Abstract
Oxovanadium(IV) Schiff base complex have been anchored onto the surface of purely siliceous MCM-41 and tested for its activity as catalyst for the oxidation of sulfides. This catalyst could alter this oxidation reaction extremity, exhibiting excellent yields with 100 % selectivity. The intercalation of the complex inside the silica matrix was supported by various characterization techniques like X-ray diffraction, differential thermogravimetric (TG-DTA), BET measurements, UV–Vis diffuse reflectance spectroscopy and Fourier transform infrared spectroscopy (FT-IR). The stability of the catalyst during the course of the reaction was confirmed from its post catalytic FT-IR and XRD analysis. The catalyst could be reused five times without notable loss of its catalytic activity and efficiency which indicates that the metal-Schiff base moiety is intact and the coordination environments are not altered during the reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the chemical industry and the fundamentals research, catalysis plays an important role. The different catalysts are in constant development to fulfill economic, political and environmental demands. The discovery of vanadate-dependent enzymes in various biological catalytic processes [1, 2] has stimulated research on the catalytic aspects of vanadium complexes. Many model vanadium show catalytic activity towards various organic transformations [3–9]. In particular, vanadium complexes are widely used as homogenous catalyst for oxidation reactions [10, 11]. The catalytic activity of these homogeneous catalysts decreases in reaction media because these catalysts cannot be recovered and reused. To avoid these problems, solid supports such as polymer, zeolite, and inorganic porous material have been used for immobilization of metal complexes. Supported vanadium catalysts are becoming increasingly important. Several publications on oxovanadium complexes supported on inorganic and organic materials have been reported for a good catalytic performance in oxidation reactions [12–15]. Immobilization of catalyst on inorganic materials have several important advantages such as stability of the inorganic supports particularly with the regards of oxidation conditions, mechanical stability, superior thermal stability and do not swell or dissolved in organic solvents. It is also interesting to know that among the inorganic supports process nano sized silica material such as MCM-41 provides excellent intrinsic properties such as low toxicity, excellent chemical stability and versatile functionalization chemistry, because MCM-41 possess uniform and tunable pore size 20–100 Å functionalizable surface, high specific surface area and large pore volume 1 cm3 g−1 for variety of potential application [16–18]. The selective oxidation of sulfides to sulfoxides is an attractive and important transformation in organic chemistry, since sulfoxides are valuable intermediate for the production of range of chemically and biologically molecules, with result they can be used as therapeutic agents such as antiulcer, antibacterial, anti-fungal, anti-alterascleortic, anti-hypertensive, and cardio tonic agents, psychotropic and vasodilators [19]. And also they play the key role in activation of enzymes [20]. In this regard; many researches have used homogenous and heterogeneous vanadium complexes as catalysts for the oxidation of organic sulfides to sulfoxides [21, 22]. Although many methods have been developed for the oxidation of sulfides, the catalytic switching of the oxidation to meet green chemical requirements still remains a major challenge.
In this paper, we report synthesis and characterization of oxovanadium complex anchored on MCM-41. This immobilized complex can be sufficiently applied for the oxidation of sulfides to sulfoxides as an active and reusable catalyst and with stability at room temperature.
2 Experimental
2.1 Introduction
The cationic surfactant cetytriemethylammoniumbromide (CTAB, 98 %), tetraethylorthosilicate (TEOS), sodium hydroxide, organic sulfides, 5-bormosalicylaldehyde, 30 % hydrogen peroxide, 3-aminopropyltriethoxysilane (3-APTES), oxovanadium acethylacetate and solvents were purchased from Merck, Flucka and Aldrich chemical companies.
2.2 Synthesis of Si-MCM-41
Nano porous Si-MCM-41 was prepared according to the literature method [23]. TEOS was used as source of silicon and CTAB was used as the structure directing agent. The molar composition for synthesis of gel was TEOS: 1, CTAB: 0.3, NaOH: 0.1 and H2O: 60. The gel mixture was stirred for 1 h at room temperature and was transferred to a Teflon lined autoclave and was statically heated at 373 K. The collected product was calcined at 823 K for 5 h. This nonporous material is designated as Si-MCM-41.
2.3 Synthesis of VO- Schiff base complex on functionalized MCM-41
Organic modification of the nonporous material was performed by stirring 4.8 g Si-MCM-41 with 4.8 g solution of 3-APTES in 100 mL of n-hexane at 80 °C for 24 h under nitrogen atmosphere. Bromo modified salen ligand was synthesized by the condensation of MCM-41-(SiCH2CH2NH2)x, (1 eq.) and 5-bromosalicyaldehyde (1 eq.) in ethanol under reflux conditions for 3 h. The resulting yellow colored solid was filtered, washed with ethanol and dried in vacuum. VO-salen- MCM-41, was prepared by stirring a solution of VO(acac)2 (1 mmol) and MCM-41-L (2 g) in ethanol (30 mL), for 24 h. The resulting solid was filtered, washed with ethanol and water using soxhlet apparatus and dried in vacuum (Scheme 1).
2.4 Catalytic oxidation of sulfides to sulfoxides
One mmol of disulfide, 5 mmol of oxidant (30 wt% H2O2) and 4 mL of solvent along with 15 mg of catalyst were added to a 10 mL two-neck flask equipped with a stirrer. The progress of the reaction was monitored by TLC. After completion of the reaction, the catalyst was removed and the products were extracted with CH2Cl2 (3 × 10 mL).
3 Results and discussion
3.1 Characterization of the catalyst
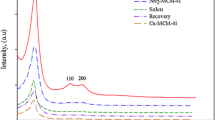
The small angle XRD patterns of MCM-41, MCM-41-nPr-NH2, 5-Br-salen-MCM-41 and VO-salen-MCM-41 are shown in Fig. 1. MCM-41 shows a very intense peak assigned to reflection at (1 0 0) and two additional peaks with low intensities at (1 1 0) and (2 0 0) reflections respectively, which can be attributed to the 2D hexagonal structure. A comparison of XRD patterns of MCM-41 with, MCM-41-nPr-NH2, 5-Br-salen-MCM-41 and VO-salen-MCM-41 shows after functionalizing nanoporous with organic groups and metal ions, some loss in intensity of peaks appears. It is noticed that decease in intensities of the peaks for the (1 1 0) and (2 0 0) plans of VO-salen-MCM-41 can be attributed to the diminishing of local order or may be the result of less scattering contrast between the channel wall of the silicate framework, organic groups and metal ion in the pores [24], The position of the (1 0 0) reflection indicated the basic hexagonal pore arrangement was sustained [25]. Finally, XRD pattern of recovered catalyst indicates that the catalyst can be recycled five times without any significant change in the structure of the catalyst or mesoporous of silicate parent.
Thermogravimetric curves of MCM-41, MCM-41-nPr-NH2, 5-Br-salen-MCM-41 and VO-salen-MCM-41 complex are shown in Fig. 2. There are two loss weights in MCM-41 curve; first step is at temperature 31–115 °C related to water absorption, second step (115–841 °C) attributed to compression of the silicate network and loss of chemical bound water weight. In this curve, 4.23 % loss weight has been observed. In addition, three stages of loss weight have also been displayed in thermo graph of MCM-41-nPr-NH2. Amino group anchored on MCM-41 had decreased in the first stage about 8.2 % which is related to absorption humidity in pores of the cavity. Sample weight loss in second and third stage is about 13.22 % which is attributed to the functionalized groups in nanoporous. And also, 5-Br-salen-MCM-41 curve showed three stage of loss weight similar to MCM-41-nPr-NH2, and this diagram confirms the formation of Schiff base ligand. In VO-salen-MCM-41 diagram four loss weights has been observed in which the first step relates to moisture loss weight, the second and third steps correspond to the analysis of the grafted functionalized group and finally the fourth step relates to coordinated metal.
N2 sorption isotherms of MCM-41, MCM-41-nPr-NH2, 5-Br-salen-MCM-41 and VO-salen-MCM-41 are shown in Fig. 1. Analysis of N2 adsorption–desorption data exhibit a typical type-IV isotherm (defined by IUPAC) with hysteresis loops indicating that mesoporous structure remained [26]. A gradual decrease in the BET surface area, pore volume, and pure diameter could be observed at each stage of modification as expected because of organic fragments or metal complexes entered the channels. The results are presented in Table 1. The nitrogen sorption study indicates that the BET surface area for MCM-41 is 986 m2 g−1 and pore volume is 0.711 cm3 g−1. MCM-41-nPr-NH2 shows uptake (BET surface area 694.98 m3 g−1) and pore volume 0.340 m3 g−1and VO-salen-MCM-41 shows further less N2 uptake (BET surface area 150.23 m2 g−1) and pore volume 0.135 cm3 g−1. This result indicate modified samples possess lower pore size distribution compared to its parents material. With an increase in bulkiness inside the pores, the hysteresis occurred at lower p/p° indicating the decrease of porous size. This is a clear indication of VO-salen-MCM-41 inside the pore channel of mesoporous silica. Similar trend also has been observed previously [27] (Fig. 3).
In Fig. 4, the UV–Vis DRS of the samples are shown. Intense n–π*, π–π* ligand charge transfer bands in the range of 220–370 nm are observed in 5-Br-salen-MCM-41 and VO-salen-MCM-41. A weak band usually appears in the ca. 600 nm region in oxovanadium(IV) complexes attributed to d–d transition for VO-salen-MCM-41indicates, this is show that oxovanadium(IV) complex have been incorporated onto the surface of MCM-41 [25]. In addition, as expected, MCM-41 does not appear any absorption bands in the wavelength region studied.
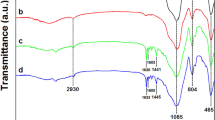
In order to verify the functionalization of the MCM-41 surface, the FT-IR spectra of the prepared MCM-41, MCM-41-nPr-NH2, 5-Br-salen-MCM-41 and VO-salen-MCM-41 were obtained and have been represented in Fig. 5. The strong and broad absorption band at about 1000–1100 cm−1 revealed the presence of Si–O–Si and Si–O–H stretching vibrations and confirmed the presence of silica in MCM-41 structure. The presence of anchored propyl chain was confirmed by C–H stretching vibrations appeared at about 2923 cm−1 and also two bands (N–H) were indicated in 1642 and 1468 cm−1. In the FT-IR spectrum of 5-Br-salen-MCM-41, the bands observed at 1655 (C=N) and 1483 (C=C) cm−1 emphasize 5-bromosalicylaldehyde were successfully formed Schiff base ligand with amino group grafted in the channels of meso structured porous silica. A red shift observed in VO-salen-MCM-41 spectra identified the imine group of 5-Br-salen-MCM-41 was coordinated with metal. Eventually, FT-IR spectrum of recovered catalyst indicates that after fifth cycle structure of the catalyst is retained.
3.2 Catalytic activity of the catalyst in sulfoxidation reaction
The catalytic activity of VO-salen-MCM-41 was evaluated in the oxidation of sulfides to sulfoxsides using hydrogen peroxide as oxidizing agent. To find the optimum reaction condition, dibenzyl sulfide was chosen as a model substrate and the influence of different amount of hydrogen peroxide, catalyst, and the nature of solvent for the oxidation of this compound were investigated. In order to choose proper reaction media various solvents were tested for the oxidation of dibenzyl sulfide. It was observed that catalytic performance was strongly affected by the type of solvent. According to Fig. 6 lowest yield was observed, when water was applied as solvent. When the conventional solvents such as n-hexane are used, the yield of dibenzyl sulfoxide was less than 64 % after 24 h. Ethanol turned out to be the best solvent providing the highest yield (97 %) in 90 min.
We have suggested in Scheme 2, the possible mechanism for the oxidation of sulfides to soulfoxides.
Based on this mechanism the presence of polar solvent such as ethanol was found to be ideal for complete conversion of sulfides to sulfoxides. To find the effect of the catalyst, blank experiment (in the presence of 1 mmol substrate, 5 mmol H2O2) in the absence of catalyst was carried out. It was observed that the oxidation reaction occurs rather slowly). However when similar oxidation reaction was conducted in the presence of catalyst (VO-salen-MCM-41, 15 mg), the reaction was completed yielding (97 %) 90 min.
To improve the conversion rate and the yield of sulfoxide, the various molar ratio of H2O2/sulfide were examined (Table 2). As it can be seen from Table 2, while the conversion of oxidation improved from 93 to 97 % the molar ratio increase from 3 to 5 mmol. In addition, when excess amount of H2O2 (7 mmol) was employed the oxidation product yield was decreased. Therefore 1 mmol of sulfide, 5 mmol of H2O2, 15 mg of catalyst and 4 mL of ethanol as a solvent is selected as the best combination and was used in subsequent oxidation reaction of sulfides. Considering the above results and usefulness of the sulfoxides in the synthesis of organic compounds, we encouraged to study the effect of VO-salen-MCM-41as catalyst in oxidation of different functional groups of sulfides under the above mentioned conditions. The results are listed in Table 3.
Table 3 shows good catalytic activity and selectivity for all chosen sulfides. One of the most important factor that affects catalytic performance in sulfoxidations is Lewis acidity behavior of oxovanadium(IV) complex. This complex can readily withdraw electron from the peroxide oxygen making it more susceptible to be attacked by sulfide compound.
The major advantage of heterogeneous catalysis over its homogenous counterpart is stability and possibility of recovering of the catalyst. For this propose the recycle test was performed using dibenzyl sulfide as model substrate in the presence of catalyst and H2O2 as oxidant in ethanol as solvent. At the end of each reaction, the solid catalyst was separated from the reaction media, washed with ethanol, and dried at 80 °C. After five cycles the immobilized catalyst retained its catalytic efficiency and selectivity, indicating the potential of immobilized catalyst in large scale process (Fig. 7).
In order to show the efficiency of described procedure in this manuscript, the results of the preparation of benzylmethyl sulfoxide with the previously reported procedure (Table 4).
4 Conclusion
VO-salen-MCM-41 is used as an excellent catalyst for high selective oxidation of sulfide to sulfoxide in ethanol as green solvent by H2O2. Under this reaction conditions the functional group such as hydroxyl group was not affected. The main advantage of this method is over oxidation into sulfone was not observed. As catalyst, oxidant and solvent are environmentally benign reagents, this method meet the needs of contemporary green chemistry and is suitable for oxidation of sulfides to sulfoxide.
References
A. Pohlmann, S. Nica, T. Kim, K. Luong, W. Plass, Inorg. Chem. Commun. 8, 289 (2005)
T.L. Turner, V.H. Nguyen, C.C. McLauchlan, Z. Dymon, B.M. Dorsey, J.D. Hooker, M.A. Jones, J. Inorg. Biochem. 96, 108 (2012)
D. Rehder, Bioinorganic Vanadium Chemistry (Wiley, New York, 2008)
S. Sandel, S.K. Weber, O. Trapp, Chem. Eng. Sci. 83, 171 (2012)
S.A. Taghavi, M. Moghadam, I. Mohammadpoor-Baltork, S. Tangestaninejad, V. Mirkhani, A.R. Khosropour, C. R. Chim. 14, 1095 (2011)
B.K. Singh, D. Adhikari, Int. J. Basic Appl. Chem. Sci. 2, 84 (2012)
M.R. Maurya, A.K. Chandrakar, S. Chand, J. Mol. Catal. A: Chem. 263, 227 (2007)
A.G.J. Ligtenbarg, R. Hage, B.L. Feringa, Coord. Chem. Rev. 89, 237 (2003)
E. Kwiatkowski, G. Romanowski, W. Nowicki, M. Kwiatkowski, Polyhedron 2809, 25 (2006)
S. Kodama, Y. Ueta, J. Yoshida, A. Nomoto, S. Yano, M. Ueshima, A. Ogawa, Dalton Trans. 44, 9708 (2009)
M. Bagherzadeh, M. Amini, J. Coord. Chem. 63, 3849 (2010)
S. Bhunia, D. Shah, S. Koner, Langmuir 27, 15322 (2011)
M.R. Maurya, M. Kumar, A. Arya, Catal. Commun. 10, 187 (2008)
M.C. Hsiao, S.T. Liu, Catal. Lett. 139, 61 (2010)
C. Pereira, A.M. Pereira, P. Quaresma, P.B. Tavares, E. Pereira, J.P. Araujo, C. Freire. Dalton Trans. 2842, 39 (2010)
J. Kim, H.S. Kim, N. Lee, T. Kim, H. Kim, T. Yu, I.C. Song, W.K. Moon, T. Hyeon. Angew. Chem. Int. Ed. 47, 8438 (2008)
J.E. Kim, J. Lee, J.H. Lee, B.C. Yu, K. Kim, Y. An, C.H. Hwang, J. Shin, G. Park, T. Hyeon, J. Am. Chem. Soc. 128, 688 (2006)
L. Zhang, S.Z. Qiao, Y.G. Jin, H.G. Yang, S. Budihartono, F. Stahr, Z.F. Yan, X.L. Wang, Z.P. Hao, G.Q. Lu, Adv. Funct. Mater. 18, 3203 (2008)
H. Golchoubian, F. Hosseinpoor, Molecules 12, 304 (2007)
B. Karimi, D. Zareyee, J. Iran. Chem. Soc. 5, 103 (2008)
S. Rayati, P. Jafarzadeh, S. Zakavi. Inorg. Chem. Commun. 29, 40 (2013)
G.P. Romanelli, D.O. Bennardi, V. Palermo, P.G. Vázquez, P. Tundo, Lett. Org. Chem. 4, 544 (2007)
H. Chen, Y. Wang, Ceram. Int. 28, 541 (2002)
M.M. Wan, L. Gao, Z. Chen, Y.K. Wang, Y. Wang, J.H. Zhu, Microporous Mesoporous Mater. 155, 24 (2012)
A.I. Ahmeda, S.E. Samraa, S.A. El-Hakama, A.S. Khderb, H.Z. El-Shenawya, W.S. Abo, El-Yazeed. Appl. Surf. Sci. 282, 217 (2013)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, T. Siemieniewska, Pure Appl. Chem. 603, 57 (1985)
A. Jabbari, H. Mahdavi, M. Nikoorazm, A. Ghorbani-Choghamarani, Res. Chem. Intermed. (2014 in press)
A. Shaabani, A. Bazgir, K. Soleimani, P. Salehi, Synth. Commun. 33, 2935 (2003)
M.M. Lakouraj, M. Tajbakhsh, Synth. Commun. 35, 775 (2005)
F. Chena, J. Wana, C. Guana, J. Yanga, H. Zhang, Synth. Commun. 26, 253 (1996)
F.R. Bisogno, A. Rioz-Martnez, C. Rodguez, I. Lavandera, G. de Gonzalo, D.E. Torres PazmiÇo, M.W. Fraaije, V. Gotor, ChemCatChem. 2, 946 (2010)
F. Toda, K. Tanaka, T. Okada, J. Chem. Soc. Chem. Commun. 6, 639 (1995)
A. Ghorbani-Choghamarani, B. Ghasemi, Z. Safari, G. Azadi, Catal. Commun. 60, 70 (2015)
K. Surendra, N.S. Krishnaveni, V.P. Kumar, R. Sridhar, K.R. Rao, Tetrahedron Lett. 46, 4581 (2005)
A. Rostamia, B. Tahmasbi, F. Abedi, Z. Shokri, J. Mol. Catal. A: Chem. 378, 200 (2013)
A. Bayat, M. Shakourian-Fard, M.M. Hashemi, Catal. Commun. 52, 16 (2014)
Acknowledgments
Financial support for this work by the research affairs of Islamic Azad University, Qeshm Branch, Qeshm, Iran is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jabbari, A., Mahdavi, H., Nikoorazm, M. et al. Oxovanadium(IV) salicylidene Schiff base complex anchored on mesoporous silica MCM-41 as hybrid materials: a robust catalyst for the oxidation of sulfides. J Porous Mater 22, 1111–1118 (2015). https://doi.org/10.1007/s10934-015-9986-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-015-9986-9