4 nanostructure and tested as an electrochemical sensor for dopamine oxidation; it operated best at a working potential of 0.17 V (vs Ag/AgCl). The developed sensor exhibited a low limit of detection (0.002 µM), a high sensitivity (126 µA. µM−1 cm−2), and a wide linear range (0.2 to 185 µM). The sensor showed a short response time of < 1 s. The sensor’s selectivity was investigated in the presence of coexisting species (uric acid, ascorbic acid, adrenaline, epinephrine, norepinephrine, histamine, serotonin, tyramine, phenethylamine, and glucose) with no effects on dopamine determination results. The developed sensor was also successfully used for determining dopamine concentrations in a real sample.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine (DA) is a vital neurotransmitter, which performs an important role in the hormonal, cardiovascular, and central nervous systems. Dopamine performs like a chemical messenger that circulates messages to parts of the brain [1, 2]. DA not only controls the release of various hormones but also performs many functions in the kidneys and bloodstream. Many diseases of the nervous system are related to the deficiency or dysfunctions of the dopaminergic system [3]. It is important to identify neurochemicals in biological fluids for the treatment, diagnosis, and prognosis of central nervous system diseases. As a result, developing sensitive, selective, and reliable methods for detecting dopamine is a fascinating subject [3, 4].
Over the years, different instrumental techniques have been utilized for dopamine detection in pharmaceutical and biological samples such as high-performance liquid chromatography (HPLC) [5], fluorimetry [6], spectrophotometry [7], capillary electrophoresis (CE) [8], electrochemiluminescence (ECL) [9], and electrochemical [10]. Among these techniques, the electrochemical technique is the preferable method owing to its high sensitivity, fast response, ease in operation, cost-efficient, and no need for sample pretreatment and skillful personnel for operation [11,12,13]. It is well-known that changing the material composition and surface features of an electrode will change its electrochemical efficiency. To improve the selectivity, sensitivity, and accuracy of dopamine determination, a well-designed working electrode is needed [14, 15].
Dopamine and its coexisting species ascorbic acid (AA) and uric acid (UA) have overlapped in the measurement process. The electrochemical analysis shows the response of the coexisting species because of the same oxidation potential [16]. To overcome this problem, different types of nanocomposites are described for DA detection. The selection and development of nanomaterial enhance the performance for the detection of biomolecules. The material which can be more efficient and have the best electrocatalytic activity is a challenge. For dopamine electroanalytical detection, metal oxides have been reported [17]. The recent research in nanotechnology has provided the direction towards new material for the sensing of DA. In nanostructured-based composites, it is easy to control the properties of the material even without changing its chemical composition. The nanocomposites of metal oxide possess several characteristics like large surface area, efficient electron transport, high electronic conductivity, high mechanical stability, low cost, and easy fabrication [18, 19]. For the fabrication of electrochemical devices, transition metal oxides (TMOs) are commonly used. Recently reported nanomaterials for dopamine sensing are Ag Au, and Pd NPs [20], MnO2 [21], Cu2O [22], and Co3O4 [23]. Among them, Co3O4 has garnered a lot of attention owing to its intriguing properties such as electrocatalytic activity, low cost, and efficiency. Cobalt oxide has a cubic close packing distribution of oxide ions, with Co(II) ions occupying the tetrahedral 8a sites and Co(III) ions occupying the octahedral 16d sites. Co2O4 is a p-type semiconductor, and the spinel structure shows attractive electrochemical performance [24, 25].
The synthesis and fabrication of doped metal oxide-based sensors have been the subject of many studies. The dopant materials are metals and non-metals. The doped metal with metal oxide has shown excellent performance in energy storage devices, fuel cells, and sensors [26,27,28,29,30]. Especially in the field of electrochemical biosensors, they have gained more interest in the doping phenomenon. The doped metal oxide shows the synergistic effect for DA detection. The doped metal has an excess of the electron when this free electron gains by the metal oxide atom; the oxidation states of the metal oxide will be changed. The oxidation states can affect the catalytic performance of the metal oxide. To tune the bandgap of the material doping is the best choice [30, 31].
The present study used a simple hydrothermal method to synthesize a highly crystalline zinc-doped cobalt oxide (Zn-Co2O4) nanostructure. The size and morphology of the nanostructure are controlled in a hydrothermal route. The synthesized nanostructure was analyzed by various techniques such as FTIR, TGA, XRD, SEM, EDX, and HR-TEM. The glassy carbon (GC) electrode surface was modified with Zn-Co2O4 nanostructure and tested as an electrochemical sensor of dopamine. For the first time, zinc-doped cobalt oxide nanostructure-based electrochemical sensor for dopamine was fabricated and gave a low limit of detection, dynamic linear range, and high sensitivity. The electrochemical impedance spectroscopy (EIS), chronoamperometry, and cyclic voltammetry (CV) techniques were used for electrochemical analysis. The developed sensor was tested for dopamine detection in the presence of coexisting species such as uric acid, ascorbic acid, adrenaline, epinephrine, norepinephrine, histamine, serotonin, tyramine, phenethylamine, and glucose. Moreover, Zn-Co2O4 nanostructure-based sensing probe was effectively employed for dopamine determination in a real sample.
Experimental
Reagents and chemicals
All reagents with high purity and analytical grade were used as obtained from Sigma-Aldrich (https://www.sigmaaldrich.com), including cobalt(II) chloride hexahydrate (98%), zinc chloride (98%), dopamine, ammonium hydroxide solution (28%), D-( +)-glucose, adrenaline, epinephrine, norepinephrine, histamine, serotonin, tyramine, phenethylamine, ascorbic acid, hydrogen peroxide solution (30% (w/w) in H2O), and ethanol (nuclear, 99.9%). Uric acid (BDH, 98%) was purchased from BDH (https://bdhme.com). Other used reagents were the physiological solution and HCl (37%, Synth). All chemicals were utilized as obtained without further purification. All solutions were prepared in deionized (DI) H2O.
Synthesis of zinc-doped cobalt oxide (Zn-Co2O4)
For the synthesis of zinc-doped cobalt oxide, a hydrothermal method was used. Zinc chloride 25 mL solution of 0.01 M was prepared in a beaker and kept under stirring for 30 min. The cobalt chloride hexahydrate solution was prepared in 50 mL deionized water with a concentration of 0.1 M and stirred for 30 min. Then both solutions were mixed and stirred for 10 min. The pH of the solution was adjusted to 10 by the drop-wise addition of ammonium hydroxide. After 10 min of stirring, the solution was transferred to a 100 mL Teflon tube, and the autoclave was tightly closed. Then it was heated for 16 h at 150 °C. After that, the reaction mixture was cooled down to 25 °C. Then the sample was washed with ethanol and deionized water. Finally, a light greenish powder was collected and dried in an oven at 80 °C for 10 h.
Choice of materials
Zinc-doped cobalt oxide nanostructure was chosen for dopamine sensing due to the facile redox tunability of the dopant nanostructure which makes it conformable and effective for electrocatalytic activity. The dopant elements increase the active sites with the synergistic effect of dopant and doped components, ultimately increasing the adsorption of dopamine molecules on the surface through facile electron enrich Zn ions which improves the electrocatalytic activity [32]. The Zn-Co2O4 has higher conductivity due to the doped element which enhances the charge transferability for dopamine sensing.
Characterization techniques
The structural analysis was performed by a PANalytical X’Pert Powder X-ray diffractometer with a monochromatic Cu–Kα radiation (1.5418 Å) source. The crystallite size of Zn-Co2O4 was calculated using the Scherer equation:
where D is the mean size of crystalline domains, κ is the shape factor (0.9), \beta is the full-width half maxima, θ is the Bragg angle, and λ is the wavelength of X-rays in nanometer (0.15418 nm). The scanning electron microscope VEGA3 TESCAN was used at 20 kV to attain information about the Zn-Co2O4 surface morphology. The average particle size was determined by the ImageJ software. Energy dispersive spectroscopy (EDX) was performed for the elemental analysis of the samples The Fourier transform infrared spectroscopy was accomplished by Thermo-Nicolet 6700 P FTIR Spectrometer (USA), for the analysis of functional group confirmation in the range of 4000 to 600 cm−1.
High-resolution transmission electron microscopy (HR-TEM) images were obtained using a Talos F200X scanning/transmission electron microscope (STEM) with a lattice-fringe resolution of 0.14 nm at an accelerating voltage of 200 kV equipped with a CETA 16 M camera. The high-resolution images of periodic structures were analyzed using TIA software. The bright field imaging was performed at spot size 3, and the same areas were marked and scanned using the STEM-HDAAF mode. The STEM model helps in providing the elemental composition as it works on the principle of mass determination. Such measurements can be performed at a low electron dose by collecting the high-angle dark-field signal using an annular detector. Elemental analysis of the samples was performed at spot size 9 and with a screen current of 60 pA. The data was analyzed using Velox analytical software.
Electrochemical measurements were performed on the Gamray Potentiostat (1010, Model No. 23045) instrument at ambient lab conditions. For electrochemical analysis, an electrochemical cell consisting of a three-electrode system was employed; GCE modified with Zn-Co3O4 nanostructures as a WE (working electrode), Ag/AgCl (3 M KCl) as a RE (reference electrode), and platinum wire was used as a counter electrode. For electrochemical activities, cyclic voltammetry (CV), chronoamperometry, and electrochemical impedance spectroscopy (EIS) measurements were performed.
Real sample preparation
A real sample solution of dopamine was used to confirm the feasibility of the proposed sensor. A commercial physiological solution (sodium chloride 0.9% w/v) Otsuka, Pakistan Ltd., was purchased. A dopamine solution of 0.01 M was prepared in a physiological solution. In an electrochemical cell, a known concentration of dopamine solution was added, and an amperogram was registered for each addition. In the concentration range of 2–120 μM, an amperogram was obtained. The procedure for dopamine determination was the same as described in the "Experimental" section.
Working electrode fabrication
For the working electrode fabrication, a wet soft polishing cloth was used to polish the glassy carbon electrode surface with alumina powders (0.03 mm). Then, the adsorbed alumina powder on the surface of GCE was removed by sonication in a mixture of ethanol and water for 2 min. Then it was washed with deionized water and dried at 400 °C in an oven. For further study, the polished GCE was ready to be modified with the 2 mg/mL of Zn-Co2O4 nanostructure dispersed in ethanol and then sonicated for 5 min for complete dispersion. Afterward, an aliquot of (5µL) of Zn-Co2O4 was drop-casted on the GCE surface and dried at room temperature. After that, the fabricated electrode Zn-Co2O4/GCE was used for electrochemical characterization and sensor applications.
Optimization of method
Influence of Zn-Co2O4 amount on GCE
To explore the loading amount of catalyst (Zn-Co2O4) on the GC electrode surface, a cyclic voltammetry technique was used. A suspension of Zn-Co2O4 (2 mg/mL) was prepared and drop-casted on the precleaned GC electrode surface. A different drop-casting quantity 1, 3, 5, 7, 9, and 11 μL was applied on the electrode surface and tested towards the oxidation of dopamine (30 μM). Figure S1a shows the graphs for the varied quantity of catalyst loading on the GCE surface. Figure S1a observes that the current response was increased up to 5 μL, when increasing the loading amount (> 5 μL), and then decreased in the current response occurred. This decrease in current response could be due to the Zn-Co2O4 heavy loading on the GCE surface; this may create resistance to electron movement [32]. Therefore, the optimum quantity of Zn-Co2O4 (5 μL) was drop-casted on the electrode surface, and this amount was used for further electrochemical experimentations.
Effect of pH
The pH of electrolytes has a substantial function in the oxidation peak current and peak potential of the analyte. Figure S1b exhibits that the anodic peak potential of DA is shifted negatively with the pH increase from 3 to 11. The slope of the plot between pH values and peak potentials shows good linearity as shown in Fig. S1c. The value of the slope for dopamine is –56.7 mV/pH, which indicates that an equal number of electrons and protons are involved in the oxidation process. The anodic peak current of DA in the pH range of 3–7 (acidic pH) increases with the increase of pH and then a negative decrease in current occurred in the pH range of 7–9 (basic pH). The electrode at pH 7.0 gave a maximum current response. From this result, it is evident that the pH has a significant influence on the oxidation potential and anodic peak current of DA. To develop a sensor for biological samples, we have selected the optimum pH 7, and potential 0.17 V was chosen for further electrochemical experiments [10, 33].
Influence of Zn doping concentration in Co2O4
To check the influence and electrochemical response of Zn doping quantity in Co2O4, different concentrations (1.6, 2.6, 3.6, and 4.6) of Zn w/w % were doped in Co2O4 material. The materials obtained were designated as 1.6 w/w% Zn-Co2O4, 2.6 w/w% Zn-Co2O4, 3.6 w/w% Zn-Co2O4, and 4.6 w/w% Zn-Co2O4. The electrochemical response of these different composites was evaluated through cyclic voltammetry in the presence of 30 µM dopamine, at pH 7 in 0.1 M PB as shown in Fig. 1. Curve (a) was recorded with the GCE modified with the 1.6 w/w % Zn-Co2O4; it gave a peak current of 14.68 µA at a potential of 0.212 V vs Ag/AgCl. Curve (b) was recorded with the GCE modified with 2.6 w/w % Zn-Co2O4; the current obtained was 18.98 µA at a potential of 0.19 V vs Ag/AgCl, and curve (c) was obtained with the GCE modified with the 3.6 w/w % Zn-Co2O4; the current obtained was 21.74 at a potential of 0.17 V against Ag/AgCl. Similarly, curve (d) was obtained with GC modified with 4.6 w/w % Zn-Co2O4; the current obtained was 20.73 at a potential of 0.23 V. From these results, it is evident that the materials containing 3.6 w/w% Zn-Co2O4 gave a well-defined anodic peak with high current value and at a lower potential value as compared to the other composites. The better electrochemical response obtained may be due to the uniform incorporation of Zn2+ ions into the crystal lattice of the host cobalt oxide. Therefore, the 3.6 w/w% Zn-Co2O4 was selected as the best material for further electrochemical analysis as evident from the obtained CV results due to higher current and lower potential.
Results and discussion
X-ray diffraction
XRD technique was performed to identify the crystallographic structure of the Co2O4 and Zn-Co2O4 samples. Figure 1 (black) portrays the XRD pattern of pure Co2O4 and depicts that the cobalt oxide has a cubic shape. It was confirmed that the undoped cobalt oxide has a pure crystalline structure. The typical diffraction peaks of undoped cobalt oxide are observed at 2θ of 19.0°, 31.27°, 36.85°, 38.54°, 44.81°, 59.65°, 65.23°, 74.11°, 77.34°, and 78.40° which are assigned to the crystal plane of (111), (220), (311), (222), (400), (511), (440), (620), (533), and (622). Figure 2 (red) shows the XRD pattern of Zn-doped Co2O4 with the diffraction peak of 19.0°, 31.27°, 34.44°, 36.85°, 38.54°, 44.81°, 47.85°, 55.60°, 59.65°, 65.23°, 74.11°, 77.34°, and 78.40° with crystal plane of (111), (220), (300), (311), (222), (400), (340), (422), (511), (440), (620), (533), and (622). The peak positions are exactly matched with the JCPDS No. 23.1390 [34]. The diffraction peaks at 34.44°, 47.85°, and 55.60° corresponding to the crystal plane of (300), (340), and (422) confirmed the existence of Zn dopant. After doping, the Zn-doped Co2O4 shows a higher intensity with crystalline behavior. In both spectra, any other impurity diffraction peaks were not observed. The ionic radii of Zn2+ (0.60 Å) and Co2+ (0.56 Å) are approximately similar, the Zn2+ ions can be successfully incorporated into the crystal lattice of the host cobalt oxide [34,35,36]. This phenomenon can be interpreted in terms of the larger covalent radius of zinc ion than cobalt ion; due to this, the unit cell volume of zinc-doped cobalt oxide is increased. The crystallite size of pure Co2O4 and Zn-Co2O4 was calculated by Scherer’s equation which is 18.50 nm and 40.60 nm, correspondingly. Figure 2, shows the materials obtained with different compositions 1.6, 2.6, 3.6, and 4.6 of Zn w/w % doped in Co2O4. It is evident from the figure that there is no change occurring in the peak position, but only the intensity changes with the increase of Zn doping concentration in Co2O4.
Fourier transform infrared spectroscopy (FTIR)
FTIR was performed for the synthesized sample of Zn-Co2O4 within the range of 4000–600 cm−1. The absorption peaks were observed at 3730, 3446, 1615, 1500, 1377, 1030, 912, and 736 cm−1. Figure 3 shows the peak at 736, 912, and 1030 cm−1 which depicts the stretching vibration of metal–oxygen bond (Co–O), confirming the formation of Co2O4 nanoparticles [32]. The peaks at 3446 and 1615 cm−1 could be assigned to the O–H stretching and bending vibration of water molecules and H2 bonded hydroxyl group in Co2O4. The absorption peaks at 1500 and 1377 cm−1 are assigned to the vibration of Zn-Co2O4 and Co–O-H, respectively [37]. These results are in close agreement with previous results. The XRD and FTIR results were used to confirm the successful formation of Zn-Co2O4.
Scanning electron microscope (SEM)
The scanning electron microscopic study provides information about the surface morphology. Figure 4a and b shows that the Zn-Co2O4 nanostructure has hexagonal shape nanoparticles. The hydrothermal process was used to prepare the nanostructure material. Because of the hydrothermal method, the material has a distinct hexagonal morphology. The SEM result was analyzed through ImageJ software and found that the average particle size was ~ 400 nm as shown in Fig. 4c.
Energy dispersive X-ray spectroscopy (EDX)
For quantitative and elemental composition analysis, energy dispersive X-ray spectrometry was used. The elemental composition of the synthesized nanostructure Zn-Co2O4 is represented in Fig. 4d. It shows that the Zn-Co2O4 nanostructure was composed of zinc, cobalt, and oxygen elements. The weight percent of Zn, Co, O, and Cl was 26.05, 45.05, 25.59, and 3.32%, respectively, observed. Similarly, the atomic percentage of Zn, Co, O, and Cl was 13.95, 26.77, 56.0, and 3.28%, respectively, as shown in the inset table. From the result of EDX, the Zn doping in Co2O4 material is confirmed.
Transmission electron microscopy (TEM)
The microstructure and crystallinity of the Zn-Co2O4 have been analyzed by HR-TEM and selected area electron diffraction (SAED). Through SAED, we observed the crystallinity of the materials. Figure 5a shows that the Zn-Co2O4 nanostructure possesses hexagonal morphology. Figure 5b shows the synthesized composite possesses a polycrystalline structure. When the electron beam was focused on individual particles, then SAED patterns were obtained. The ring pattern shows that the Zn-Co2O4 particles are randomly oriented, and strong intensities indicate the high crystallinity of the sample. Furthermore, the labeled diffraction rings can be associated with the five major crystal planes (111), (220), (400), (422), and (440) of cubic. This result agrees with the X-ray diffraction pattern.
Electrochemical study on the surface of zinc-doped cobalt oxide electrode
The sensing properties were investigated using electroanalytical techniques. A three-electrode setup was employed for electrochemical study, consisting of a GC electrode modified with Zn-Co2O4 nanostructure as the working electrode, platinum wire as the counter electrode, and Ag/AgCl (3 M KCl) as the reference electrode. For electrochemical measurements, the supporting electrolyte 0.1 M phosphate buffer (PB) was used. The dopamine was determined through CV and chronoamperometry. Electrochemical impedance spectroscopy (EIS) was used to determine the working electrode’s resistivity.
Cyclic voltammetric study
The cyclic voltammetric experiments were conducted on a three-electrode system in 0.1 M phosphate buffer in the electrochemical cell. The GC electrode modified with the synthesized Co2O4 and Zn-Co2O4 nanostructure materials was tested for electrocatalytic oxidation of dopamine. The electrochemical measurements were conducted with Ag/AgCl as a reference electrode in the potential range of − 0.05 to 0.4 V vs Ag/AgCl. Figure 6a illustrates the cyclic voltammograms of bare GCE in the blank solution (absence of analyte) as curve (a), Co2O4 /GC electrode in the blank solution as curve (b), and Zn-Co2O4/GC electrode in the blank solution as curve (c). All three electrodes did not show any redox peak in the blank solution, but the Zn-Co2O4/GC electrode shows higher current values with a maximum area under the curve as compared to the Co2O4 /GC electrode and bare GCE. It confirms that the GCE modified with Zn-Co2O4 nanostructure gave higher current values and maximum area under the curve due to the electron-rich Zn-Co2O4 nanostructure.
Cyclic voltammogram obtained in 0.1 M PB (pH 7.0): a curve (a) bare GCE in the blank solution, curve (b) Co2O4/GC electrode in blank solution, and curve (c) Zn-Co2O4/GC electrode in blank solution. b curve (a) bare GCE in 30 µM of dopamine, curve (b) Co2O4/GCE in 30 µM, 0.1 M PB (pH 7.0), and curve (c) Zn-Co2O4/GCE in 30 µM, 0.1 M PB (pH 7.0). c Cyclic voltammogram obtained with Zn-Co2O4/GCE in different concentrations of dopamine 10–70 µM, in 0.1 M PB (pH 7.0)
Figure 6b shows the CV recorded in the presence of dopamine (30 μM); curve (a) was obtained through bare GCE in the presence of dopamine (30 μM), curve (b) with Co2O4/GC electrode, and curve (c) with Zn-Co2O4 /GC electrode with the same concentration of dopamine. The Co2O4/GC electrode showed an undefined dopamine oxidation peak at a higher potential (0.25 V vs Ag/AgCl). On the other hand, the Zn-Co2O4/GC electrode exhibits a prominent and well-defined oxidation peak of dopamine at a lower potential (0.17 V vs Ag/AgCl) as compared to the Co2O4/GCE. Furthermore, Zn-Co2O4/GC electrode gave a higher anodic peak current as compared to the bare GCE and Co2O4/GC electrode in the same concentration of dopamine. The conductivity of Zn-Co2O4 nanostructure increased because of the synergistic effect, and the anodic peak current value of the Zn-Co2O4/GC electrode is twofold higher than that of the bare GCE and onefold higher than the Co2O4/GC electrode.
To further check the response of the fabricated sensing probe, different dopamine concentrations (10–70 μM) were added to the electrochemical cell. The oxidation peak current increases linearly as the dopamine concentration rise, as shown in Fig. 6c. It is confirmed that the proposed sensor Zn-Co2O4/GC electrode possesses an optimistic electrocatalytic activity towards dopamine detection in the same oxidation potential.
Amperometric sensing of dopamine by proposed sensor
The amperometric detection technique is the best choice for the electrochemical determination of low concentrations of analytes. The chronoamperometric techniques were used to check the electrochemical response of the fabricated electrode by applying a fixed potential. The amperometric response of Zn-Co2O4/GC electrode to successive addition of dopamine in 0.1 M PB at a fixed interval of 50 s is depicted in Fig. 7a. At a fixed applied voltage of + 0.170 V vs Ag/AgCl, the modified electrode exhibits a fast response by increasing the current after each addition of dopamine. This indicates that the sensing probe detects dopamine effectively at the applied voltage. The calibration plot obtained from the amperogram acquired at various dopamine concentrations is shown in Fig. 7b. The sensor responded linearly to dopamine in the concentration range of 0.2 to 185 µM, with a linear correlation coefficient (R2) as shown in the following equation:
The limit of detection (3 standard deviations of the blank/slope) was estimated for dopamine [38]. For DA, the LOD value was found to be 0.002 μM, using Zn-Co2O4/GC electrode. The sensitivity of the sensor was obtained (126 µA. μM−1 cm−2) from the slope of the calibration plot. The amperometric response time of the Zn-Co2O4/GC electrode after the addition of 50 µM dopamine is less than 1 s, which confirms that the proposed sensor is very fast for dopamine sensing. Table 1 compares the proposed sensor’s output to that of a previously recorded dopamine sensor in the literature. [20, 23, 39,40,41,42,43,44,45].
Selectivity, reproducibility, and stability of Zn-Co2O4 sensing probe
The interference study was conducted to confirm the selectivity of various interfering species that co-exist with dopamine in the blood, such as uric acid, ascorbic acid, adrenaline, epinephrine, norepinephrine, histamine, serotonin, tyramine, phenethylamine, and glucose. Human blood contains 30 times more ascorbic acid and uric acid than dopamine. Figure S2 depicts the recorded amperometric response of consecutive addition of dopamine (10 µM) and uric acid, adrenaline, epinephrine, norepinephrine, histamine, serotonin, tyramine, phenethylamine, glucose (20 µM), and ascorbic acid (300 µM) solution after every 50 s. It is evident from Fig. S2 that each injection of DA shows a prominent increase in the current, while the interfering species did not show any change in current after their addition. Furthermore, they doubled the concentrations of analytes, but they did not show any change in current. The amperogram shows that the interfering species did not respond to the proposed sensor, also it does not affect the result of dopamine oxidation even in much higher concentrations. These findings demonstrate that the sensing probe has high selectivity and sensitivity for quantifying dopamine, even in the presence of other electroactive organisms. Amperometry was used to assess the stability and repeatability of the proposed sensor Zn-Co2O4/GC electrode. The stability of the sensor was checked with 5 consecutive measurements in the presence of 60 µM dopamine and gave the 1.95% RSD of limiting current, indicating that the proposed sensor is extremely stable. Besides, the sensor was used to measure the solution after 1 month under the same experimental conditions, and the current response did not change (1.92% RSD). Additionally, to check the repeatability of the fabrication of the sensor, five different Zn-Co2O4/GC electrodes were prepared and assessed their current in 0.1 M PB in the presence of dopamine (70 µM). It provided a current RSD value of 2%, indicating that the sensor fabrication process was highly repeatable. It proved that the sensor fabrication process is extremely repeatable.
Electrochemical impedance spectroscopy (EIS) study
The electron transferability between the electrolyte and the electrode surface was investigated using electrochemical impedance spectroscopy. The electrochemical impedance spectra of bare GCE and fabricated Zn-Co2O4/GC electrode were obtained in 0.1 M KCl and 5 mM potassium ferro-ferricyanide, in PB as supporting electrolyte. At high frequencies, EIS has a semicircular component, and at low frequencies, it has an inclined line. The diffusion limit step is related to the inclined line, also known as the Warburg (W) section, and the diameter of the semicircular portion is directly related to the electrode surface where electron transfer resistance (Rct) occurs. The Nyquist plots of the bare GCE and Zn-Co2O4/GC electrode, respectively, were performed in the frequency range of 0.1 to 100 kHz, as shown in Fig. 8a and b. This has a low Rct value, indicating that the electrode surface is transferring the maximum electrons. The proposed equivalent circuit from the electrochemical impedance spectrum is shown in the inset Fig. 8a. The CV measurements were also carried out in a potassium ferro-ferricyanide solution. Figure 8c shows the bare GCE and modified Zn-Co2O4 electrode response in 5 mM ferro-ferricyanide solution. Because of the electron-rich nanostructure material and faster electron transfer kinetics, the modified electrode Zn-Co2O4/GC electrode displays a higher redox current value than the bare GCE.
Real samples analysis
To check the practical applicability of the proposed sensing probe Zn-Co2O4/GC electrode for dopamine determination in physiological solution, DA concentration was measured by adopting an already established protocol as reported earlier [39]. For practical analysis, a known concentration of dopamine 20 µM was prepared in a physiological solution. The amperometric technique was applied for dopamine analysis in a real sample. The applied potential of + 0.170 V vs Ag/AgCl was applied for the amperometric determination of dopamine. Figure 9 represents the response of dopamine in the physiological solution, with the addition of 2 µM, and afterwards after every 50 s, and then 10 µM physiological solution was added into 0.1 M PB. The amperometric curve was obtained in the linear range from 2 to 120 µM. The fabricated electrode had a good response, indicating that the DA was electrochemically oxidized by the Zn-Co2O4/GC electrode sensing probe in physiological solution. The developed sensing probe exhibited an excellent response towards dopamine sensing. Because of its ease of synthesis, scalability, and reproducibility, this non-enzymatic electrochemical sensor may be useful in practical applications. This research shows that the fabricated sensor has high selectivity and sensitivity for detecting DA.
Conclusion
In this work, a hexagonal zinc-doped cobalt oxide (Zn-Co2O4) nanostructure was used to modify the glassy carbon electrode and was investigated as a non-enzymatic electrochemical sensor for dopamine determination. The Zn-Co2O4 nanostructure was synthesized using a simple hydrothermal process. Because of the synergistic effect of electron-rich zinc with Co2O4, which in turn increases the active sites of the materials, the doped nanostructure material exhibited high conductivity and fast transfer kinetics. The Zn-Co2O4/GCE sensing probe shows a good response to the oxidation of dopamine in the wide linear range. The sensor has good repeatability and a fast response time of about 1 s, with stability of up to 6 months. The sensor is extremely accurate and selective for dopamine. There is no evidence of interference from other electroactive species in the blood. Therefore, the probe was efficiently employed for the determination of DA in a physiological solution as a real sample.
References
Chauhan N et al (2020) Recent advancement in nanosensors for neurotransmitters detection: present and future perspective. Process Biochem 91:241–259
Mani V et al (2016) Determination of dopamine using a glassy carbon electrode modified with a graphene and carbon nanotube hybrid decorated with molybdenum disulfide flowers. Microchim Acta 183(7):2267–2275
Govindasamy M et al (2018) Determination of neurotransmitter in biological and drug samples using gold nanorods decorated f-MWCNTs modified electrode. J Electrochem Soc 165(9):B370
Devasenathipathy R et al (2014) Electrodeposition of gold nanoparticles on a pectin scaffold and its electrocatalytic application in the selective determination of dopamine. RSC Adv 4(99):55900–55907
Carrera V et al (2007) A simple and rapid HPLC–MS method for the simultaneous determination of epinephrine, norepinephrine, dopamine and 5-hydroxytryptamine: application to the secretion of bovine chromaffin cell cultures. J Chromatogr B 847(2):88–94
Wu H-P, Cheng T-L, Tseng W-L (2007) Phosphate-modified TiO2 nanoparticles for selective detection of dopamine, levodopa, adrenaline, and catechol based on fluorescence quenching. Langmuir 23(14):7880–7885
Mamiński M et al (2005) Spectrophotometric determination of dopamine in microliter scale using microfluidic system based on polymeric technology. Anal Chim Acta 540(1):153–157
Lapainis T et al (2007) A multichannel native fluorescence detection system for capillary electrophoretic analysis of neurotransmitters in single neurons. Anal Bioanal Chem 387(1):97–105
Abdolmohammad-Zadeh H, Zamani-Kalajahi M (2020) A novel chemiluminescent-based nano-probe for ultra-trace quantification of dopamine in human plasma samples. Microchem J 155:104704
Govindasamy M et al (2016) Simultaneous determination of dopamine and uric acid in the presence of high ascorbic acid concentration using cetyltrimethylammonium bromide–polyaniline/activated charcoal composite. RSC Adv 6(102):100605–100613
Kim Y-R et al (2010) Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens Bioelectron 25(10):2366–2369
Keerthi M et al (2019) A core-shell molybdenum nanoparticles entrapped f-MWCNT s hybrid nanostructured material based non-enzymatic biosensor for electrochemical detection of dopamine neurotransmitter in biological samples. Sci Rep 9(1):1–12
Kogularasu S et al (2017) 3D graphene oxide-cobalt oxide polyhedrons for highly sensitive non-enzymatic electrochemical determination of hydrogen peroxide. Sens Actuators, B Chem 253:773–783
Kim D-S et al (2018) Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci Rep 8(1):1–10
Abdelwahab AA, Shim Y-B (2015) Simultaneous determination of ascorbic acid, dopamine, uric acid and folic acid based on activated graphene/MWCNT nanocomposite loaded Au nanoclusters. Sens Actuators, B Chem 221:659–665
Xie Y-L et al (2015) Facile ultrasonic synthesis of graphene/SnO2 nanocomposite and its application to the simultaneous electrochemical determination of dopamine, ascorbic acid, and uric acid. J Electroanal Chem 749:26–30
Aparna T, Sivasubramanian R, Dar MA (2018) One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J Alloy Compd 741:1130–1141
Chen D et al (2018) Electrochemical determination of dopamine using a glassy carbon electrode modified with a nanocomposite consisting of nanoporous platinum-yttrium and graphene. Microchim Acta 185(2):1–7
Oztekin Y et al (2012) Copper nanoparticle modified carbon electrode for determination of dopamine. Electrochim Acta 76:201–207
Hsieh Y-S, Hong B-D, Lee C-L (2016) Non-enzymatic sensing of dopamine using a glassy carbon electrode modified with a nanocomposite consisting of palladium nanocubes supported on reduced graphene oxide in a nafion matrix. Microchim Acta 183(2):905–910
He Q et al (2019) A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim Acta 296:683–692
Rajaji U et al (2020) A nanocomposite consisting of cuprous oxide supported on graphitic carbon nitride nanosheets for non-enzymatic electrochemical sensing of 8-hydroxy-2′-deoxyguanosine. Microchim Acta 187(8):1–10
Numan A et al (2017) Binary nanocomposite based on Co 3 O 4 nanocubes and multiwalled carbon nanotubes as an ultrasensitive platform for amperometric determination of dopamine. Microchim Acta 184(8):2739–2748
Chen J, Wu X, Selloni A (2011) Electronic structure and bonding properties of cobalt oxide in the spinel structure. Phys Rev B 83(24):245204
Mani V et al (2020) Growth of large-scale MoS 2 nanosheets on double layered ZnCo 2 O 4 for real-time in situ H 2 S monitoring in live cells. J Mater Chem B 8(33):7453–7465
An C et al (2019) Metal oxide-based supercapacitors: progress and prospectives. Nanoscale Adv 1(12):4644–4658
Nguyen T, Montemor MdF (2019) Metal oxide and hydroxide–based aqueous supercapacitors: from charge storage mechanisms and functional electrode engineering to need-tailored devices. Adv Sci 6(9):1801797
Suneetha RB, Selvi P, Vedhi C (2019) Synthesis, structural and electrochemical characterization of Zn doped iron oxide/grapheneoxide/chitosan nanocomposite for supercapacitor application. Vacuum 164:396–404
Wang B et al (2017) Semiconductor-ionic membrane of LaSrCoFe-oxide-doped ceria solid oxide fuel cells. Electrochim Acta 248:496–504
George JM, Antony A, Mathew B (2018) Metal oxide nanoparticles in electrochemical sensing and biosensing: a review. Microchim Acta 185(7):1–26
Baye AF et al (2020) Graphene oxide interlayered Ga-doped FeSe2 nanorod: a robust nanocomposite with ideal electronic structure for electrochemical dopamine detection. Electrochim Acta 363:137245
Manjula N et al (2021) Facile synthesis of hexagonal-shaped zinc doped cobalt oxide: application for electroanalytical determination of antibacterial drug ofloxacin in urine samples. J Electroanalyt Chem 885:115101
Sakthinathan S et al (2016) Reduced graphene oxide non-covalent functionalized with zinc tetra phenyl porphyrin nanocomposite for electrochemical detection of dopamine in human serum and rat brain samples. Electroanalysis 28(9):2126–2135
Mani V et al (2019) ZnCo2O4 nanoflowers grown on Co3O4 nanowire-decorated Cu foams for in situ profiling of H2O2 in live cells and biological media. ACS Appl Nano Mater 2(8):5049–5060
Long H et al (2016) Nanowire-assembled hierarchical ZnCo2O4 microstructure integrated with a low-power microheater for highly sensitive formaldehyde detection. ACS Appl Mater Interfaces 8(46):31764–31771
Liu X et al (2018) Nanoporous Zn-doped Co3O4 sheets with single-unit-cell-wide lateral surfaces for efficient oxygen evolution and water splitting. Nano Energy 44:371–377
Jincy C, Meena P (2020) Synthesis, characterization, and NH3 gas sensing application of Zn doped cobalt oxide nanoparticles. Inorg Chem Commun 120:108145
Jayabal S, Ramaraj R (2013) Synthesis of core/shell Au/Ag nanorods embedded in functionalized silicate sol–gel matrix and their applications in electrochemical sensors. Electrochim Acta 88:51–58
Younus AR et al (2019) Nonenzymatic amperometric dopamine sensor based on a carbon ceramic electrode of type SiO 2/C modified with Co 3 O 4 nanoparticles. Microchim Acta 186(7):1–10
Zhuang X et al (2018) Reduced graphene oxide functionalized with a CoS 2/ionic liquid composite and decorated with gold nanoparticles for voltammetric sensing of dopamine. Microchim Acta 185(3):1–9
Xing L, Ma Z (2016) A glassy carbon electrode modified with a nanocomposite consisting of MoS 2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 183(1):257–263
Mei L-P et al (2016) A glassy carbon electrode modified with porous Cu 2 O nanospheres on reduced graphene oxide support for simultaneous sensing of uric acid and dopamine with high selectivity over ascorbic acid. Microchim Acta 183(6):2039–2046
Numan A et al (2017) Facile fabrication of cobalt oxide nanograin-decorated reduced graphene oxide composite as ultrasensitive platform for dopamine detection. Sens Actuators, B Chem 238:1043–1051
Ejaz A, Joo Y, Jeon S (2017) Fabrication of 1, 4-bis (aminomethyl) benzene and cobalt hydroxide@ graphene oxide for selective detection of dopamine in the presence of ascorbic acid and serotonin. Sens Actuators, B Chem 240:297–307
Umapathi S et al (2020) Electrochemical sensor based on CuSe for determination of dopamine. Microchim Acta 187(8):1–13
Firooz AA et al (2021) High electrochemical detection of dopamine based on Cu doped single phase hexagonally ZnO plates. Mater Today Commun 26:101716
Durai L, Gopalakrishnan A, Badhulika S (2021) One-pot hydrothermal synthesis of NiCoZn a ternary mixed metal oxide nanorod based electrochemical sensor for trace level recognition of dopamine in biofluids. Mater Lett 298:130044
Acknowledgements
The authors are thankful to the Higher Education Commission (HEC), Pakistan, for the NRPU Project No. 5358 as financial assistance and King Fahd University of Petroleum and Minerals, Kingdom of Saudi Arabia, under Project No. DF191016.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, M.I., Muhammad, N., Tariq, M. et al. Non-enzymatic electrochemical dopamine sensing probe based on hexagonal shape zinc-doped cobalt oxide (Zn-Co2O4) nanostructure. Microchim Acta 189, 37 (2022). https://doi.org/10.1007/s00604-021-05142-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05142-z