Abstract
With increased use of sodium-glucose co-transporter 2 (SGLT2) inhibitors as antidiabetic agents, the risk of serious fungal urinary tract infection (UTI) may be increased. We present the case of a 67-year-old Caucasian female who was admitted for emphysematous pyelitis and found to have a fungal ball in the renal pelvis. Candida glabrata was cultured and the patient was managed with percutaneous nephrostomy tube placement and antifungal treatment. The fungal ball persisted and required surgical removal with ureteroscopy and basket extraction. Fungal balls can be a difficult sequelae of UTIs requiring a combination of antifungal and surgical intervention for definitive management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jardiance, or empagliflozin, is a sodium-glucose co-transporter 2 (SGLT2) inhibitor approved for patients with type 2 diabetes. SGLT2 inhibitors act by decreasing glucose reabsorption in the proximal tubule of the kidney, leading to glucosuria and improved glycemic control. Empagliflozin has been shown to reduce glycated hemoglobin (HbA1c) levels as a monotherapy, combined with metformin, or as add-on therapy. The United State Food and Drug Administration (FDA) warnings for empagliflozin include hypotension due to volume contraction, ketoacidosis, impairment of renal function, urosepsis, and genital mycotic infections.
During phase 3 trials, empagliflozin was associated with increased frequency of urinary tract infections (UTIs) in both men and women (7.6% vs 9.3% with placebo or empagliflozin 10 mg, respectively). The risk was higher in patients with a history of chronic/recurrent UTIs. Female patients or patients > 75 years of age taking empagliflozin are also at increased risk of UTIs. Empagliflozin-monotherapy was associated with higher rates of genital mycotic infections compared to placebo (5.4% vs. 1.5% and 3.1% vs. 0.4%, for women and men, respectively) [1]. Fournier’s gangrene was also noted to be a rare complication associated with empagliflozin. Retrospective analysis of the FDA adverse event reporting system identified 12 cases of Fournier’s gangrene from 2013 to 2018 in patient’s taking SGLT2 inhibitors, whereas only 6 cases of Fournier’s gangrene were found in patient taking other antidiabetic agents from 1984 to 2019 [2].
Notably, fungal urinary tract infections were not thought to be associated with empagliflozin despite the theoretical risk of glucosuria promoting fungal growth. Herein, a difficult case of funguria and fungal bezoar/ball in a patient taking empagliflozin is presented.
Case presentation
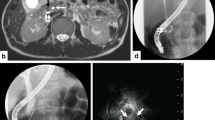
A 67-year-old woman with a past medical history of diabetes mellitus type 2, kidney stone requiring surgical intervention 10 years prior, hypertension, morbid obesity, and diabetic neuropathy presented to an outside hospital for gross hematuria. She reported increased urinary frequency but denied dysuria, back pain, nausea, vomiting, diarrhea, shortness of breath, and chest pain. Her HbA1c at her last primary care visit was 7.9%, and home medications include empagliflozin 25 mg, atorvastatin, insulin glargine, insulin lispro, ramipril, and liraglutide. She began taking empagliflozin 11 months prior to presenting to the emergency department without complications. In the emergency department, she was afebrile and hypertensive. Her physical exam was unremarkable, including a soft, nontender abdomen without suprapubic or flank pain. A urine culture and abdominal CT were obtained. The culture grew proteus mirabilis and the CT demonstrated emphysematous pyelitis with a 5 mm stone in the right ureteropelvic junction with moderate hydronephrosis (Fig. 1).
The patient underwent right ureteral stenting and began a 14-day course of ciprofloxacin. Subsequent ureteroscopy for stone removal was attempted 5 days later. Holmium laser lithotripsy was performed and pyeloscopy demonstrated a heterogenous appearing mass in the renal pelvis. Biopsy of the mass confirmed a fungal ball with numerous yeasts and gram-positive bacterial colonies. At this point, the patient began a course of oral fluconazole, continued ciprofloxacin, and was referred to Indiana University for further management.
The patient had her stent removed and was scheduled for a CT urogram as an outpatient, but prior to the appointment presented to the emergency department with severe right flank pain and intractable nausea and vomiting. She was febrile to 101.3°F, but otherwise hemodynamically stable. Her WBC count was 15.5 × 103/µL (Table 1). A CT abdomen/pelvis demonstrated right hydroureteronephrosis with perinephric stranding and no obstructing stone. She was admitted and underwent percutaneous nephrostomy (PCN) drain placement. The drain placement was successful, but she became hypotensive and was admitted to the SICU for resuscitation. IV fluids, amphotericin B, piperacillin-tazobactam, and vancomycin were initiated. Blood and urine cultures grew Candida glabrata and extended spectrum beta-lactamase (ESBL) E. coli. Urine culture from the nephrostomy tube grew lactobacillus species and ESBL E. coli, and she was transitioned to ertapenem and micafungin. Chest X-ray, ocular exam, and oral exam did not demonstrate evidence of infection. The patient improved on the therapy and follow-up CT urogram demonstrated a hyperdense mass in the renal pelvis and perinephric fat stranding. She remained stable and was discharged on hospital day 7. A 5-day course of amphotericin B infusion was completed during the inpatient stay and micafungin was continued for 14 days total. Empagliflozin was not restarted upon discharge due to concern that it may have contributed to fungal bezoar development. Further management of the fungal bezoar was deferred pending outpatient follow-up and completion of antibiotic course.
Approximately 1 month later, she was readmitted with intractable diarrhea and tested positive for Clostridium difficile. She began a course of oral vancomycin. A urine culture again grew ESBL E. coli, but blood cultures showed no growth. She was restarted on ertapenem but was not started on micafungin because fungal urine and blood cultures were negative. During this admission, a CT abdomen/pelvis and antegrade nephrostogram demonstrated a 3–4 cm proximal ureter filling defect consistent with a dislodged fungal ball (Fig. 2). The patient remained afebrile after starting antimicrobial agents and antegrade amphotericin B irrigations via nephrostomy tube. Due to the negative fungal urine and blood cultures, further IV antifungal therapy was not used. An outline of the clinical course is seen in Fig. 4.
As the consulting Urology team, we discussed a percutaneous removal through the established nephrostomy tract versus a ureteroscopic approach. Approximately 2 weeks after discharge, she was taken to the OR and we began with ureteroscopy using the Dornier Axis (Dornier, Germany) disposable flexible ureteroscope. The ureter was widely dilated due to obstruction from the large fungal ball and, therefore, we felt ureteroscopic extraction with a large sheath was feasible. A 13/15 F ureteral access sheath (Boston Scientific, Boston, MA, USA) was placed in atraumatic fashion. Large amounts of fungus ball were removed using the 1.9F ZeroTip Basket (Boston Scientific, USA) (Fig. 3). A thorough pyeloscopy/ureteroscopy was performed at the end of case to ensure there were no residual fungal bezoars. The PCN tube was removed at the conclusion of the case and a 6 Fr × 24 cm ureteral stent on a dangle was placed. The patient tolerated the procedure well and her infections have resolved.
Discussion and literature review
Fungal urinary tract infections include: cystitis, pyelitis, pyelonephritis, and obstructive uropathy. Fungal UTIs are more common in patients with diabetes mellitus, those with exposure to antibiotic/corticosteroid therapy, and urinary stasis. Glucosuria from SGLT2 inhibitors has also been shown to increase the risk of fungal UTIs in proportion to both treatment duration and dosage in mice [3]. Proposed mechanisms include increased biofilm formation, higher hemolytic enzymatic activity, and more adhesive capabilities mediated through agglutinin-like sequence proteins [4]. Fungal bezoars (i.e., balls) are a rare complication of fungal UTI and most commonly occur in the setting of disseminated fungal infection. However, ascending infection, such as in this patient, have been reported [5]. While large studies of fungal bezoars are not present in the literature, case reports and case series suggest that diabetes, particularly uncontrolled diabetes, immunosuppression, and decreased urinary flow such as from an obstructing stone are risk factors. Immunosuppression is associated with increased risk of fungemia, while obstruction or poor urinary flow is more consistent with ascending infection. Treatment of fungal bezoars can be difficult and typically require medical and surgical intervention, including systemic antifungal treatment, antegrade antifungal irrigations through nephrostomy tracts, or even surgical removal via a percutaneous or ureteroscopic approach [6]. Percutaneous access through a PCN tube allows the surgeon to easily debulk or remove proximal fungal infections [7]. In our case, because of the degree of ureteral dilation from the obstructing fungal ball, a ureteroscopic approach with a large ureteral access sheath was attempted (Fig. 4).
C. glabrata isolates are frequently resistant to fluconazole, and other antifungals such as micafungin have poor urinary penetration. Therefore, The Infectious Disease Society of America currently recommends antegrade irrigation of nephrostomy tubes using amphotericin B as treatment for fungal bezoars (strong recommendation, low level of evidence), but a recommended treatment length is not given [8]. Different case reports have utilized treatment from 5–6 days of irrigation and 14–19 days of IV antifungal therapy [7]. Amphotericin B is nephrotoxic through direct damage to the proximal tubules (systemic or irrigation), therefore, antegrade irrigations in the setting of negative fungal cultures could have led to unnecessary renal injury in our patient [9]. Furthermore, since our patient was clinically stable, we felt early intervention with definitive fungal bezoar removal in a single surgical setting offers an alternative to prolonged antibiotic/antifungal therapy without systemic side effects. Surgical removal was effective for this patient and she has not had any recurrence of funguria despite months of follow-up.
Conclusion
Although a rare complication, we present a difficult case of fungal genitourinary infection requiring invasive management in a patient taking empagliflozin. As the frequency of SGLT2 inhibitor use increases in patients with diabetes, recognizing such serious complications promptly will continue to be important for the management of these patients. After developing a fungal complication, empagliflozin should be discontinued alternative agents for glucose control should be selected in collaboration with the patients’ primary care physician or endocrinologist.
References
Levine MJ. Empagliflozin for type 2 diabetes mellitus: an overview of phase 3 clinical trials. Curr Diabetes Rev. 2017;13(4):405–23. https://doi.org/10.2174/1573399812666160613113556.
FDA. FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes. US Food and Drug Administration, Drug Safety Communications. 2018. https://www.fda.gov/media/115602/download.
Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. infections in patients with diabetes mellitus. J Clin Med. 2019. https://doi.org/10.3390/jcm8010076.
Nett JE, Lepak AJ, Marchillo K, Andes DR. Time course global gene expression analysis of an in vivo Candida biofilm. J Infect Dis. 2009;200(2):307–13. https://doi.org/10.1086/599838.
Zeineddine N, Mansour W, El Bitar S, Campitelli M, Mobarakai N. Fungal bezoar: a rare cause of ureteral obstruction. Case Rep Infect Dis. 2017;2017:6454619. https://doi.org/10.1155/2017/6454619.
Das MK, Rangrajan Pakshi R, Kalra S, Elumalai A, Theckumparampil N. Fungal balls mimicking renal calculi: a zebra among horses. J Endourol Case Rep. 2019;5(4):167–70. https://doi.org/10.1089/cren.2019.0012.
Viswambaram P, Misko J, Rawlins M, Clark S, Dyer J, Hayne D. Multi-route antifungal administration in the management of urinary Candida glabrata bezoar. Urol Case Rep. 2020;33: 101275. https://doi.org/10.1016/j.eucr.2020.101275.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-50. https://doi.org/10.1093/cid/civ933.
Ashley ESD, Lewis R, Lewis JS, Martin C, Andes D. Pharmacology of systemic antifungal agents. Clin Infect Dis. 2006;43(1):S28–39. https://doi.org/10.1086/504492.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Woloshuk, A., Lee, M., Assmus, M. et al. A case of ureteral fungal mass removal in a patient taking empagliflozin. CEN Case Rep 10, 603–607 (2021). https://doi.org/10.1007/s13730-021-00616-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-021-00616-8