Abstract
Two new palladium catalysts immobilized on modified magnetic nanoparticles containing NNN and NNS Schiff base ligands were synthesized and characterized by FT-IR spectroscopy, thermogravimetric analysis, X-ray diffraction, X-ray photoelectron spectroscopy, transmission electron microscopy, CHNS and ICP. These catalytic systems, containing Pd nanoparticles, showed high activity in the Suzuki–Miyaura cross-coupling of phenylboronic acid with aryl halides. The activity, Pd loading, reusability and Pd leaching of these catalysts were compared. The supported catalysts have the advantage to be completely recoverable with the simple application of an external magnetic field. The immobilized palladium catalysts could be reused several times without significant loss of their catalytic activities.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The heterogenization of homogeneous catalysts and their use in the synthesis of fine chemical has become a major area of research. This is due to the potential advantages of these materials such as simplified recovery and reusability, the potential for incorporation in continuous reactors and microreactors over homogeneous systems [1, 2]. Recently, interest in catalysis by metal nanoparticles (NPs) is increasing dramatically [3,4,5,6]. Semi-heterogeneous catalysis is between homogeneous and heterogeneous catalysis, and progress has been made in the efficiency and selectivity of reactions and recovery and reusability of the catalyst [7].
Biaryl units as molecular components in biologically active molecules and functional polymers have attracted enormous interest [8, 9]. The Suzuki cross-coupling reaction is an increasingly popular approach for the construction of unsymmetrical biaryl compounds since the arylhalides used are environmentally safer than other organometallic reagents [10,11,12,13]. Toxic and expensive homogeneous palladium phosphine complexes, which are rarely recoverable and produce impure products [14], are used as catalysts for the Suzuki reaction [15,16,17].
Nanoparticles have attracted significant interest as efficient supports for homogeneous catalyst immobilization because of their high surface area [18,19,20,21,22,23,24]. The use of nanoparticles as a support can lead to significant enhancement of catalyst activity, because nanoparticle materials have very small size and are easily dispersed in solution [25, 26]. When the size of the support is decreased to the nanometer scale, the activity of the supported catalyst can be dramatically improved compared to homogeneous catalysts immobilized on conventional supports under conditions where internal diffusion is rate limiting [27, 28]. However, the facile separation and recycling of the nanoparticle materials from the reaction media remain a challenge [7, 27]. The problem can be solved by employing magnetic supports. Catalysts supported magnetic nanoparticles (MNPs) can be readily isolated from the product solution merely by applying an external magnetic field [4, 29]. The magnetic nanoparticles offer advantages over conventional catalyst supports and should be of interest to the chemical industry. Magnetic separation has emerged as a robust, highly efficient and fast separation tool with many advantages compared with product/catalyst isolation by means of other chemical or physical procedures, such as liquid–liquid extraction, chromatography, filtration or centrifugation [30].
Modifying of magnetic nanoparticles surface with amines allow us to make a Schiff base and improve support properties. Feng and coworkers modified the magnetic particle surfaces by (3-aminopropyl) triethoxysilane and PEG diacid coat. There results showed that these nanoparticles appear to be a promising vehicle for MR imaging [31]. Our previous work on new palladium catalyst immobilized on modified magnetic nanoparticles containing NNO donor atoms showed desirable activity in the Suzuki–Miyaura cross-coupling reaction of phenylboronic acid with aryl halides [32]. Bui and coworkers prepared a palladium complex catalyst immobilized on CoFe2O4 nanoparticles with NNN Schiff base ligand and investigated its activity in Suzuki reaction [22]. Firuzabadi et al. [33] modified the Fe3O4@SiO2 nanoparticles with (3-chloropropyl)-trimethoxysilane in order to synthesis chloro-functionalized magnetic nanoparticles (CPS-MNPs) that yields the production of CPS-MNPs-NNN ligand. Good yields of products were obtained in the Heck and Sonogashira coupling reactions.
In this paper, we synthesized new palladium catalysts immobilized on modified magnetic nanoparticles containing NNN and NNS donor Schiff base ligands. So heterogeneous catalysts with high Pd loading were produced and then characterized. Here, we gathered the advantages of heterogeneous catalysis, thermally stable, oxygen insensitive, air and moisture stable, magnetic separation and enhanced catalytic activity of palladium without added phosphine ligands to synthesize a new catalyst for Suzuki cross-coupling reaction. The magnetic catalyst could be facilely isolated from the reaction mixture by simple magnetic decantation, and could be reused without significant degradation in activity several times.
Experimental
Materials and methods
The preparation of catalysts was carried out under an inert argon or nitrogen atmosphere. All chemicals were purchased from Merck Chemical Company.
Gas chromatography experiments (GC) were performed on an Agilent GC 6890 equipped with a 19096C006 80/100 WHP packed column and a flame ionization detector (FID). FT Infrared (FT-IR) spectra were obtained as potassium bromide pellets in the range of 400–4000 cm−1 with a Jasco 6300D spectrophotometer. X-ray diffraction (XRD) analyses were carried out on a D8 Advanced Bruker instrument using Cu Kα radiation (λ = 1.5406 Å). The transmission electron microscopy (TEM) was carried out on a Philips CM10 transmission electron microscope operating at 200 kV. The ICP analyses were carried out by a PerkinElmer optima 7300 DV spectrometer. Elemental analysis was performed on a LECO, CHNS-932 analyzer. Thermogravimetric analysis (TGA) were carried out on a Mettler TG50 instrument under air flow at a uniform heating rate of 5 °C min−1 in the range 25–800 °C. The X-ray photoelectron spectroscopy (XPS) measurements were performed using a Gammadata-scienta ESCA200 hemispherical analyzer equipped with an Al (K α = 1486.6 eV) X-ray source.
Synthesis of the magnetic core (Fe3O4@SiO2 nanoparticles)
A co-precipitation method was utilized for the synthesis of the catalyst support comprised of silica-coated magnetic NPs. These nanoparticles were prepared by chemical co-precipitation of Fe3+ and Fe2+ ions with a molar ratio of 2:1. Typically, an appropriate amount of FeCl3∙6H2O (5.8 g) and FeCl2∙4H2O (2.15 g) were dissolved in 38 mL degassed HCl 0.4 molar. Then, this solution was added to 300 mL of degassed ammonia 0.7 molar solutions quickly and carefully in one portion while stirring in ultrasound bath. The addition of the base to the Fe2+/Fe3+ salt solution resulted in the formation of the black precipitate of MNPs immediately that is dispersed in solvent. After stirring in ultrasound bath for another 30 min, black solid collected by external magnet and washed with ethanol and distilled water three times. The as obtained sediment dispersed in 150 ml distilled water.
To modifying surface of MNPs, amine, thiol and chloride terminated silane compounds are available commercially and can be modified depending on the conjugation tactics of the catalytic species. Since Fe3O4 nanoparticles are delicate to heat and oxidation milieus, to protect their surface and to preventing oxidation to Fe2O3, the surface modification of magnetic nanoparticles was also performed by coupling silane reagents to the surface hydroxyl group, Fe–OH, of magnetic nanoparticles to produce a thin layer of SiO2 around particles.
This SiO2 shell can effectively inhibit chemical degradation and on the other hand easy dissolution property of Fe3O4 particles in nanoscale making the photocatalyst recyclable after multiple reaction cycles. Following for synthesis of silica-coated Fe3O4 nanoparticles, 50 ml of previous suspension added to a solution of 250 ml 2-propanol that contained ammonium hydroxide (4.5 ml) and mixture was stirred in ultrasonic vibration. Then, 6 ml tetraethoxysilane (TEOS) was added. The resulting silica-coated Fe3O4 nanoparticles were washed with copiously amount of deionized water and collected by magnetic separation. The obtained sediment was dried at 45 °C under vacuum for 12 h.
Preparation of Fe3O4/SiO2-NH2 nanoparticles
The silica-coated superparamagnetic nanoparticles (Fe3O4/SiO2 nanoparticles) were functionalized with N-(2-aminoethyl)-3-aminopropyltriethoxysilane (AEAPS) as following: Fe3O4/SiO2 nanoparticles (2 g) were dispersed in a solution of dry toluene (100 ml). The suspension was sonicated for 30 min at room temperature. Then, AEAPS (2 ml) was added and the mixture was heated at 70 °C under vigorous stirring for 24 h. The final product was separated by a magnet and washed with toluene (2 × 20 ml) and acetone (2 × 20 ml) by magnetic decantation, and dried at 45 °C under vacuum overnight.
Synthesis of Schiff base immobilized on Fe3O4/SiO2-NH2
The Fe3O4/SiO2-NH2 nanoparticles (3 g) were added to round-bottom flask containing absolute ethanol (80 ml). The mixture was sonicated for 10 min and then 2-pyridinecarbaldehyde or 2-thiophenecarbaldehyde (10 mmol) was added to the reaction mixture. The resulting mixtures were sonicated for 15 min and then stirred for 48 h. For the flasks containing 2-pyridinecarbaldehyde and 2-thiophenecarbaldehyde, the temperature reduced to 2–5 °C by ice bath, because these materials are sensitive to high temperature. After that, the reaction temperature was promoted to 60 °C slowly and stirring was continued for another 24 h. At the end of the reaction, the reaction mixture was cooled to room temperature and the solid was separated by magnetic decantation. The magnetic precipitations were washed with copious amounts of ethanol and acetone and dried under vacuum at 45 °C to yield the immobilized Schiff bases.
Synthesis of Palladium Immobilized on Functionalized MNPs
Each of the immobilized Schiff bases (0.5 g) was added to the round-bottom flask containing 80 ml absolute methanol separately and sonicated for 30 min. The flacks putted in ice bath to reduce their temperature to 2–5 °C. Then solution of palladium acetate (0.010 g) in acetone (10 ml) was added dropwise to each flask. The mixture was then stirred vigorously at 2–5 °C for 8 h under nitrogen atmosphere. Then temperature raised up to 65 °C and stirring continued for another 20 h. The solid was separated by magnetic decantation and then the magnetic catalyst was washed with copious amounts of ethanol and acetone, respectively, and dried under vacuum at 45 °C overnight to yield the immobilized palladium catalysts.
Results and discussion
Catalyst characterization
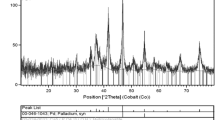
The preparation routes for modified magnetic nanoparticles are shown in Scheme 1. The Pd supported Fe3O4 magnetic nanoparticles, Fe3O4/SiO2-NH2/PC-Pd and Fe3O4/SiO2-NH2/TC-Pd, were characterized by X-ray powder diffraction (XRD). Seven diffraction peaks were observed in 2θ = 21.41, 35.25, 41.7, 50.78, 63.18, 67.64 and 74.62 corresponds to crystalline magnetic nanostructures (Fig. 1). This pattern is corresponding to crystalline Fe3O4 magnetic nanostructures (JCPDS card no. 75-0449). Since the loading of Pd is low, no diffraction peak related to Pd nanoparticles was observed; therefore, the XRD pattern of the immobilized palladium catalyst is similar to that of magnetic nanoparticles.
Moreover, the sharp diffraction peaks show that the obtained nanoparticles have high crystallinity. The particle size of product was calculated from the major diffraction peaks using the Scherrer equation [34]:
In this equation, D XRD is the average size of the particles, assuming particles are spherical, k = 0.9, λ is the wavelength of radiation (1.54 Å, for Cu Kα radiation), β is the full width at half maximum (FWHM) of the diffracted peak and θ is the angle of diffraction. The crystallite sizes of obtained powder after were calculated about 19 nm which is in close agreement with the sizes obtained from TEM studies.
Figure 2 shows the FT-IR spectra for Fe3O4/SiO2, Fe3O4/SiO2-NH2, Fe3O4/SiO2-NH2/PC-Pd and Fe3O4/SiO2-NH2/TC-Pd nanoparticles. The significant features in the FT-IR spectrum of the Fe3O4/SiO2 nanoparticles (Fig. 2a) were the appearance of bands at 1035 and 423 cm−1 for Si–O stretching, and the Fe–O stretching vibration was observed at 590 cm−1. After modification of Fe3O4/SiO2 with AEAPS, the bands at 2900 cm−1 due to the –CH2 and aromatic C–H stretching vibrations, and the bands at 1584 and 3288 cm−1 attributed to bending vibration and stretching vibration of N–H bonds, respectively, were observed (Fig. 2b). When the surface was modified with 2-pyridinecarbaldehyde or 2-thiophenecarbaldehyde, the peaks of the imine (C=N) stretching vibration appeared at 1637 and 1622 cm−1, respectively, which showed the successful formation of Schiff bases (Fig. 2c, d). In the FT-IR spectra of the immobilized palladium catalysts, the imine characteristic peaks appeared with a slight shift. This is probably due to the fact that the chemical environment of the imine has been changed by the palladium.
The nitrogen and sulfur content of Fe3O4/SiO2-NH2, Fe3O4/SiO2-NH2/PC and Fe3O4/SiO2-NH2/TC were determined by CHNS analysis. The results are shown in Table 1. Increasing the amount of nitrogen in the case of Fe3O4/SiO2-NH2/PC and the presence of sulfur in the case of Fe3O4/SiO2-NH2/TC are good indication for attachment of aldehydes to Fe3O4/SiO2-NH2.
X-ray photo electron spectroscopy (XPS) was performed to determine the oxidation state of Pd in the Fe3O4/SiO2-NH2/PC. Extended XPS spectrum showed the carbon, palladium, nitrogen, oxygen and iron elements in the matrix of Fe3O4/SiO2-NH2/PC (Fig. 3a). High-resolution XPS spectrum for palladium showed that the oxidation number of Pd is zero and Pd nanoparticles have been anchored on the surface (Fig. 3b). XPS image shows the presence of weakly related doublet peaks to Pd(II) 3d in 337 and 342 eV relating to residual palladium salt precursor in the catalyst. But the main peaks are related to the zero oxidation state of palladium species. Previous investigations demonstrated that methanol will reduce the palladium at 27 °C [35]. Therefore, the proposed catalyst structure may be like the structure shown in Fig. 4. Since the synthesize process was similar, we think that the oxidation state of Pd in catalyst having NNS ligand is zero too.
The TEM images of the as-prepared catalysts are presented in Fig. 5. These micrographs show that most of the particles are quasi-spherical and a thin layer of silica has been coated on them. The size of these nanoparticles, estimated from the images, was in the range of 8–14 nm, which is in close agreement with the sizes obtained from XRD studies. TEM images of catalysts also show the core–shell structure of the particles. Interestingly, the magnetic core is visible as a dark spot inside the bright spherical SiO2 thin shell in the TEM images. Since Pd nanoparticles have low concentration, the identification of palladium nanoparticles in TEM images is difficult.
The amount of organic moieties of Fe3O4/SiO2-NH2/PC, Fe3O4/SiO2-NH2/TC and Schiff bases was also measured by TGA analysis under nitrogen atmosphere at the heating rate of 5 °C/min−1 (Fig. 6). The weight loss of the Schiff bases between 30 and 700 °C as a function of temperature was determined using TGA, which is an irreversible process because of thermal decomposition. The TGA plots depict a two-step thermal decomposition. The first step of weight loss (below 230 °C) in the case corresponds to the removal of physically adsorbed water, whereas, in the other cases, the main weight loss in the second step (above 260 °C) is due to the removal of organic moieties on the surface. The TGA results are summarized in Table 2. As shown in Fig. 6, when palladium loaded on the Schiff base supports, the residual weigh had more amounts in comparison with previous step.
The palladium content for each catalyst was determined by inductively coupled plasma–optical emission spectrometry (ICP-OES). In comparison with Fe3O4/SiO2-NH2/TC-Pd sample, results showed more palladium values for Fe3O4/SiO2-NH2/PC-Pd catalyst (0.31 and 0.35 mmol Pd g−1, respectively).
Suzuki–Miyaura cross-coupling of aryl halides with phenylboronic acid catalyzed by Fe3O4/SiO2-NH2/PC-Pd and Fe3O4/SiO2-NH2/TC-Pd
To optimization the reaction parameters such as the base, solvent type, temperature and catalyst amount, the coupling of iodobenzene with phenylboronic acid in the presence of Fe3O4/SiO2-NH2/PC-Pd and Fe3O4/SiO2-NH2/TC-Pd was chosen as model reaction. Initially the model reaction was performed in the presence of different single and mixed solvents (Table 3, entries 1–7). Among the solvents examined, MeOH/H2O (2:1) was the best reaction media for both catalysts.
Then, the same reaction was carried out in different organic and inorganic bases such as K2CO3, Na3PO4, Na2CO3, Et3N, KOH and K3PO4 (Table 3, entries 8–14). Among them, K2CO3 was the best base for Fe3O4/SiO2-NH2/PC-Pd and Fe3O4/SiO2-NH2/TC-Pd, while in the absence of catalyst no appreciable yield was detected.
Investigation of the effect of reaction temperature showed that temperature of 65 °C is best for both catalysts. Increasing the reaction temperature to 70 °C did not affect the yield, but by lowering the reaction temperature the yield was decreased (Table 3, entries 15–18). The effect of palladium content on the model reaction was also investigated; the best results for both catalysts were obtained using 1.5 mol% Pd (Table 3, entries 19–23). Therefore, it was concluded that the optimum reaction conditions involved aryl halide (1 mmol), phenylboronic acid (1.2 mmol), K2CO3 (2 mmol) catalyst (1.5 mol% Pd) in MeOH/H2O (2:1) at 65 °C.
Afterward, under the optimized conditions, the immobilized palladium catalysts were assessed for their activity in the Suzuki cross-coupling reaction. In the presence of both catalysts, a diversity of aryl halides containing electron-donating and electron-withdrawing substituents efficiently reacted with phenylboronic acid to afford the desired cross-coupling products. The catalyst separation at the end of the reaction was performed exclusively by using a permanent magnet to recover the catalysts. The results are listed in Table 4. As expected, it was observed that the presence of an electron-withdrawing group on the benzene ring of the aryl halide accelerated the Suzuki reaction rate, while the presence of an electron-donating group decreased the reaction rate.
Catalyst recycling
The magnetic properties of catalysts allow fast separation of the catalysts from reaction media and simplify the catalyst separation and reusing them in successive reactions which is an important issue from economical points of view. This operation can be done by applying an external magnet. The catalyst is collected inside the reactor wall, which minimizes the use of auxiliary substances, the use of solvents, catalyst loss, catalyst oxidation and the time consumption in laborious filtration procedures.
The catalysts recycling was carried out in the reaction of bromobenzene (1 mmol), phenylboronic acid (1.2 mmol), K2CO3 (2 mmol), Fe3O4/SiO2-NH2/PC-Pd or Fe3O4/SiO2-NH2/TC-Pd (1.5 mol% Pd) in MeOH/H2O (2:1) at 65 °C. After each run, the catalyst was removed easily by an external magnet and washed by ethanol, water and acetone, successively, and reused in the next catalytic cycle with fresh reactants. The results are shown in Fig. 7a, b. The Fe3O4/SiO2-NH2/PC-Pd and Fe3O4/SiO2-NH2/TC-Pd catalysts were reused nine and six consecutive cycles, respectively. The amount of Pd leached was measured by ICP-OES analysis before and after reaction. The results showed that about 2.1 and 2.7% of the initial palladium content of Fe3O4/SiO2-NH2/PC-Pd and Fe3O4/SiO2-NH2/TC-Pd samples have been leached after the final run.
To show the advantages and the merit of the prepared catalysts in comparison with similar reported catalysts, some of the results for Suzuki cross-coupling reaction of iodobenzene and phenylboronic acid are summarized in Table 5. Results indicate that the Fe3O4/SiO2-NH2/PC-Pd catalyst has more advantages with respect to reaction condition, time, Pd loading, leaching and yield than some of the previously reported ones [36,37,38].
Conclusions
Two palladium catalysts immobilized on modified silica-coated superparamagnetic nanoparticles (Fe3O4/SiO2) were synthesized and characterized by FT-IR, XRD, TGA, XPS, TEM and ICP analysis. They were phosphine free ligands and used as catalysts for the Suzuki cross-coupling reaction of several aryl halides with phenylboronic acid. The presence of pendant Schiff base groups on the catalyst support seems to play a special role in the catalytic activity of the Pd nanoparticles, resulting in higher selectivity, conversion and reusability. Amine and sulfur groups are donors and stabilize the palladium active species. The differences in electronegativity of S and N atoms can have difference impact on palladium loading in catalyst and the catalyst activity resulting to clear differences in the TOF of two catalysts. All reactions proceed in mild thermal condition and green solvent. The recovery of the catalysts was easily achieved by simple magnetic decantation using an external magnetic field. The catalysts can be reused several times without significant loss of their catalytic activities.
References
J.H. Clark, D. Macquarrie, Handbook of Green Chemistry and Technology (Wiley-Blackwell, Hoboken, 2002)
P.T. Anastas, M.M. Kirchhoff, T.C. Williamson, Appl. Catal. A 221, 3 (2001)
S. Miao, C. Zhang, Z. Liu, B. Han, Y. Xie, S. Ding, Z. Yang, J. Phys. Chem. C 112, 774 (2008)
C.W. Lim, I.S. Lee, Nano Today 5, 412 (2010)
A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset, V. Polshettiwar, Chem. Soc. Rev. 40, 5181 (2011)
G.R. Bardajee, R. Malakooti, I. Abtin, H. Atashin, Microporous Mesoporous Mater. 169, 67 (2013)
D. Astruc, F. Lu, J.R. Aranzaes, Angew. Chem. Int. Ed. Engl. 44, 7852 (2005)
S. Paul, J.H. Clark, Green Chem. 5, 635 (2003)
K.C. Nicolaou, P.G. Bulger, D. Sarlah, Angew. Chem. Int. Ed. 44, 4442 (2005)
Á. Molnár, Chem. Rev. 111, 2251 (2011)
R. Narayanan, Molecules 15, 2124 (2010)
A. Fihri, P. Meunier, J.-C. Hierso, Coord. Chem. Rev. 251, 2017 (2007)
N. Miyaura, A. Suzuki, Chem. Rev. 95, 2457 (1995)
M. Cai, J. Sha, Q. Xu, J. Mol. Catal. A Chem. 268, 82 (2007)
F. Bellina, A. Carpita, R. Rossi, Synthesis (2004). doi:10.1055/s-2004-831223
N.T.S. Phan, M. Van Der Sluys, C.W. Jones, Adv. Synth. Catal. 348, 609 (2006)
J.-P. Corbet, G. Mignani, Chem. Rev. 106, 2651 (2006)
J. Wu, P. Su, J. Huang, S. Wang, Y. Yang, J. Colloid Interface Sci. 399, 107 (2013)
B. Tamami, H. Allahyari, S. Ghasemi, F. Farjadian, J. Organomet. Chem. 696, 594 (2011)
N.T.S. Phan, H.V. Le, J. Mol. Catal. A Chem. 334, 130 (2011)
P. Das, D. Sharma, A.K. Shil, A. Kumari, Tetrahedron Lett. 52, 1176 (2011)
N.T. Bui, T.B. Dang, H.V. Le, N.T.S. Phan, Chin. J. Catal. 32, 1667 (2011)
P. Wang, Q. Lu, J. Li, Mater. Res. Bull. 45, 129 (2010)
G. Fan, B. Zou, S. Cheng, L. Zheng, J. Ind. Eng. Chem. 16, 220 (2010)
M. Kooti, M. Afshari, Catal. Lett. 142, 319 (2012)
S. Paul, J.H. Clark, J. Mol. Catal. A Chem. 215, 107 (2004)
N.T.S. Phan, C.W. Jones, J. Mol. Catal. A Chem. 253, 123 (2006)
W. Yan, S.M. Mahurin, Z. Pan, S.H. Overbury, S. Dai, J. Am. Chem. Soc. 127, 10480 (2005)
N.T.S. Phan, C.S. Gill, J.V. Nguyen, Z.J. Zhang, C.W. Jones, Angew. Chem. Int. Ed. 45, 2209 (2006)
N.J.S. Costa, P.K. Kiyohara, A.L. Monteiro, Y. Coppel, K. Philippot, L.M. Ross, J. Catal. 276, 382 (2010)
B. Feng, R.Y. Hong, L.S. Wang, L. Guo, H.Z. Li, J. Ding, Y. Zheng, D.G. Wei, Colloids Surf. A 328, 52 (2008)
H. Khojasteh, V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork, J. Nanostruct. 5, 271 (2015)
F.D. Firuzabadi, Z. Asadi, F. Panahi, RSC Adv. 6, 101061 (2016)
H.P. Klug, L.E. Alexander, X-Ray Diffraction Procedures: For Polycrystalline and Amorphous Materials (Wiley, New York, 1974)
V.M. Nosova, Y.A. Ustynyuk, L.G. Bruk, O.N. Temkin, A.V. Kisin, P.A. Storozhenko, Inorg. Chem. 50, 9300 (2011)
Z. Yinghuai, S.C. Peng, A. Emi, S. Zhenshun, R.A. Kemp, Adv. Synth. Catal. 349, 1917 (2007)
P. D. Stevens, G. Li, J. Fan, M. Yen, Y. Gao, Chem. Commun. 4435 (2005). doi:10.1039/B505424A
U. Laska, C.G. Frost, G.J. Price, P.K. Plucinski, J. Catal. 268, 318 (2009)
Acknowledgment
We are thankful to the Research Council of the University of Isfahan for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khojasteh, H., Mirkhani, V., Moghadam, M. et al. Suzuki–Miyaura cross-coupling reaction by palladium immobilized on functionalized magnetic nanoparticles with NNN and NNS Schiff base ligands in a mild reaction condition. J IRAN CHEM SOC 14, 1139–1150 (2017). https://doi.org/10.1007/s13738-017-1064-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1064-6