Abstract
In recent times, increasing reports of exit site infections (ESI) in peritoneal dialysis (PD) patients related to environmentally acquired atypical organisms, such as nontuberculous mycobacterium (NTM), have been reported in the literature. Among these NTM, Mycobacterium abscessus (M. abscessus) is unique and is associated with high morbidity and treatment failure rates. The international society of PD guidelines suggests individualizing therapeutic options for NTM-related ESI. Moreover, the guidelines encourage simultaneous catheter removal and reinsertion (SCRR) in isolated ESI, not responding to antimicrobial therapy to avoid PD interruptions. Physicians should be aware of the limitations of such approaches as delay in appropriate PD catheter intervention can be fraught with complications in patients with M. abscessus ESI. We report an M. abscessus ESI in a PD patient who underwent SCRR in conjunction with targeted antimicrobial therapy, and developed M. abscessus peritonitis requiring PD catheter removal and conversion to hemodialysis. The patient also developed ESI at the new exit site long after the PD catheter was removed, requiring prolonged antimicrobial therapy. Our case, taken together with available published case reports, highlights the futility of the SCRR approach towards the M. abscessus ESI and makes the cases for early PD catheter removal in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritoneal dialysis (PD) technique failure is the second largest cause of attrition on PD after death. Infections and catheter malfunction comprise a vast majority of preventable PD technique failures [1, 2]. Avoiding transfer to hemodialysis during such complications is important as it is cost-effective and patient-centric but also, once on hemodialysis, most patients do not return to PD [2, 3]. Simultaneous catheter removal and reinsertion (SCRR) can allow patients to continue PD without interruption, with several reports showing successful outcomes for certain mechanical and infectious complications [4].
The current International Society for Peritoneal dialysis (ISPD) guidelines suggest removing PD catheter in isolated exit site infections/tunnel infections (ESI/TI) without peritonitis that persist after 3 weeks of effective antimicrobial therapy [5]. They further recommend considering SCRR in these circumstances to minimize PD interruptions and alleviate the need for temporary hemodialysis. However, this approach may not be generalizable to all ESI/TI. Recognizing conditions where such an approach is not feasible is important to avoid potential severe complications secondary to infection.
In recent times, increasing reports of ESI/TI related to environmentally acquired atypical organisms, such as nontuberculous mycobacterium (NTM), have been reported in the literature. The ISPD guidelines suggest individualizing therapies for such NTM ESI. We recently reported that salvaging PD catheter with a conservative 'antimicrobial alone' approach is futile in one such NTM, M. abscessus ESI. The patient had a recurrence of M. abscessus ESI after stopping the antimicrobials necessitating removal of PD catheter [6]. We now report a case of M. abscessus ESI in whom we performed SCRR in an attempt to continue PD without interruption. Our patient developed secondary M. abscessus peritonitis shortly after the procedure and ESI at the new contralateral exit site, requiring prolonged antimicrobial therapy and permanent transfer to hemodialysis. The current report aims to demonstrate that SCRR is not a feasible option in an otherwise uncomplicated M. abscessus ESI.
Case discussion
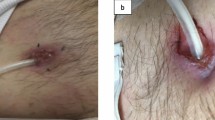
A 73-year-old man with a history of end-stage renal disease (ESRD) secondary to diabetes on PD for 5 years presented with granulating right-sided PD exit site over 1 month (Fig. 1a, b). Since starting the PD, the patient had moderately controlled diabetes with HbA1c values ranging between 7 and 9% and was on gentamycin based exit site care. The patient did not have any systemic symptoms, including abdominal pain, fever, or weight loss. Examination revealed stable hemodynamics without signs of peritonitis and an erythematous granulating, circumferential ulcer at the catheter exit site. No discharge, tenderness or erythema was noted on the tunnel site. PD fluid showed a white blood cell count (WBC) of 6/mm3, and cultures were negative for any growth. The exit site cultures yielded M. abscessus confirming isolated ESI.
Based on the strong patient preference to avoid hemodialysis and the multi-disciplinary team's opinions, a decision was taken to initiate the patient on an antimicrobial regimen guided by the sensitivity report (oral azithromycin and linezolid, and intraperitoneal amikacin and meropenem) and proceed with SCRR. Intraoperatively, there were no signs of tunnel infection during the original PD catheter removal, confirming isolated ESI. A new PD catheter was inserted on the contralateral side (left side) using the well-described method of inserting a new catheter (clean step) before removal of the old catheter (dirty step) in patients with PD catheter infections [4]. Postoperatively, the patient was continued on dose-adjusted antimicrobials and high-dose diuretic regimen with close monitoring for dialysis indications. Low-volume, supine PD was restarted 1 week after the SCRR procedure.
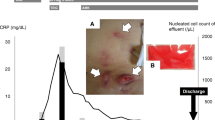
The patient developed abdominal pain with rebound tenderness 2 weeks after SCRR. PD fluid showed a WBC count of 680/mm3 with 45% neutrophils raising the suspicion for peritonitis. Urgent removal of PD catheter was arranged, and the patient was switched to hemodialysis. The peritoneal fluid cultures were positive for M. abscessus, and the patient was continued on the antimicrobial agents. The postoperative course on the antimicrobial regimen was complicated with the development of new ulceration and serosanguinous discharge at the contralateral left-side PD site after 10 weeks (Fig. 1c). Though wound cultures at the new exit site did not yield any organism, M. abscessus ESI was suspected based on the prolonged wound healing. His hemoglobin A1c during the course of treatment varied from 7 to 8.5%. The patient completed 8 months of intravenous antimicrobials, amikacin, and meropenem. His oral medications, including azithromycin and linezolid, were discontinued after 11 months, 1 month beyond the full clinical resolution of the ESI (Fig. 1d). The complete timeline and course of the ESI infection and treatment are depicted in Fig. 2.
Discussion
Equivalent survivals with better patient-centered and health services outcomes have led to the desire for increasing the use of PD as a modality for renal replacement therapy in several countries, including the US [7]. Maintaining a high fraction of the ESRD population on PD will require addressing the high rates of PD technique failure [8]. SCRR allows for continuing PD not only in catheter-related mechanical PD complications but also in infectious complications, especially the isolated ESI/TI [4, 5]. While SCRR may be appropriate for the vast majority of ESI related to the conventional organisms, our report argues that ESI/TI related to atypical organisms such as M. abscessus should not be managed by this approach.
Prophylactic use of topical antimicrobial agents such as gentamicin and mupirocin has substantially reduced the incidence of ESI caused by conventional gram-positive and gram-negative organisms. However, several recent reports including our report, show that regular use of gentamycin is associated with an increased incidence of ESI related to certain environmental organisms commonly found in soil, and water, such as NTM, pseudomonas, or enterobacteriaceae [5, 9, 10]. NTM causing ESI typically belong to Runyon class IV, rapidly growing organisms consisting of M. fortuitum, M. chelonae, and M. abscessus [11]. While of the same class, these organisms have significantly different clinical behavior and response patterns to antimicrobials. M. fortuitum and M. chelonae exit site infections may be amenable to conservative approaches with antibiotic therapy with or without surgical debridement [12, 13]. M. abscessus, on the contrary, is a virulent organism intrinsically resistant to many antimicrobials and can develop acquired resistance to many drugs. These characteristics render M. abscessus to be most pathogenic and extremely challenging to treat organism amongst the NTM, with treatment failures reported as high as 60–70% [12, 14, 15].
The treatment recommendations for M. abscessus PD catheter-related infections by professional organizations are not consistent. The Infectious Diseases Society of America (IDSA) guidelines recommend combination therapy of oral (clarithromycin or azithromycin) and parenteral (Amikacin, cefoxitin, or Imipenem) antimicrobials for 4–6 months and suggest removal of the foreign body [12]. The ISPD guidelines recommend removing PD catheters for NTM-related peritonitis but are vague for NTM-related ESI and advise for individualizing therapeutic options in ESI/TI [5, 16]. We analyzed isolated ESI/TI cases secondary to M. abscessus reported in the literature (Table 1), broadly categorizing them into three groups by the primary approach to catheter management (antimicrobials alone, antimicrobials with removal of PD catheter and transitioning to HD, antimicrobials with SCRR).
To date, we found that there are only seven reported M. abscessus ESI/TI cases that were managed without primary PD catheter removal (Table 1). Six cases were treated with antimicrobial therapy alone, whereas one patient deemed too sick for treatment died on palliative care. The outcomes in the six cases were heterogeneous but suboptimal. Four patients developed substantial complications, including prolonged wound healing, need for surgical deroofing, development of secondary peritonitis, and either required permanent transfer to hemodialysis or died. To date, only two cases have been reported to have full resolution of M. abscessus isolated ESI without interruption of PD though; one of these was treated for only 8 weeks of therapy, raising the concerns for skin colonization rather than an ESI in this report.
Similarly, we found a total of four case reports of M. abscessus ESI/TI, including our case that were managed by antimicrobials along with SCRR. We found that this approach failed in all reported cases, including the current one, with the development of varying complications such as chronic sinus formation, bacteremia, disseminated cutaneous infections, peritonitis, and ileus. All cases eventually required removal of the newly inserted PD catheter and permanent transfer to hemodialysis, with one reported death [17,18,19]. While prior reports had not initiated targeted antimicrobials prior to the SCRR procedure, starting antimicrobials prior to the SCRR procedure did not prevent the spread of the infection to the peritoneum in our patient. Additionally, prior reports have shown that M. abscessus ESI/TI can spread through the para-catheter route to cause peritonitis [20]. Together, these results suggest that while surgical interventions such as cuff shaving, deroofing, and SCRR may have promising outcomes in PD technique survival in otherwise uncomplicated ESI, these approaches appear inappropriate for M. abscessus ESI/TI [4, 5, 21].
Against these, we found a total of eight case reports with M. abscessus ESI/TI, where the initial approach involved prompt removal of the PD catheter and transition to hemodialysis. Among these, one patient refused further treatment and chose palliative care. The remaining seven cases were able to successfully resolve M. abscessus ESI/TI without any residual complications. From the perspectives of PD failure, two were permanently transferred to hemodialysis whereas, five of the total eight patients eventually returned to PD, ranging 6 weeks–6 months after the ESI resolution. No disseminated infections or additional mortality were reported in these patients.
Our report, in conjunction with the reported literature, highlights several important points. Isolated M. abscessus ESI/TI are challenging to treat and require prolonged antimicrobial therapy. It further indicates that a conservative (antimicrobial alone) and semi-conservative (SCRR) approach towards these ESI/TI may not be optimal, even when the infection is isolated or shows an early clinical response. Patients who underwent early catheter removal and temporary transition to hemodialysis with close follow-up appeared to have a favorable course, with greater probabilities for return to PD. Thus, caution should be exercised in generalizing the current ISPD ESI guidelines for atypical organisms like M. abscessus.
In conclusion, M. abscessus ESI/TI are associated with significant morbidity and mortality. We suggest considerations for early PD catheter removal with transfer to hemodialysis, without simultaneous PD catheter insertion in addition to targeted antimicrobial therapy. Patients can be reinitiated on PD once clinical resolution is achieved. Considering the rising trends for the NTM ESI, we believe that these considerations should shape the future ISPD ESI guidelines.
References
Lan PG, Clayton PA, Johnson DW, Mcdonald SP, Borlace M, Badve SV, et al. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: Proposal for a standardized definition of technique failure. Perit Dial Int. 2016;36(6):623–30. https://doi.org/10.3747/pdi.2015.00218.
Shen JI, Mitani AA, Saxena AB, Goldstein BA, Winkelmayer WC. Determinants of peritoneal dialysis technique failure in incident US patients. Perit Dial Int. 2013;33(2):155–66. https://doi.org/10.3747/pdi.2011.00233.
Jaar BG, Plantinga LC, Crews DC, Fink NE, Hebah N, Coresh J, et al. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: a prospective study. BMC Nephrol. 2009;10(1):3. https://doi.org/10.1186/1471-2369-10-3.
Crabtree JH, Siddiqi RA. Simultaneous catheter replacement for infectious and mechanical complications without interruption of peritoneal dialysis. Perit Dial Int. 2016;36(2):182–7. https://doi.org/10.3747/pdi.2014.00313.
Szeto C-C, Li PK-T, Johnson DW, Bernardini J, Dong J, Figueiredo AE, et al. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int. 2017;37(2):141–54. https://doi.org/10.3747/pdi.2016.00120.
Chamarthi G, Kamboj M, Archibald LK, Shukla AM. Mycobacterium abscessus exit-site infection in peritoneal dialysis patients: should we ever aim to salvage the catheter? CEN Case Rep. 2020. https://doi.org/10.1007/s13730-020-00506-5.
Sloan CE, Coffman CJ, Sanders LL, Maciejewski ML, Lee S-YD, Hirth RA, et al. Trends in peritoneal dialysis use in the United States after medicare payment reform. Clin J Am Soc Nephrol. 2019;14(12):1763–72. https://doi.org/10.2215/cjn.05910519.
Flanagin EP, Chivate Y, Weiner DE. Home dialysis in the United States: a roadmap for increasing peritoneal dialysis utilization. Am J Kidney Dis. 2020;75(3):413–6. https://doi.org/10.1053/j.ajkd.2019.10.013.
Lo MW, Mak SK, Wong YY, Lo KC, Chan SF, Tong GM, et al. Atypical mycobacterial exit-site infection and peritonitis in peritoneal dialysis patients on prophylactic exit-site gentamicin cream. Perit Dial Int. 2013;33(3):267–72. https://doi.org/10.3747/pdi.2011.00184.
Pierce DA, Williamson JC, Mauck VS, Russell GB, Palavecino E, Burkart JM. The effect on peritoneal dialysis pathogens of changing topical antibiotic prophylaxis. Perit Dial Int. 2012;32(5):525–30. https://doi.org/10.3747/pdi.2011.00183.
Renaud CJ, Subramanian S, Tambyah PA, Lee EJ. The clinical course of rapidly growing nontuberculous mycobacterial peritoneal dialysis infections in Asians: A case series and literature review. Nephrology (Carlton). 2011;16(2):174–9. https://doi.org/10.1111/j.1440-1797.2010.01370.x.
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. https://doi.org/10.1164/rccm.200604-571ST.
Jo A, Ishibashi Y, Hirohama D, Takara Y, Kume H, Fujita T. Early surgical intervention may prevent peritonitis in cases with Tenckhoff catheter infection by nontuberculous mycobacterium. Perit Dial Int. 2012;32(2):227–9. https://doi.org/10.3747/pdi.2011.00080.
Johansen MD, Herrmann J-L, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18(7):392–407. https://doi.org/10.1038/s41579-020-0331-1.
Lee M-R, Sheng W-H, Hung C-C, Yu C-J, Lee L-N, Hsueh P-R. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015. https://doi.org/10.3201/2109.141634.
Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36(5):481–508. https://doi.org/10.3747/pdi.2016.00078.
Mooren V, Bleeker MWP, van Ingen J, Hermans MHA, Wever PC. Disseminated Mycobacterium abscessus infection in a peritoneal dialysis patient. IDCases. 2017;9:6–7. https://doi.org/10.1016/j.idcr.2017.05.001.
Kameyama H, Mori Y, Kimura T, Sugishita C, Adachi T, Sonomura K, et al. A case report of Mycobacterium abscessus peritonitis in a peritoneal dialysis patient. Ther Apher Dial. 2007;11(6):449–51. https://doi.org/10.1111/j.1744-9987.2007.00526.x.
Yoshimura R, Kawanishi M, Fujii S, Yamauchi A, Takase K, Yoshikane K, et al. Peritoneal dialysis-associated infection caused by Mycobacterium abscessus: a case report. BMC Nephrol. 2018;19(1):341. https://doi.org/10.1186/s12882-018-1148-2.
Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury*. Crit Care Med. 2013;41(2):464–71. https://doi.org/10.1097/ccm.0b013e31826ab3a1.
Meng C, Beco A, Oliveira A, Pereira L, Pestana M. Peritoneal dialysis cuff-shaving-a salvage therapy for refractory exit-site infections. Perit Dial Int. 2019;39(3):276–81. https://doi.org/10.3747/pdi.2018.00193.
Maeda Y, Uno T, Yoshida A, Takahashi A, Inaba N, Shiigai T. Nontuberculous mycobacterial peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis. J Rural Med. 2009;4(2):75–9. https://doi.org/10.2185/jrm.4.75.
Marzuk SM, Rohit A, Nagarajan P, Nzana V, Katuraga VM, Parthasarathy R, et al. An unusual case of unresolving tunnel infection in a patient on continuous ambulatory peritoneal dialysis. Indian J Med Microbiol. 2018;36(4):600–2. https://doi.org/10.4103/ijmm.IJMM_18_425.
Tsai S-F. Catheter related infection due to Mycobacterium abscessus in a patient under peritoneal dialysis. Ther Apher Dial. 2013;17(3):349–50. https://doi.org/10.1111/1744-9987.12005.
Hibi A, Kasugai T, Kamiya K, Ito C, Kominato S, Mizuguchi K, et al. Peritoneal dialysis-associated catheter infection caused by Mycobacterium abscessus in an elderly patient who was successfully treated with catheter removal. CEN Case Rep. 2017;6(2):175–9. https://doi.org/10.1007/s13730-017-0270-5.
Ellis EN, Schutze GE, Wheeler JG. Nontuberculous mycobacterial exit-site infection and abscess in a peritoneal dialysis patient. A case report and review of the literature. Pediatr Nephrol. 2005;20(7):1016–8. https://doi.org/10.1007/s00467-005-1870-4.
Inoue H, Washida N, Morimoto K, Shinozuka K, Kasai T, Uchiyama K, et al. Non-tuberculous mycobacterial infections related to peritoneal dialysis. Perit Dial Int. 2018;38(2):147–9. https://doi.org/10.3747/pdi.2017.00172.
Funding
A. M. Shukla reports the ongoing grant support from the Department of Veterans Affairs. The grant support is unrelated to and has no conflicts with the work published here.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest exists.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individuals participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Chamarthi, G., Modi, D., Andreoni, K. et al. Simultaneous catheter removal and reinsertion, is it acceptable in M. abscessus exit site infection?. CEN Case Rep 10, 483–489 (2021). https://doi.org/10.1007/s13730-021-00593-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-021-00593-y