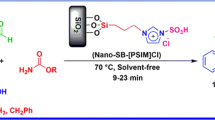
Abstract
A vanadium Schiff base complex on nano silica was prepared by reaction of nano silica NH2-functionalized, 2,4-dihydroxy benzaldehyde and VO(acac)2. The VSBC@NS was used as an efficient and reusable heterogeneous catalyst for the selective oxidation of sulfides to sulfoxides and alcohols to aldehydes with H2O2 as an oxidant in methanol.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organosulfur compounds such as sulfoxides and sulfones are very prominent compounds in organic chemistry. For instance, sulfoxides have found use as final products, reagents, intermediates and catalysts. In particular, sulfoxides are important intermediates in the synthesis of natural and biological materials [1–4].
The first synt hesis of sulfoxides was reported by Märker in 1865, since a large number of methods for the oxidation of sulfides to sulfoxides have been reported [5]. Because sulfides are much more nucleophilic than sulfoxides, usually there is a reasonably good selectivity for the oxidation of sulfides. However, the selective preparation of sulfoxides over sulfones is an important knot in the oxidation of sulfides [6, 7].
Various oxidants are used for the oxidation of sulfides [8–11]. Because of high cost, production of by-products and the low content of effective oxygen, most oxidants are not sufficient for large-scale synthesis. Among all of them aqueous hydrogen peroxide is the most attractive, as it is both environmentally benign and economical.
The discovery of new catalysts and their use in organic reactions is of much current interest in the chemical research of heterogeneous catalysis, primarily due to advantages like easy and cheap recovery of catalyst, simple workup and reduction of waste [12]. In recent years, selective oxidation of sulfides to sulfoxides has been reported with heterogeneous systems [13–17], and competition to find new and efficient processes using a capable and recyclable catalyst continues. Herein, we report a heterogeneous catalytic system of vanadium Schiff base complexed on nano silica. These were prepared by the reaction of nano silica NH2-functionalized, 2,4-dihydroxybenzaldehyde and VO(acac)2, and was used for the selective oxidation of sulfides to sulfoxides with H2O2 as oxidant in methanol in quantitative yields at room temperature (Scheme 1).
Experimental
Characterization
Scanning electron microscope VSBC@NS was performed using Hitachi SEM SU3500. The thermal stability of the VSBC@NS was determined using a thermogravimetric analyzer (TGA/DTA BAHR: STA 503) under air and a heating rate of 10 °C min−1. Elemental analysis of VSBC@NS nanocomposite was performed using an Elementar Analysensysteme GmbH VarioEL CHNS. Gas chromatography was performed on a Trace GC ultra from the Thermo Company equipped with FID detector and Rtx®-1 capillary column. 1H-NMR spectra were recorded on a BRUKERDRX-300AVANCEspectrometer. NMR spectra were obtained. Melting points of products were measured on an Electrothermal 9100 apparatus and are uncorrected. The concentration of vanadium was estimated using an inductively coupled plasma optical emission spectrometer (ICP-OES) Varian Vista PRO Radial. IR spectra were recorded on a Bomem MB-Series FT-IR spectrophotometer.
Materials
Aqueous solutions of hydrogen peroxide (30 %), toluene, dichloromethane, methanol, ethanol, HCl, 2,4-dihydroxybenzaldehyde, (3-aminopropyl) triethoxysilane were purchased from Merck, and used as provided. Silica nanoparticles were purchased from Merck.
Fictionalization of nano silica (NS)
A suspension of (3-aminopropyl) triethoxysilane (2 mmol, 0.443 g) and activated nano silica (2 g) in toluene (15 mL) was heated under reflux with stirring under nitrogen atmosphere. After heating for 24 h, the powder was filtered, washed with diethyl ether and dried under vacuum.
Synthesis of VSBC@NS
The mixture of NH2-functionalized nano silica previously obtained (2 g) and 2,4-dihydroxybenzaldehyde (1 mmol, 0.14 g) in ethanol (20 mL) was heated at reflux for 1 h. Then VO (acac)2 (1 mmol, 0.27 g) was added and stirring was continued at 40 °C for 1 h. After cooling to room temperature, H2O2 (1 mmol, 0.1 mL, 30 % aq) was added and the mixture stirred for an additional 30 min. The reaction mixture was filtered, washed with ethanol and Et2O (3x) and dried under vacuum. The loading of nitrogen was determined to be 0.5 mmol g−1 from elementary analysis (CHN). The ICP analysis gave the actual V contents as 0.44 mmol g−1 for VSBC@NS nanocomposite.
General procedure for the oxidation of sulfide
To a stirred suspension of the selected sulfide (0.5 mmol) and the heterogeneous catalyst VSBC@NS (0.1 mmol of V) in methanol (2 mL), H2O2 (0.06 mL, 0.6 mmol, 30 % aq) was added in one portion. The slurry was stirred at room temperature for 2 h. The catalyst was filtered off and washed with acetone (10 mL). The filtrate was diluted with ethyl acetate (10 mL) and the resulting solution was dried with anhydrous sodium sulfate, filtered and evaporated in vacuum to afford the crude product which was purified by column chromatography on silica gel (10 % EtOAc in hexane) to afford the pure sulfoxide.
General procedure for the oxidation of alcohols
To a stirred suspension of the selected alcohol (0.5 mmol) and the heterogeneous catalyst VSBC@NS (0.1 mmol of V) in methanol (2 mL), H2O2 (0.06 mL, 0.6 mmol, 30 % aq) was added in one portion. The slurry was stirred under reflux condition for 4 h. The catalyst was filtered off and washed with acetone (10 mL). The filtrate was diluted with ethyl acetate (10 mL) and the resulting solution was dried with anhydrous sodium sulfate, filtered and evaporated in vacuum to afford the crude product which was purified by column chromatography on silica gel (5 % EtOAc in hexane) to afford the pure aldehyde.
All compounds are known compounds and were reported previously.
Methyl phenyl sulfoxide
Mp.: 26–29 °C (Lit [18] Mp.: 28–29 °C),. 1H NMR (300 MHz,CDCl3) δ: 7.65 (m, 2H), 7.48 (m, 2H), 7.30 (m, 1H), 2.64 (s, 3H).
1-(Ethylsulfinyl)-4-methylbenzene
Mp.: 140–142 °C (Lit [19] Mp.: 142–143 °C), 1H NMR (300 MHz,CDCl3) δ: 7.49 (d, 2H, j = 8.1), 7.31 (d, 2H, j = 8.1), 2.63–2.93 (m, 2H), 2.40 (s, 3H), 1.15–1.25 (m, 3H).
1-(Benzylsulfinyl)benzene
Mp.: 120–124 °C (Lit [20] Mp.:122–123 °C), 1HNMR (300 MHz,CDCl3) δ: 7.38–7.49 (m, 5H), 7.26–7.35 (m, 4H), 6.99 (d, 2H, j = 6), 4.12 (d, 1H, j = 12.6), 4.02 (d, 1H, j = 12.6).
Results and discussion
Vanadium complexes formed from tri-dentate Schiff base ligands with an ONO donor are widely used as catalyst in the oxidation of sulfides. Because of outbreak synergic effect of silica on some catalytic reaction, To explore whether this was the case, we first attempted an in situ synthesis of a vanadium Schiff base complex with a bi-dentate ligand (NO donor) by the mixing of nano silica NH2-functionalized, aldehyde and VO(acac)2 (Table 1). As shown in Table 1, the presence of a hydroxyl group at the ortho position had an intensely positive effect on the yield of reaction (Table 1, entry 2). Also a 2,4-dihydroxy-substituted aromatic was slightly better than a 2-hydroxy-substituted aromatic group with respect to a beneficial effect on the yield of reaction.

To probe the effect of synthesized catalyst on the sulfoxidation reaction a series of experiments were performed, Table 2. First, in the absence of catalyst only a small amount of sulfoxide was formed (Table 2, entry 1). This did not change dramatically if only SiO2 or vanadium catalyst in the absence of SiO2 were used (Table 2, entries 2 and 3). However, when the in situ catalyst was prepared the yield improved dramatically (Table 2, entry 4). When a pre-synthesized catalyst (entry 5) in the presence of H2O2 (1.2 eq.) was used a modest increase in yield was observed.

To try and probe the structure of our catalyst the FT-IR spectra of nano silica, nano silica NH2-functionalized and VSBC@NS were taken and are shown in Fig. 1. In the FT-IR spectroscopy of nano silica, the characteristic bands at around 3500 cm−1 is attributed to the O–H stretching absorbance of Si–OH while the peak at around 1100 cm−1 is assigned to the Si–O stretching bond. After NH2-functionalization of nano silica, the peak intensity at 3500 cm−1 decreased greatly. The appearance of new peak at around 1630 cm−1 confirmed the formation of the vanadium complex on nano silica in the VSBC@NS FT-IR spectra.
The density and distribution of the complex on the nano silica, quantitative energy dispersive X-ray spectroscopy (EDS) mapping was done. As shown in Fig. 2c–e, analysis of element distribution demonstrates that C, N and V appear to be uniformly distributed on the catalyst surfaces. The weight percent of C, N, V, O, Si from EDS analysis is reported in Table 3.
Thermogravimetric analysis (TGA) was further used to study the thermal behavior and stability of the VSBC@NS. This nanocomposite showed high thermal stability (Fig. 3). The total weight lost for VSBC@NS was only 4 % at temperatures 300 °C.
The effects of the catalyst loading (Table 4, entries 1–3), solvent (Table 4, entries 3–6), temperature (Table 4, entries 6–8), reaction time (Table 4, entries 8–13) and amount of oxidant (Table 4, entries 13–16) were also evaluated. As shown in Table 4, methanol as solvent at room temperature and 1.2 eq H2O2 as oxidant was selected as the optimum reaction condition.

In an effort to explore the scope of the reaction, a series of sulfides were used in the oxidation reaction, and the results are presented in Table 5.

The recyclability and reusability of immobilized catalyst (VSBC@NS) was examined in the oxidation of methyl phenyl sulfide. After the reaction was complete the catalyst was filtered off and washed with acetone, dried and reused for a subsequent reaction (five runs) by adding fresh substrate and oxidant under identical reaction procedure. In all cases, reaction times and yield (99, 99, 91, 85, 76 %) of the methyl phenyl sulfoxide slightly decreased. ICP results of the used VSBC@NS nanocomposite catalyst indicate the leaching of 2.3 % of vanadium in the oxidation of methyl phenyl sulfide after the fifth cycle.
Table 6 compares efficiency of VSBC@NS nanocomposite (reaction conditions, time, and yield) with efficiency of other reported heterogeneous vanadium based catalysts in the oxidation of methyl phenyl sulfide.
Inspired by the high activity and stability of VSBC@NS nanocomposite the oxidation of sulfides was further employed as another model reaction to test further the performance of VSBC@NS nanocomposite. We chose the oxidation of benzyl alcohol as an optimum reaction in MeOH as solvent with a catalyst loading of 20 mol% of V for 4 h under reflux condition (Table 7, entry 3). Under this condition, we found that the oxidation proceeds well, affording the excellent yield (95 %) of the corresponding aldehyde.

To extend the scope of the prepared catalyst, the oxidation of the alcohols to the related carbonyl compounds have been investigated. The results of the mentioned reactions have been presented in the Table 8. The reactions were done with good to high yields. Selectivity for the oxidation of the alcohols to the corresponding carbonyl compounds had been observed, too (Table 8).
Conclusion
In summary a facile, eco-friendly and economical process for the selective oxidation of sulfides to sulfoxides and alcohols to aldehydes by introducing a heterogeneous vanadium Schiff bases complex supported on nano silica as a selective oxidative catalyst was developed. Mild reaction conditions excellent yields and selectivity are other advantages of this protocol. Moreover, the catalyst was chemically stable and could be recycled for at least five times in the corresponding reaction without a significant reduction in the catalytic activity.
References
E.N. Prilezhaeva, Russ. Chem. Rev. 70, 897 (2001)
M.C. Carreño, Chem. Rev. 95, 1717 (1995)
S. Burrage, T. Raynham, G. Williams, J.W. Essez, C. Allen, M. Cardno, V. Swali, M. Bradley, Chem. Eur. J. 6, 1455 (2000)
A. Padwa, M.D. Danca, Org. Lett. 4, 715 (2002)
C. Marcker, J. Liebigs Ann. Chem. 136, 75 (1865)
J.E. Bäckvall, Selective oxidation of amines and sulfides chapter in modern oxidation methods (Weinheim, Wiley, 2010)
N. Hall, M. Orio, A. Jorge-Robin, B. Gennaro, C. Marchi-Delapierre, C. Duboc, Inorg. Chem. 52, 13424 (2013)
P. Kowalski, K. Mitka, K. Ossowska, Z. Kolarska, Tetrahedron 61, 1933 (2005)
K. Kaczorowska, Z. Kolarska, K. Mitka, P. Kowalski, Tetrahedron 61, 8315 (2005)
K.C. Nicolau, R.L. Magolda, W.J. Sipio, W.E. Barnette, Z. Lysenko, M.M. Joullie, J. Am. Chem. Soc. 102, 3784 (1980)
L.A. Paquette, R.V.C. Carr, Org. Synth. 64, 157 (1985)
L. Gröschel, R. Haidar, A. Beyer, K.H. Reichert, R. Schomäcker, Catal. Lett. 95, 67 (2004)
B. Karimi, M. Ghoreishi-Nezhad, J.H. Clark, Org. Lett. 7, 625 (2005)
A. Shaabani, Z. Hezarkhani, E. Badali, RSC Adv. 5, 61759 (2015)
P. Veerakumar, Z.Z. Lu, M. Velayudham, K.L. Lu, S. Rajagopal, J. Mol. Catal. A Chem. 332, 128 (2010)
A. Ghorbani-Choghamarani, G. Azadi, J. Iran. Chem. Soc. 8, 1082 (2011)
A. Ghorbani-Choghamarani, J. Zeinivand, J. Iran. Chem. Soc. 7, 190 (2010)
A. Seshadri, B.J. Jyoti, P.S. Prasad, S. Rani, I. Nashreen, Green. Chem. 15, 2044 (2013)
S. Oae, K. Tsujihara, N. Furukawa, Tetrahedron. Lett. 11, 2663 (1970)
Ch. Banek, V. Nemykin, A. Yoshimura, M. Zhdankin, J. Org. Chem. 76, 3812 (2011)
F. Gregori, I. Nobili, F. Bigi, R. Maggia, G. Predieri, G. Sartori, J. Mol. Catal. A Chem. 286, 124 (2008)
R. Ando, T. Yagyu, M. Maeda, Inorg. Chim. Acta 357, 2237 (2004)
S.L. Jain, B.S. Rana, B. Singh, A.K. Sinha, A. Bhaumik, M. Nandi, B. Sain, Green Chem. 12, 374 (2010)
Acknowledgments
We gratefully acknowledge the financial support from the Research Council of Shahid Beheshti University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dabiri, M., Koohshari, M., Shafipour, F. et al. Supported vanadium Schiff bases complex on nano silica: a heterogeneous catalyst for the selective oxidation of sulfides and alcohols. J IRAN CHEM SOC 13, 1265–1272 (2016). https://doi.org/10.1007/s13738-016-0840-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0840-z