Abstract
Chronic active antibody-mediated rejection (CAAMR) is a frequent cause of late graft loss. However, effective treatment for CAAMR after kidney transplantation has not yet been established. Here, we present the case of a kidney transplant recipient who recovered from CAAMR after administration of rabbit anti-thymocyte globulin. A 61-year-old man underwent ABO-compatible living-donor kidney transplantation for end-stage kidney disease; the kidney was donated by his wife. Five years after the transplant, the patient’s serum creatinine level and urine protein-to-creatinine ratio increased. He was subsequently diagnosed with CAAMR based on the kidney allograft biopsy and the presence of donor-specific human leukocyte antigen antibodies. Rabbit anti-thymocyte globulin treatment was administered following steroid pulse therapy. Subsequently, his serum creatinine levels and urine protein to creatinine ratio improved. There was also an improvement in the pathological findings seen on biopsy and the mean fluorescence intensity of donor-specific antibodies. In conclusion, this report describes the case of a kidney transplant recipient who developed CAAMR, treated using rabbit anti-thymocyte globulin. This strategy might be a viable treatment option for CAAMR after a kidney transplant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic active antibody-mediated rejection (CAAMR) is the most common cause of late graft loss in kidney transplant recipients. Treatments for acute antibody-mediated rejection, such as plasmapheresis, intravenous immunoglobulin (IVIg), and rituximab, have been proven to be effective [1]. Rabbit anti-thymocyte globulin (rATG) has been widely used as induction immunotherapy for kidney transplant recipients in the United States. However, there are few published reports about the treatment of CAAMR with rATG.
In this report, we present the case of a kidney transplant recipient who was treated with rATG and recovered from the kidney injury caused by CAAMR. This case report regarding the clinical effects of rATG will make an important contribution in establishing effective treatment strategies for CAAMR.
Case report
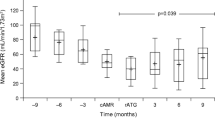
A 61-year-old male underwent an ABO-compatible living kidney transplant for end-stage kidney disease caused by diabetic nephropathy in February 2012. The donor was his wife. Human leukocyte antigen (HLA) loci of A, B, and DRB1 showed 3/6 mismatches. The lymphocyte cytotoxicity test and flow cytometry crossmatching were negative. Additionally, HLA antibody screening using a flow panel reactive antibody (Flow PRA™) screening test (One Lambda, CA, US) was negative. Extended-release tacrolimus (TAC-ER), mycophenolate mofetil (MMF), methylprednisolone (mPSL), and basiliximab were administered as induction immunosuppressive agents. The maintenance immunosuppressive regimen was 3 mg/day of TAC-ER (trough level: 3.3 ng/mL), 1000 mg/day of MMF, and 5 mg/day of prednisolone. Although the patient’s graft function remained stable (serum creatinine level 1.5 mg/dL and urine protein to creatinine ratio 0.1 g/gCr) until 5 years after surgery, an increase in the serum creatinine level (1.75 mg/dL) and urinary protein to creatinine ratio (1.4 g/gCr) were then observed. His blood pressure was stable (Systolic blood pressure: 120–130 mmHg), and he was not on any antihypertensive medication. Flow PRA™ screening tests indicated class I negative/class II positive (19.3%), and a LABscreen™ single antigen test (One Lambda, CA, US) showed donor-specific HLA antibodies (DSA) for DQB1*05:01. The mean fluorescence intensity (MFI) was 2916. A kidney allograft biopsy was performed. The microscopic findings indicated transplant glomerulopathy with severe glomerulitis and severe microvascular inflammation. Basement membrane duplexing and mesangial cell proliferation were observed in the glomerulus. Additionally, the electron microscopic analysis showed moderate multilayering of the peritubular capillary basement membrane. Hence, the patient was diagnosed with CAAMR (Banff 2013 classification: t1, i1, g3, v0, ptc3, C4d1, ci0, ct0, cg2, mm2, cv0, ah1, aah1 and ptcbm2). Increase in MMF dosage and rabbit anti-thymocyte globulin treatment following steroid pulse therapy was planned. The patient was first administered mPSL pulse therapy (500 mg/day) for 3 days. Subsequently, we administered rATG (1.5 mg/kg/day) for 7 days. Moreover, the dose of MMF was increased from 1000 to 1500 mg/day. Just before the start of treatment, his serum creatinine level increased to a maximum value of 2.07 mg/dL. Following this treatment, his serum creatinine levels improved to 1.75 mg/dL, and his urine protein to creatinine ratio improved to 0.20 g/gCr.
Three weeks after rATG treatment, the patient developed fever (38.0 °C), and polymorphonuclear leukocytes were detected on the cytomegalovirus (CMV) antigenemia test (positive cell count: 1508/1630). His fever abated after initiating treatment with valganciclovir. This treatment was continued for 30 days, after which the CMV antigenemia test was negative. Six months after treatment, microscopic findings revealed that transplant glomerulopathy and microvascular inflammation had improved from severe to moderate. The infiltration of inflammatory cells in the glomerulus was markedly improved. In addition, glomerulitis changed from global to segmental. Basement membrane duplexing of capillaries in the glomerulus and mesangial cells remained unchanged. No change in the moderate peritubular capillary basement membrane multilayering on electron microscopic analysis. (Banff 2013 classification: t0, i0, g2, v0, ptc3, C4d1, ci0, ct0, cg1, mm3, cv 0, ah1, aah2, and ptcbm2). Twelve months post-treatment, the MFI decreased to 661. Finally, at 2 years after rATG treatment, the patient’s kidney function was stable (serum creatinine levels 1.6 mg/dL and urine protein to creatinine ratio 0.20 g/gCr). Pathological findings had not worsened, and the electron microscopic analysis showed a slight improvement in the peritubular capillary basement membrane multilayering. (Banff classification [2017]: i0, t0, v0, g3, ptc2, ti0, i-IFTA0, C4d1 [3% of ptc], cg1b, mm0, ah3, aah3 cv0, ci0, ct0 and ptcbm1).
Discussion
Currently, the published evidence regarding a successful treatment strategy for CAAMR is scarce. Patients with acute antibody-mediated rejections are usually treated with drugs, such as rituximab, plasmapheresis, IVIg, complement inhibitor, and Imlifidase. Similar treatments have been used in cases with CAAMR. However, these treatments are not yet approved under the Japanese insurance system.
In contrast, rATG is approved under the Japanese insurance system for the treatment of acute rejection. rATG is a polyclonal gamma immunoglobulin against human thymocytes produced in rabbits. rATG has a high affinity for T cell surface antigens (CD2, CD3, CD4, CD5, CD7, CD8, CD25, and TCRαβ) and leukocyte surface antigen (CD11a). It is considered to have a T cell-damaging effect through a “complement-dependent pathway,” “apoptosis,” and “opsonin action” [2, 3]. Additionally, rATG also has antibodies against natural killer cells, B cells, plasma cells, adhesion molecules between cells, and chemokine receptors [4]. Therefore, it might have various immunosuppressive effects and cellular immunity.
Pascual et al. indicated that using rATG as an induction immunosuppressive therapy reduces the development of de novo DSA and the rate of acute antibody-mediated rejection [5]. Zand et al. confirmed that rATG leads to apoptosis of B cells and plasma cells in vitro [6]. Therefore, rATG might have a therapeutic effect on antibody-mediated rejection.
There are a few published reports of rATG treatment for antibody-mediated rejection. Cihan et al. reported that rATG treatment for patients with chronic antibody-related rejection significantly improved the estimated glomerular rate (eGFR). In addition, DSA disappeared in 50% of the cases [7]. Furthermore, Nanmoku et al. reported that rATG treatment for patients with CAAMR significantly improved the serum creatinine and urinary protein levels [8]. Hence, we selected rATG treatment following steroid pulse therapy for CAAMR. In this case, the possibility of improvement only due to the steroid pulse therapy cannot be denied. However, the pathological findings showed microvascular inflammation, and we were concerned about the probability of irreversible deterioration of the kidney function without additional treatment. Hence, we planned rATG following steroid pulse therapy in advance.
In the present case, microvascular inflammation (g and ptc) and C4d positivity persisted after the administration of rATG. Although the percentage of glomeruli with transplant glomerulitis did not improve markedly, for individual glomerulus, the infiltration of inflammatory cells was improved after treatment. Furthermore, considering that the patient’s kidney function improved, the acute damage caused by antibody-mediated rejection could have been treated. On the other hand, basement membrane duplexing and mesangial cell proliferation in the glomerulus continued after the treatment. We think that acute changes may be transitioning to chronic changes.
It has also been reported that transplant glomerulopathy did not improve with treatment using agents, such as rituximab, plasmapheresis, IVIg, and others. Billing et al. reported that the kidney function in pediatric patients diagnosed with CAAMR was successfully maintained after treatment with IVIg and rituximab. However, in a subgroup analysis, the therapeutic effect was found to be insufficient in patients with transplant glomerulopathy [9, 10]. Bchelet et al. reported that treatment using IVIg and rituximab in patients with CAAMR with severe transplant glomerulopathy did not improve the course of transplant glomerulopathy. In contrast, it was found to increase the complications significantly [11]. Moreso et al. demonstrated that there were no significant differences in the eGFR, urine protein levels, and MFI between patients with CAAMR treated with IVIg and rituximab and those treated with placebo [12]. Therefore, IVIg and rituximab treatment for patients with CAAMR who have transplant glomerulopathy might be ineffective. Piñeiro et al. conducted a retrospective study in which rituximab, plasma exchange, and immunoglobulins were found to be ineffective in treating chronic active ABMR [13].
Similarly, it might be difficult to see a dramatic improvement in microvascular inflammation with rATG treatment once it has developed. However, considering that the level of MFI decreased and the patient's kidney function has remained stable for a long duration, we think that it may have some effect on humoral immunity and not only on cellular immunity. The effects of rATG are diverse, and it is difficult to clarify where the effects actually occurred. Therefore, we think that rATG administration might be a treatment option for CAAMR. We think that this case might make an important contribution to the treatment of CAAMR using rATG in the future because there is scanty literature about the pathological findings of renal biopsy during the post-treatment long-term follow-up of approximately 2 years.
Common adverse events associated with rATG include hypersensitivities, such as cytokine release syndrome, serum sickness, febrile neutropenia, thrombocytopenia, infection, lymphoproliferative disease, interstitial pneumonia, and liver damage. In cases of infection, it is necessary to pay particular attention to those associated with CMV [14]. Prophylactic administration of antiviral drugs for 6 weeks after rATG treatment is recommended in some countries [15], although there is no insurance coverage for this treatment in Japan. Therefore, close monitoring with CMV antigenemia test might be necessary after rATG treatment (Figs. 1, 2).
In conclusion, we presented the case of a renal transplant recipient who developed CAAMR and was treated with rATG following steroid pulse therapy. This case makes a significant contribution in establishing a strategy that rATG following steroid pulse therapy might be a treatment option for CAAMR. However, more research is necessary to further establish optimal treatment strategies.
Abbreviations
- CAAMR:
-
Chronic active antibody-mediated rejection
- CMV:
-
Cytomegalovirus
- DSA:
-
Donor-specific antibodies
- eGFR:
-
Estimated glomerular filtration rate
- HLA:
-
Human leukocyte antigen
- IVIg:
-
Intravenous immunoglobulin
- MFI:
-
Mean fluorescence intensity
- MMF:
-
Mycophenolate mofetil
- mPSL:
-
Methylprednisolone
- PRA:
-
Panel reactive antibody
- rATG:
-
Rabbit anti-thymocyte globulin
- TAC-ER:
-
Extended-release tacrolimus
References
Bartel G, Schwaiger E, Bohmig GA. Prevention and treatment of alloantibody-mediated kidney transplant rejection. Transpl Int. 2011;24:1142–55.
Genestier L, Fournel S, Flacher M, et al. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91:2360–8.
Préville X, Flacher M, LeMauff B, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71:460–8.
Mourad G, Morelon E, Noël C, et al. The role of thymoglobulin induction in kidney transplantation: an update. Clin Transpl. 2012;26:E450–64.
Pascual J, Zuckermann A, Djamali A, Hertig A, Naesens M. Rabbit antithymocyte globulin and donor-specific antibodies in kidney transplantation: a review. Transpl Rev. 2016;30:85–91.
Zand MS, Vo T, Huggins J, et al. Polyclonal rabbit anti-thymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005;79:1507–15.
Cihan Y, Kanzelmeyer N, Drube J, et al. Rabbit anti-human thymocyte immunoglobulin for the rescue treatment of chronic antibody-mediated rejection after pediatric kidney transplantation. Pediatr Nephrol. 2017;32:2133–42.
Nanmoku K, Shinzato T, Kubo T, Shimizu T, Yagisawa T. Effect of rabbit antithymocyte globulin on acute and chronic active antibody-mediated rejection after kidney transplantation. Transpl Proc. 2019;51:2602–5.
Billing H, Rieger S, Susal C, et al. IVIG and rituximab for treatment of chronic antibody-mediated rejection: a prospective study in pediatric renal transplantation with a 2-year follow-up. Transpl Int. 2012;25:1165–73.
Billing H, Rieger S, Ovens J, et al. Successful treatment of chronic antibody-mediated rejection with IVIG and rituximab in pediatric renal transplant recipients. Transplantation. 2008;86:1214–21.
Bachelet T, Nodimar C, Taupin JL, et al. Intravenous immunoglobulins and rituximab therapy for severe transplant glomerulopathy in chronic antibody-mediated rejection: a pilot study. Clin Transpl. 2015;29:439–46.
Moreso F, Crespo M, Ruiz JC, et al. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: a multicenter, prospective, randomized, double-blind clinical trial. Am J Transpl. 2018;18:927–35.
Gaston J, Erika D, Manel S, et al. Rituximab, plasma exchange and immunoglobulins: an ineffective treatment for chronic active antibody-mediated rejection. BMC Nephrol. 2018;19:261.
Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48:772–86.
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transpl. 2009;9:1–155.
Acknowledgements
We are grateful to Dr. Kyo M. for the discussions of pathological findings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tanaka, R., Tsutahara, K., Inoguchi, S. et al. Clinical effect of rabbit anti-thymocyte globulin for chronic active antibody-mediated rejection after kidney transplantation. CEN Case Rep 11, 79–83 (2022). https://doi.org/10.1007/s13730-021-00633-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-021-00633-7