Abstract
In this study, a new analytical strategy was developed to determine trace cadmium in aqueous samples with high sensitivity and accuracy. A combination of magnetic nickel nanoparticles (Ni-MNPs) based dispersive solid-phase extraction (DSPE) and flame atomic absorption spectrometry fitted with a slotted quartz tube (SQT-FAAS) lowered the detection limit of cadmium. The magnetic Ni nanoparticles were synthesized, characterized, and thoroughly optimized in a stepwise approach. The quartz tube was custom cut in the laboratory to suit the specifics of the flame burner. Using the optimized conditions, a limit of detection value of 0.58 μg/L and limit of quantification value of 1.93 μg/L were obtained. To demonstrate accuracy and applicability of the developed method, well water samples were analyzed for their Cd content, and matrix effect on the extraction yield was investigated. The percent recovery results calculated ranged from 93.8 to 108.2%, with corresponding standard deviation values ranging from 1.7 to 7.7. These results established the developed method as sensitive, accurate, and precise for determination of cadmium at trace levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium is one of the most common industrial pollutants that can cause critical health and environmental problems (Registry 2012). The release of cadmium into natural resources may cause serious effects on human health and the ecosystem. Even though cadmium causes no significant harm to the well-being of living organisms at low concentrations, its accumulation could lead to serious health problems such as different cancer types (Nawrot et al. 2006). Therefore, a sensitive and accurate quantification method for trace cadmium is essential to determine its levels in food and environmental samples and to take the necessary precautions on time.
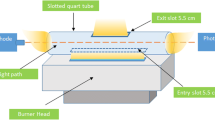
Modern technology provides high-end instruments such as inductively coupled plasma-optical emission spectrometry (ICP-OES) (de la Rosa et al. 2004), inductively coupled plasma-mass spectrometry (ICP-MS) (Djedjibegovic et al. 2012), and electrothermal-atomic absorption spectrometry (ET-AAS) (Zhong et al. 2016) for the determination of toxic elements. These instruments are well-known for determination of cadmium at trace and ultra-trace levels for decades now. Despite their highly accurate and sensitive determination capabilities, these instruments having complex user interfaces are expensive and relatively hard to access in many regions (Akkaya et al. 2017). Thus, a more common, cheap, and easy to operate instrument, flame atomic absorption spectrometry (FAAS), comes into prominence. However, FAAS has its own drawbacks, and these drawbacks cause relatively low detection performance when compared with the abovementioned determination instruments (Keskin et al. 2015). To overcome this, instrumental improvements have been developed, including the attachment of a slotted quartz tube (SQT) onto the burner head of the FAAS instrument (Çelik et al. 2018). This attachment helps to retain analyte atoms in the pathway of radiation from light source by 2 to 5 times more than regular period, and this improves absorbance signals (Arslan et al. 2011).
There are also different sample treatment techniques that are used to decrease matrix effect and increase the concentration of the analyte. These are achieved by extracting the analyte of interest to a different and\or smaller volume of media. Some of these techniques are dispersive solid-liquid extraction (Álvarez Méndez et al. 2015), single-drop microextraction (Wen et al. 2013), and solid-phase extraction (Huang and Hu 2008). Solid-phase microextraction utilizes solid materials to adsorb the analyte onto its surface and release it with an appropriate eluent. Magnetic particles based dispersive solid-phase extraction is one of the sub-branches of SPME which relies on magnetic particles’ surfactant and magnetic properties. Magnetite (Li et al. 2005), maghemite (Rajput et al. 2017), nickel (Ai et al. 2017), and cobalt (Akkaya et al. 2019) are the most common magnetic particles and can find broad application areas (Wierucka and Biziuk 2014). Magnetic nickel nanoparticles (Ni-MNPs) show high analyte selectivity and high magnetic properties, which makes them preferable as DSPE agents.
In this study, Ni-MNPs were used as DSPE agent to extract cadmium from environmental water samples. Ni-MNPs showed high extraction efficiency and, thus, high percent recoveries at different concentrations. To the best of our knowledge, this is the first study in the literature that employed Ni-MNPs as DSPE agent for the sensitive and accurate extraction/preconcentration of cadmium from aqueous media prior to quantification by SQT-FAAS.
Experimental
Chemicals and reagents
Ni-Al alloy and sodium hydroxide used for Ni-MNP synthesis were purchased from Merck, Germany, and Sigma-Aldrich, USA, respectively. Cadmium standard stock solution (1000 mg/L) used for optimization studies during the method development was obtained from High-Purity Standards, USA. Ultrapure water used for dilution and cleaning processes was obtained from a Milli-Q® Reference Ultrapure Water Purification System. Potassium dihydrogen phosphate, sodium bicarbonate, and di-sodium tetraborate decahydrate and other chemicals including ethanol, nitric acid, and hydrochloric acid were purchased from Merck, Germany.
Apparatus
PerkinElmer AAnalyst 400 AAS was used to determine Cd. To improve the residence period of atoms in the light path, a lab-cut quartz tube was attached to the flame unit of the FAAS (14 mm outer diameter, 35 mm exit slot, and 115 mm entrance slot). Cadmium hollow cathode lamp (2.0 mA, 228.8 nm) was applied as light source, and a deuterium lamp was used as background corrector tool. A hot plate (IKA RCT model) for the heating purposes, a pH meter (Hanna brand) for pH measurement, and a scale (OHAUS brand) for weighing the nanoparticles were used. Sample agitation was done using a HAPA M-100 model ultrasonicator and a Benchmark Scientific Inc. (USA) BV1000 Vortex mixer. The morphological properties of nanoparticles were observed by using a Zeiss EVO® LS 10 scanning electron microscopy. PANanalytical XRD system was used to determine phase composition of the prepared nanoparticles. In the separation of magnetic nanoparticles from the sample/standard solution, different sizes of neodymium magnets were used.
Synthesis of coated magnetic nanoparticles
The procedure reported in the literature by Michalcová et al. was modified in this study for the synthesis of Ni-MNPs (Michalcová et al. 2014). Briefly, 10 g NaOH and 200 mL ultrapure water were added into a beaker and heated on a hot plate. The solution was stirred at 900 rpm, and when it reached 80 °C, 20 g NiAl (50:50 wt%) was added to the beaker and stirred for 2.0 h at 80 °C. Then, the nanoparticles formed were successively washed 4 to 5 times with ultrapure water and ethanol. In the last step, the washed nanoparticles were dried overnight in an oven set to 50 °C.
Extraction procedure
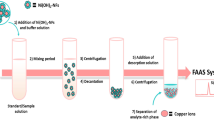
Seventy-five milligrams of Ni-MNPs was weighed and transferred to a 15.0 mL centrifuge tube containing 8.0 mL of Cd standard solution. The solution was vortexed for 120 s for an efficient dispersion and optimum nanoparticle-sample interaction. Cadmium-adsorbed Ni-MNPs were then gathered to the inner wall of the tube by means of an external magnetic field (neodymium magnet). The supernatant was discarded while the particles were held still. Finally, 150 μL of conc. HNO3 was used to release the adsorbed analyte, and the eluent was sent to SQT-FAAS system for absorbance reading.
Results and discussion
In order to lower the detection power of the determination system, all experimental parameters were optimized using the one-parameter-at-a-time design. To be precise, one parameter was optimized, while the others were held constant. In all optimization studies, triplicate measurements were performed, and the highest average absorbance value was used in selecting the optimum value.
Characterization of magnetic nickel nanoparticles
X-ray diffraction was used to analyze the structure of the prepared Ni-MNPs. Figure 1 shows the XRD pattern of the produced Ni-MNPs. The intensity was determined in the range of 40° < 2θ < 80° with 0.02° step size. The three characteristic peaks observed for nickel 2Ɵ = 44.45, 51.78, and 76.42° correspond to the 2θ values for the planes (111), (200), and (220), respectively. This indicates that the resulting powders are cubic structure nickel (PDF #04-016-6268). As seen from the XRD plot, the (111) reflection is the maximum intensity for the peaks.
SEM (scanning electron microscopy) was used to observe whether the particles are formed in nano-size or not. As seen in Fig. 2, the size of the particles ranged below 100 nm which clearly indicates that the particles are nano-sized.
Optimization of pH of the solution and buffer amount
pH of a solution is one of the important parameters in extraction studies because it determines the magnitude of the affinity between the analyte and the solid adsorbent. In this optimization step, pH 2.0, 5.0, 9.0, 11, and 13 buffer solutions were added to standard solutions. 2.0 mL of buffer solution was added each time, and to avoid any differentiation on the Cd concentration, final volume after buffer addition was taken into account. An un-buffered sample was also tested to see the extraction performance of the solution’s natural pH (6.95), and the highest response was obtained from the pH 6.95 solution as can be seen in Fig. 3.
Optimization of the amount of magnetic Ni nanoparticle
The amount of magnetic nanoparticles has a significant effect on the performance of the extraction procedure. More MNP means more surface area which provides more active centers for the analyte to interact, but it also causes the need for excess amount of elution solvent to recover all the analyte from the particle surface. The use of excess eluent would cause a decrease in the final analyte concentration by diluting it. On the other hand, inadequate amounts of MNP would not be able to efficiently collect the analyte in the standard/sample. Therefore, different amounts (25, 50, 75, and 100 mg) of MNP were tested, and the optimum one was determined as demonstrated in Fig. 4. As can be seen in the figure, 75 mg was selected as optimum amount. In the usage of 100 mg, signal of the analyte decreased. It might be due to the low desorption of the analyte from the MNP because same volume of eluent was used in all amount usages.
Duration and type of mixing
After the determination of the optimum MNP amount, the next step was to determine the optimum interaction type and period between the particles and the sample. Selection of the proper mixing type to increase the extraction yield is crucial in extraction studies because the right type of mixing provides an efficient interaction, therefore, a higher yield. For this purpose, ultrasonication and vortex mixing were tested, and the latter was selected as the optimum mixing type due to its higher absorbance values. Different interaction periods were also tested to observe their effect on extraction yield. Insufficient interaction period may hamper the maximum adsorption, and excess mixing on the other hand may result with desorption of the analyte from the adsorbent surface. Thus, 15, 30, 45, 60, and 120 s mixing periods were tested, and 120 s was selected for its highest outcome.
Type and amount of the eluent
The selection of the amount and the type of eluent is as crucial as other optimization parameters. The elution solvent is responsible for desorption of the analyte from the surface of the MNPs. HCl and HNO3 were tested in this optimization, and the analyte recovery performance of HNO3 was approximately two times higher than the results obtained from HCl; thus, HNO3 emerged as optimum eluent due to its high elution performance. Different concentrations of HNO3 were also tested (75, 100, 150, 200, and 250 μL) in the selection of optimum amount. The results indicated that 150 μL of HNO3 was efficient to elute appreciable amounts of the analyte. Excess amounts of the eluent would dilute the final concentration, and inefficient amounts would fail to desorb all of the analyte. A Gaussian-like shape that demonstrates this phenomenon can be seen in Fig. 5.
Analytical figures of merit
Instrumental parameters and SQT height were investigated, and the optimized values obtained are presented in Table 1 together with optimum extraction parameter values.
A wide linear range (2.0–100 μg/L) with a high correlation coefficient factor of 0.9995 as shown in Fig. 6 and very low detection (LOD)/quantification (LOQ) limits (0.58/1.93 μg/L) were obtained using the optimum experimental conditions. Table 2 shows the improvement achieved by comparing the LOD-LOQ values of the conventional FAAS with the optimized systems. Table 2 also presents the analytical figures of merit values of similar determination methods published in the literature.
The following equations were used for the calculations made in this study:
Limit of detection (LOD) = 3*Std deviation of the lowest conc.\slope of cal. plot
Limit of quantification (LOQ) = 10*Std deviation of the lowest conc.\slope of cal. Plot
Enhancement factor = (LOQ value of direct analysis)/(LOQ value of Ni-MNPs-SPE-SQT-FAAS)
Recovery studies
Different sample matrices have their own characteristic properties, and these may cause interferences by either suppressing or enhancing the signal of the analyte of interest. To observe matrix interferences, an artificially aggravated matrix solution or a real sample solution can be used. In this study, well water samples obtained from three different sources were analyzed under the optimum conditions for their Cd content, and there was no signal obtained for the samples. Hence, spiking experiments with known concentration of analyte were performed. Table 3 presents the results obtained. The closeness of the recovery results to 100% shows that the real sample matrix did not hinder the extraction performance of the developed method, which is a good indicator that applicability of the developed method is high for the selected matrix.
Conclusions
An efficient, simple, accurate, and cheap analytical method for the determination of Cd was developed utilizing Ni-MNPs as SPE adsorbent agents. This was achieved by combining Ni-MNPs-DSPE with SQT-FAAS. Recovery studies showed that the developed method is applicable to real samples and can be safely used for the quantitative determination of Cd with high accuracy and precision under the optimum conditions. The method is also practical with its few extraction steps and presents low time consumption. This study is the first one in the literature where magnetic Ni nanoparticles as SPE agent was used to extract/preconcentrate Cd from aqueous samples prior to its accurate quantification by SQT-FAAS system.
Data availability
Not applicable.
References
Ai, L., Tian, T., & Jiang, J. (2017). Ultrathin graphene layers encapsulating nickel nanoparticles derived metal–organic frameworks for highly efficient electrocatalytic hydrogen and oxygen evolution reactions. ACS Sustainable Chemistry & Engineering, 5(6), 4771–4777.
Akkaya, E., Aylin Kasa, N., Çetin, G., & Bakirdere, S. (2017). A new method for the determination of cadmium at ultratrace levels using slotted quartz tube-flame atomic absorption spectrometry after preconcentration with stearic acid coated magnetite nanoparticles. [Article]. Journal of Analytical Atomic Spectrometry, 32(12), 2433–2438. https://doi.org/10.1039/c7ja00303j.
Akkaya, E., Erulas, F. A., Büyükpinar, Ç., & Bakirdere, S. (2019). Accurate and sensitive determination of lead in black tea samples using cobalt magnetic particles based dispersive solid-phase microextraction prior to slotted quartz tube-flame atomic absorption spectrometry. Food Chemistry, 297, 124947. https://doi.org/10.1016/j.foodchem.2019.06.014.
Álvarez Méndez, J., Barciela García, J., García Martín, S., Peña Crecente, R. M., & Herrero Latorre, C. (2015). Determination of cadmium and lead in urine samples after dispersive solid-liquid extraction on multiwalled carbon nanotubes by slurry sampling electrothermal atomic absorption spectrometry. [Article]. Spectrochimica Acta - Part B Atomic Spectroscopy, 106, 13–19. https://doi.org/10.1016/j.sab.2015.01.008.
Arslan, Y., Kendüzler, E., & Ataman, O. Y. (2011). Indium determination using slotted quartz tube-atom trap-flame atomic absorption spectrometry and interference studies. Talanta, 85(4), 1786–1791. https://doi.org/10.1016/j.talanta.2011.07.006.
Çelik, B., Akkaya, E., Bakirdere, S., & Aydin, F. (2018). Determination of indium using vortex assisted solid phase microextraction based on oleic acid coated magnetic nanoparticles combined with slotted quartz tube-flame atomic absorption spectrometry. [Article]. Microchemical Journal, 141, 7–11. https://doi.org/10.1016/j.microc.2018.04.031.
de la Rosa, G., Peralta-Videa, J. R., Montes, M., Parsons, J. G., Cano-Aguilera, I., & Gardea-Torresdey, J. L. (2004). Cadmium uptake and translocation in tumbleweed (Salsola kali), a potential Cd-hyperaccumulator desert plant species: ICP/OES and XAS studies. Chemosphere, 55(9), 1159–1168.
Djedjibegovic, J., Larssen, T., Skrbo, A., Marjanović, A., & Sober, M. (2012). Contents of cadmium, copper, mercury and lead in fish from the Neretva river (Bosnia and Herzegovina) determined by inductively coupled plasma mass spectrometry (ICP-MS). Food Chemistry, 131(2), 469–476.
Huang, C., & Hu, B. (2008). Silica-coated magnetic nanoparticles modified with γ-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy, 63(3), 437–444. https://doi.org/10.1016/j.sab.2007.12.010.
Keskin, G., Bakirdere, S., & Yaman, M. (2015). Sensitive determination of lead, cadmium and nickel in soil, water, vegetable and fruit samples using STAT-FAAS after preconcentration with activated carbon. [Article]. Toxicology and Industrial Health, 31(10), 881–889. https://doi.org/10.1177/0748233713484650.
Li, Z., Wei, L., Gao, M., & Lei, H. (2005). One-pot reaction to synthesize biocompatible magnetite nanoparticles. Advanced Materials, 17(8), 1001–1005.
Michalcová, A., Svobodová, P., Nováková, R., Len, A., Heczko, O., Vojtěch, D., Marek, I., & Novák, P. (2014). Structure and magnetic properties of nickel nanoparticles prepared by selective leaching. Materials Letters, 137, 221–224. https://doi.org/10.1016/j.matlet.2014.09.012.
Mirabi, A., Dalirandeh, Z., & Rad, A. S. (2015). Preparation of modified magnetic nanoparticles as a sorbent for the preconcentration and determination of cadmium ions in food and environmental water samples prior to flame atomic absorption spectrometry. [Article]. Journal of Magnetism and Magnetic Materials, 381, 138–144. https://doi.org/10.1016/j.jmmm.2014.12.071.
Nawrot, T., Plusquin, M., Hogervorst, J., Roels, H. A., Celis, H., Thijs, L., Vangronsveld, J., van Hecke, E., & Staessen, J. A. (2006). Environmental exposure to cadmium and risk of cancer: A prospective population-based study. [Article]. Lancet Oncology, 7(2), 119–126. https://doi.org/10.1016/S1470-2045(06)70545-9.
Rajput, S., Singh, L. P., Pittman Jr., C. U., & Mohan, D. (2017). Lead (Pb2+) and copper (Cu2+) remediation from water using superparamagnetic maghemite (γ-Fe2O3) nanoparticles synthesized by flame spray pyrolysis (FSP). Journal of Colloid and Interface Science, 492, 176–190.
Registry, A. f. T. S. a. D. (2012). Toxicological Profile for Cadmium. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15.
Saljooghi, A. S., & Saljoghi, Z. S. (2011). Natural analcime zeolite modified with 2,3,5,6-tetra(2-pyridyl)pyrazine for preconcentration and determination of trace amounts of cadmium by flame atomic absorption spectrometry. Toxicology and Industrial Health, 28(9), 771–778. https://doi.org/10.1177/0748233711422730.
Wen, X., Deng, Q., Wang, J., Yang, S., & Zhao, X. (2013). A new coupling of ionic liquid based-single drop microextraction with tungsten coil electrothermal atomic absorption spectrometry. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 105, 320–325.
Wierucka, M., & Biziuk, M. (2014). Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. TrAC Trends in Analytical Chemistry, 59, 50–58.
Zhong, W.-S., Ren, T., & Zhao, L.-J. (2016). Determination of Pb (lead), Cd (cadmium), Cr (chromium), Cu (copper), and Ni (nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. Journal of Food and Drug Analysis, 24(1), 46–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yağci, Ö., Akkaya, E. & Bakirdere, S. Nano-sized magnetic Ni particles based dispersive solid-phase extraction of trace Cd before the determination by flame atomic absorption spectrometry with slotted quartz tube: a new, accurate, and sensitive quantification method. Environ Monit Assess 192, 583 (2020). https://doi.org/10.1007/s10661-020-08548-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08548-z