Abstract
A microextraction method, namely temperature-controlled/assisted ionic liquid-based dispersive liquid-phase microextraction (DLPME) for manganese(II) determination in environmental water samples is presented. Manganese(II) was extracted from aqueous solution into an ionic liquid (IL) after complexation with 1-(2-thiazolylazo)-2-naphthol (TAN) and directly introduced for electrothermal atomic absorption spectrometry (ETAAS) determination. Several factors that might affect ETAAS signal and extraction efficiency, such as pyrolysis and atomization temperature; and pH adjustment, concentration of TAN, extraction time, volume of IL and stirring rate were studied. Within optimal experimental conditions, the detection limit (3σ), the quantification limit (10σ), the enrichment factor and the relative standard deviation for five replicate determinations at 0.5 μg L−1 of manganese(II) were calculated as 0.023 μg L−1, 0.076 μg L−1, 58.7 and 6.3 %, respectively. The presented method was also successfully applied to the determination of manganese(II) in real samples. The main benefits of the method are simplicity, low cost, rapidity, high recovery, powerful extraction efficiency and the proposed approach is free of volatile organic solvents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Manganese (Mn) is a necessary element for human life. It is a cofactor for several enzymes including kinases, phosphatases and oxidoreductases. It is a potential, toxic element at high concentrations. The chronic exposure to this element can cause a neurodegenerative disease called manganism with symptoms such as mental and emotional conflicts and muscle stiffness [1, 2]. Manganese is usually present at trace or ultra-trace levels in various environmental samples. As an example, concentration ranges of manganese in fresh waters are 0.02–130 µg L−1 [3]. According to the World Health Organization (WHO), adult daily intake of manganese from food is between 2 and 9 mg. Manganese intake can be as high as 20 mg daily without apparent ill effect. With an intake of 12 mg per day, a 60-kg adult would receive 0.2 mg kg−1 of body weight per day. Accounting for about 20 percent of the drinking water, and using an uncertainty factor of 3 to allow for possible increased bioavailability of manganese from water, gives a value of 0.4 mg L−1. Therefore, very sensitive methods are necessary for monitoring this element in water samples. Electrothermal atomic absorption spectrometry (ETAAS) is a powerful and useful technique for this aim [4]. But the direct determination of manganese(II) at very low concentrations is often difficult because of insufficient sensitivity of this technique as well as the matrix interferences occurring in real samples. Because of this, an elementary separation and preconcentration step is often required. Liquid-liquid extraction (LLE) is among the oldest of the preconcentration and matrix isolation techniques used in analytical chemistry [4]. However, it is time-consuming even when automated and requires large amounts of high-purity organic solvents. Solid-phase extraction (SPE) [5], solid-phase microextraction (SPME) [6] and stir bar sorption extraction (SBSE) [7, 8] are other techniques that have been developed and used in sample pretreatment.
In last two decades, liquid-phase microextraction (LPME) has attracted increasing attention as a novel sample preparation technique. LPMEs include (1) single-drop microextraction (SDME), in which the extraction phase is a drop of water-immiscible solvent suspended in the aqueous sample or in the headspace above it [9, 10]; (2) hollow fiber LPME (HF-LPME), where the target analytes are extracted from aqueous samples into an organic solvent sustained in the pores and the lumen of supported liquid membrane (2-phase HF-LPME) or into an aqueous acceptor situated only in lumen of HF (3-phase HF-LPME) [11, 12]; and (3) dispersive liquid-phase microextraction (DLPME), which is based on the ternary component solvent system [13, 14] similar to homogeneous liquid-liquid extraction (HLLE) and cloud point extraction (CPE) [15]. DLPME as a high revenue, powerful, simple, fast and inexpensive microextraction method has been developed by Assadi and co-workers [13]. Recently, DLPME was also performed using several automation variants [16–18]. The basic principle of such method is powerful disruption of extractant/disperser mixture within aqueous solution, leading to a very high contact area between both aqueous and extraction phases. However, the amount of disperser is often enormous, which may lead to decrease of extraction recovery of hydrophobic compounds. Because of that, the alternative liquid-phase microextraction methods were developed, avoiding the use of the dispersing solvent. These approaches achieve cloudy state by multiplying air bubbling [19], magnetic stirring within syringe barrel [20, 21], vigorous injection of solvent into the sample phase [22], pressure assistance [23], ultra-sonication [24, 25] and by repeated up-and-down shaking [26].
Another approach, also belonging to this group of DLPME is called temperature-controlled/assisted IL-based DLPME (TCIL-DLPME) [27]. In this method, the disruption of IL extraction phase is driven by temperature control. In the present work, the IL 1-Hexyl-3-methylimidazolium hexafluorophosphate [C6MIM][PF6] was used as a solvent for TCIL-DLPME of manganese(II) as 1-(2-thiazolylazo)-2-naphthol (TAN) complex. The extracted analyte was directly injected for ETAAS.
Experimental
Instrumentation
A model A Analyst 800 atomic absorption spectrometer (Perkin-Elmer, Shelton, CT, USA) well-appointed with a deuterium lamp as background correction system and a graphite furnace atomizer was used. A manganese hollow cathode lamp (Perkin-Elmer, USA) was used as the radiation source and the operating conditions of the hollow cathode lamp were recommended by the performer. Pyrolytically plated graphite tubes were employed all over Argon 99.999 % (Roham Gas Co., Tehran, Iran) with 1.5 L min−1 fluency rate was used as a preserver and purge gas. The detailed graphite furnace temperature program used for the determination of manganese(II) is shown in Table 1. A 50 µL microsyringe (Hamilton, Germany) was employed to shot IL extracting phase to the solution. A model 691 pH-meter (Metrohm, Switzerland) was used for pH measurements and a Jeio Tech BW-50G water bath were obtained from Hettich (Kirch Lengern, Germany). Liquid-liquid separation was conducted with a Universal 320R refrigerated centrifuge (Hettich, UK).
Standard solutions and reagents
All the chemicals used were of analytical-reagent grade and all solutions were diluted with bi-distilled deionized water obtained from Ghazi Serum Co., Tabriz, Iran. 1-Hexyle-3-methylimidazolium hexafluorophosphate [C6MIM][PF6] was purchased from Merck (Darmstadt, Germany) and used as obtained. A stock solution of manganese(II) was prepared by dissolving an appropriate amount of Mn(NO3)2, 4H2O (Merck) into a 100 mL flask with distilled water and hoarded at 4 °C in dark glass bottles. The working solutions of manganese(II) were made by suitable dilution of the stock solution with bi-distilled water. The 1 × 10−3 mol L−1 solutions of 1-(2-thiazolylazo)-2-naphthol (TAN) were provided by dissolving suitable amount of TAN (Fluka) in 25 mL of methanol. A stock buffer solution (0.1 mol L−1) was prepared by dissolving appropriate sums of boric acid in deionized water and regulating to pH 9.5 by adding diluted NaOH solution.
Sample preparation
In this work, environmental water samples like underground water, river water and tap water were gathered for manganese(II) determination in real samples. Tap water was obtained from our laboratory. River water sample was obtained from southwest Iran and underground water sample was collected from Bonab, Iran. The collected water samples were filtered through a 0.45 µm micropore membrane immediately and were kept in dark glass bottles, then stored at a temperature of 4 °C.
The procedure
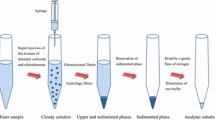
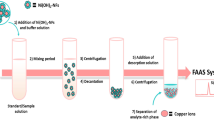
For this microextraction procedure, 10 mL of water sample (ultra-pure water or environmental water) was added into a 15 mL capacity syringe. Then 100 µL borate buffer and 500 µL of 1 × 10−3 mol L−1 TAN solution was added. A 50 µL of [C6MIM][PF6] as extraction solvent was introduced rapidly into the aqueous solution. Then the syringe was heated in a water bath at 35 °C. The IL was dissolved completely and mixed absolutely with the solution. The vial was then cooled with ice water for 5 min and the solution became cloudy and the analytes migrated into the IL phase. Thereafter the solution was centrifuged for 5 min at 4000 rpm. As a result, the fine droplets of IL settled at the bottom of the tube. Bulk aqueous phase was removed simply by a syringe. Then, 30 µL of 50 % (V/V) HNO3 and 105 µL of 95 % ethanol were added to the sedimented phase, giving a total volume of 150 µL, which is suitable for direct analysis using ETAAS. The procedure is depicted in Fig. 1. The enrichment factor (EF) was calculated as the proportion between the manganese(II) concentration of the IL phase in the final solution (C sed) after extraction and the primary concentration of the manganese(II) (C 0) within the sample (EF = C sed/C 0). The extraction efficiencies summarized as Ec were calculated by the following equation:
where V sed and V aq are the final volume of sedimented phase (150 µL) and the volume of the aqueous sample, respectively.
Results and discussion
Optimization of ETAAS conditions
The selection of a suitable pyrolysis temperature is very important for removing as much the matrix as possible and preventing the pyrolysis loss of the analyte former to atomization. This lowers the possibility of chemical interference and decreases the magnitude of the background signal. In this work, pyrolysis and atomization steps were optimized using 0.4 µg L−1 manganese(II) solutions presented to the TCIL-DLPME process [28]. Figure 2a shows the resulting pyrolysis curve. It can be seen that when the pyrolysis temperature was up to 600 °C, the maximum attraction was acquired. At lower pyrolysis temperature, the background signal was high, which is possibly due to evaporation of excess TAN and IL itself at the atomization step. This causes an important signal suppression, which resulted in the low absorbance values for low pyrolysis temperature. Increasing pyrolysis temperature above 600 °C leads to loss of analyte and hence decreases analytical signal. Therefore, 600 °C was chosen as the optimized pyrolysis temperature for the determination of manganese(II). The effect of pyrolysis time on the attraction of manganese(II) was also inquired. The results pointed that, keeping the pyrolysis temperature at chosen value, the absorbance was increased with increasing pyrolysis time up to 40 s and no perceptible advances were incurred for longer times. As a result, a pyrolysis time of 40 s was chosen. The atomization temperature was similarly optimized (Fig. 2b). As can be seen, the maximum signal was obtained at about 2500 °C, and then diminished with the further increasing of temperature. So, the atomization temperature of 2500 °C was chosen for the further experiments. Since atomization time had little effect on atomic signal, an atomization time of 4 s was selected for atomization of manganese(II).
a Pyrolysis curve (pyrolysis temperature, 600 °C; pyrolysis time, 40 s) and b atomization curve (atomization temperature, 2500 °C and atomization time, 4 s) for DLPME of 0.4 µg L−1 manganese. Other conditions are given in Table 1
Effect of IL volume
The effect of IL volume on the extraction efficiency was studied by dissolving a certain volume of [C6MIM][PF6] in an aqueous sample under conditions described above. Results shown in Fig. 3 marked that the extraction efficiency of two compounds IL volume increased with the IL volume within the range 30–50 µL and decreased in the range 50–60 µL. The best extraction efficiency was obtained at 50 µL. The larger volume of IL affected in lower extraction transfer because the amount of [C6MIM][PF6] exceeded the target dispersed amount and excess IL attracted on to the wall of the tube. At the same time, part of the IL was dissolved in the aqueous solution and led to a few losses of analytes. According to the results, 50 µL of IL was chosen as optimum volume.
Effect of pH
As all know, pH plays an important role in the extraction of manganese(II) in water sample since the formation and extraction yield of Mn-TAN complex depends on the pH of the aqueous solution. Therefore, the effect of sample pH on the extraction efficiency of manganese(II) in the range 3–12 was investigated. As shown in Fig. 4, the extraction increased dramatically when the pH was increased from 6 to 9.5 and then remained almost constant across the range 9–12. At low pH, TAN is mainly in the protonated form, hence the amount of deprotonated TAN for the formation of Mn-TAN complex would be limited. This together with the inclination of hydrophilic metal ion to septum in aqueous phase leads to low extraction efficiency. Based on the obtained results, a pH value of 9.5 was selected for further studies. This pH was adjusted by using buffer solution. Influence of buffer amount was also studied. The results showed that the addition of 50 µL or larger amount of 0.1 mol L−1 borate buffer into the sample solutions were sufficient for keeping attraction at highest value. Therefore, 100 µL buffer solutions were chosen for experiments.
Effect of TAN concentration
The influence of the TAN concentration on the extraction efficiency of manganese(II) was also investigated and results are shown in Fig. 5. The extraction efficiency increased with increasing TAN concentration from 0.5 to 5 × 10−5 mol L−1 as a result of the high extraction efficiency of the manganese(II)-TAN complex to IL. For TAN concentration above 5 × 10−5 mol L−1, the attraction remained unchanged, as excess TAN did not improve extraction efficiency or mass transfer. So, 5 × 10−5 mol L−1 of TAN was selected as the optimum value.
Effect of extraction time
Extraction time is one of the most important factors in most of the extraction procedures, especially in microextraction methods such as SPME and LPME. In this study, extraction time means the time from the moment that the solution containing completely dissolved IL was put into ice water bath to the set interval. Results shown in Fig. 6 stated that the extraction efficiency increased for the studied analytes in the first 6 min, and then increased very little during the time range 6–10 min. Therefore, 6 min was admitted as the optimal condition. It is divulged that the surface area between extraction solvent and aqueous phase (sample) is infinitely large. Thereby, transfer of analytes from aqueous phase (sample) to extraction phase is fast. Afterwards, balance state is achieved quickly; as a result of that the extraction time is very short. This is the most important benefit of offered method.
Effect of temperature
Temperature is the driving force for the complete dispersion of [C6MIM][PF6] into the aqueous solution because ILs are dissolved more easily at the temperature above 30 °C. Therefore, it plays an important role on the extraction efficiency of manganese(II) water samples. A series of experiments were designed for the optimization of temperature. As can be seen from the result; the extraction efficiency was greatest at 35 °C and then decreased with the increase of temperature. Because of that, the solubility of manganese(II) in aqueous solution has been shown to increase with temperature.
Effect of centrifuge conditions
Centrifugation controls the phase separation and is one of the crucial steps in suggested method. The study of optimal centrifugation rate showed 4000 rpm as the most appropriate (Fig. 7). To find out the optimal centrifugation time, such interval was investigated and results showed that over 4 min IL phase was completely transferred to the bottom part of centrifuge tube. Therefore, the optimum centrifugation time was chosen as 5 min.
Study of interferences
In order to demonstrate the selectivity of the developed microextraction method, the effect of alkali and alkaline earth metals and several heavy metals on the extraction and determination of manganese(II) has been investigated. Different amounts of ions were added to the test solution containing 1.0 μg L−1 of manganese(II) using suggested procedure. The interference was taken into account when tolerable deviation exceeded more than 5 %. All studied ions were found not to affect manganese(II) signal in the DLPME-ETAAS system when they are present in 500-fold excess. However, the higher concentrations of alkali and alkaline earth metals can be also tolerated. As shown from results, the application of suggested method offers interference-free determination of manganese(II) at trace levels in water samples [28]. The results are shown in Table 2.
Method assessment
Important parameters like linear range, correlation coefficient (R), detection limit (LOD), quantification limit (LOQ) were assessed to evaluate method performance. Under optimal conditions, linear dynamic range was investigated obeying the Beer’s law within the concentration range 0.1–3.0 μg L−1. The R value was calculated as 0.998, the LOD and LOQ were found to be 0.023 μg L−1 and 0.076 μg L−1, respectively. In order to study the accuracy of the proposed method a series of six solutions containing 0.5 μg L−1 manganese(II) were measured on the same day. The relative standard deviation (RSD) was 6.3 % and the enrichment factor was 58.7.
Real sample analysis
In order to study the applicability of the proposed TCIL-DLPME method, the determination of manganese(II) was performed using underground, river and tap water samples by recovery test. Table 3 shows obtained results. As can be seen, the recoveries within 95–105 % were obtained confirming the accuracy of the suggested DLPME procedure.
Conclusion
In this study, a temperature-controlled/assisted ionic liquid-based dispersive liquid-phase microextraction (TCIL-DLPME) was developed. This system maintained the green analytical chemistry requirements. The results showed the possibility of the high extraction efficiency and trace manganese(II) determination using TCIL-DLPME and ETAAS, respectively. Comparison with previously reported ETAAS-preconcentration works (Table 4) indicates that linear range and LOD are better or comparable. Only the work from [29] makes an exception. Among other benefits, the developed method offers also to be simple, cheap and free of volatile organic solvents.
References
L. Quintanar, Inorg. Chim. Acta 361, 875–884 (2008)
Toxicological Profile for Manganese, U.S. ATSDR, http://www.atsdr.cdc.gov/toxprofiles/tp151.html (2000)
K.C. Teo, J. Chen, Analyst 126, 534–537 (2001)
ASTM, D 858-95: Standard Test Methods for Manganese in Water (2002)
J. Munoz, J.R. Baena, M. Gallego, M. Vaicarcel, J. Chromatogr. A 1023, 175–181 (2004)
M.L. Gac, G. Lespes, M.P. Gautier, J. Chromatogr. A 999, 123–134 (2003)
J. Vercauteren, C. Peres, C. Devos, P. Sandra, F. Vanhaecke, L. Moens, Anal. Chem. 73, 1509–1514 (2001)
A. Prieto, O. Zuloaga, A. Usobiaga, N. Etxebarria, L.A. Fernandez, C. Marcic, A.D. Diego, J. Chromatogr. A 1185, 130–138 (2008)
V. Colombini, C.B. Montigny, L. Yang, P. Maxwell, R.E. Sturgeon, Z. Mester, Talanta 63, 555–560 (2004)
H. Shioji, S. Tsunoi, H. Harion, M. Tanaka, J. Chromatogr. A 1048, 81–88 (2004)
A. Sarafraz-yazdi, A.H. Amiri, Trends Anal. Chem. 29, 1–14 (2010)
S.P. Bjergaard, K.E. Rasmussen, J. Chromatogr. A 1184, 132–142 (2008)
M. Rezaee, Y. Assadi, M.R.M. Hosseini, E. Ahmadi, S. Berijani, J. Chromatogr. A 1116, 1–9 (2006)
A.P. Birjandi, A. Bidari, F.R.M. Hosseini, Y. Assadi, J. Chromatogr. A 1193, 19–25 (2008)
D. Citak, M. Tuzen, Food Chem. Toxicol. 48, 1399–1404 (2010)
M. Chamsaz, J. Akhoundzadeh, M.H. Arbab-zavar, J. Adv. Res. 4, 361–366 (2013)
B. Horstkotte, M. Alexovič, F. Maya, C.M. Duarte, V. Andruch, V. Cerdá, Talanta 99, 349–356 (2012)
A. Bulatov, K. Medinskaia, D. Aseeva, S. Garmonov, L. Moskvin, Talanta 133, 66–70 (2015)
M. Alexovič, V. Andruch, I.S. Balogh, J. Šandrejová, Anal. Methods 5, 2497–2502 (2013)
B. Horstkotte, R. Suárez, P. Solich, V. Cerdà, Anal. Chim. Acta 788, 52–60 (2014)
N. ullah, T. G. Kazi, M. Tuzen, Food Chem. 172, 161-165 (2015)
M. Alexovič, M. Wieczorek, J. Kozak, P. Kościelniak, I.S. Balogh, V. Andruch, Talanta 133, 127–133 (2015)
N. ullah, M. Tuzen, T. G. Kazi, D. Citak, M. Soylak, J. Anal. At. Spectrom. 28, 1441-1445 (2013)
A. Gong, X. Zhu, Talanta 131, 603–608 (2015)
M. Tuzen, O.Z. Pekiner, Food Chem. 188, 619–624 (2015)
P.S. Chen, W.Y. Haung, S.D. Huang, J. Chromatogr. B 955–956, 116–123 (2014)
Q.X. Zhao, H.H. Bai, J.P. Xiao, J. Chromatogr. A 1177, 43–49 (2008)
J. Manzoori, M. Amjadi, J. Abulhassani, Talanta 77, 1539–1544 (2009)
K. Benkhedda, E. Ivanova, F. Adams, J. Anal. At. Spectrom. 14, 957–961 (1999)
Y. Okamoto, Y. Nomura, H. Nakamura, K. Iwamaru, T. Fujiwara, T. Kumamaru, Microchem. J. 65, 341–346 (2000)
P. Liang, H. Sang, Z. Sun, J. Colloid Interf. Sci. 304, 486–490 (2006)
M. Okumura, T. Anate, K. Fujinaga, Y. Seike, Anal. Sci. 18, 1093–1097 (2002)
E. Ivanova, K. Benkhedda, F. Adams, J. Anal. At. Spectrom. 13, 527–531 (1998)
Acknowledgments
The Islamic Azad University, Tabriz Branch, college of science, laboratory of chemistry department supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farzadbeh, N., Vardini, M.T. & Sheikhloie, H. Trace determination of manganese(II) by temperature-controlled/assisted ionic liquid-based dispersive liquid-phase microextraction and electrothermal atomic absorption spectrometry. J IRAN CHEM SOC 13, 715–722 (2016). https://doi.org/10.1007/s13738-015-0783-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0783-9