Abstract
Occasionally, over-anticoagulation with warfarin induces acute kidney injury (AKI) characterized by glomerular hemorrhage with tubular obstruction by red blood cell casts, which is widely acknowledged as warfarin-related nephropathy. Owing to extensive use of direct oral anticoagulants, similar AKI cases have been reported among patients treated with dabigatran. Dabigatran is primarily excreted by the kidneys; thus, renal impairment is one of the risk factors for dabigatran-induced bleeding complications. Nevertheless, risk factors for dabigatran-induced anticoagulant-related nephropathy (ARN) remain partially clarified. Here, we report a histologically established case of dabigatran-induced ARN with undiagnosed IgA nephropathy in a patient with normal baseline renal function. In addition, we summarize previously published cases of biopsy-proven, dabigatran-related ARN. A 67-year-old female with normal preexisting renal function developed macrohematuria and AKI. She had been treated with dabigatran for deep vein thrombosis. A renal biopsy diagnosed ARN with inactive IgA nephropathy. After dabigatran withdrawal, her macrohematuria and renal function improved. This report demonstrates that ARN could occur in patients with normal baseline renal function. Our case and prior reports suggest that IgA nephropathy could be a risk factor for dabigatran-induced ARN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anticoagulant therapy is essential to evade thromboembolic events in patients with atrial fibrillation or deep vein thrombosis. Currently, direct oral anticoagulants (DOACs) are being rapidly used owing to their safety, efficacy, and convenience [1, 2]. Nevertheless, the risk of kidney injury caused by DOACs remains unclear. Here, we report a histologically established case of dabigatran-induced anticoagulant-related nephropathy (ARN) with newly diagnosed IgA nephropathy.
Case report
A 67-year-old female was referred to our hospital with persistent macrohematuria and acute kidney injury (AKI). At the age of 62, she started dabigatran (150 mg twice daily) for deep vein thrombosis. She had no history of easy or severe bleeding indicative of an underlying bleeding disorder before the administration of dabigatran. Her other routine medications comprised amlodipine, imidapril, metformin, and teneligliptin. A medical checkup two years ago detected microhematuria with no proteinuria. She presented with macrohematuria two months before admission; however, no urological abnormalities were detected by ultrasonography and computed tomography. She had no signs and symptoms of recent infections including tonsillitis before developing the macrohematuria. Her creatinine level increased from 0.5 to 2.16 mg/dL over 10 days, and she was admitted for the assessment of AKI.
Upon admission, her blood pressure was 144/86 mmHg, and the pulse rate was 67 bpm. We noted no tonsillar hypertrophy, peripheral edema, purpura, and petechiae. Urinalysis revealed a urine protein level of 2 + (2.09 g/gCr) and a urine blood level of 3+ with red blood cell (RBC) casts and granular casts. In addition, laboratory tests revealed a hemoglobin level at 9.6 g/dL with normal leukocyte and platelet counts. The prothrombin time was 29.9 s, and prothrombin time international normalized ratio (PT-INR) was 2.47. In addition, activated partial thromboplastin time (APTT) was markedly prolonged at 96.7 s. The serum creatinine level was 3.67 mg/dL, and the estimated glomerular filtration rate was 10 mL/min/1.73 m2. Moreover, liver function test results were normal with aspartate aminotransferase and alanine aminotransferase levels of 32 IU/L and 16 IU/L, respectively. The total protein and the albumin levels were 7.3 g/dL and 3.5 g/dL, respectively. The HbA1c level was 5.2%. Autoimmune disease screening was negative, and serum complement levels in the normal range. Furthermore, ultrasonography revealed normal kidneys and no abnormalities of other organs.
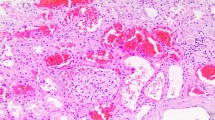
After stopping dabigatran, a percutaneous renal biopsy was performed. Hemorrhagic lesions were evident macroscopically in the renal biopsy specimens (Fig. 1a). In addition, light microscopy revealed that three of 25 glomeruli were globally sclerosed. Other glomeruli were unremarkable with normal mesangial matrix and cellularity (Fig. 1b). Moreover, some tubules were obstructed by RBC casts, and we observed diffuse acute tubular injury and interstitial hemorrhage (Fig. 1c,1d). Besides, immunofluorescence studies revealed granular staining for IgA in the mesangial area (Fig. 1e). No glomerular staining was noted for IgG, IgM, and C3. Furthermore, electron microscopy revealed a few scattered small deposits in the mesangial area (Fig. 1f). Based on these findings, ARN with IgA nephropathy was diagnosed.
a Macroscopic hemorrhagic lesions in renal biopsy specimens (arrows). b Glomerulus with normal mesangial matrix and cellularity (periodic acid-Schiff stain, magnification × 400). c Red blood cells casts and acute tubular injury (hematoxylin–eosin stain, magnification × 200). d Interstitial hemorrhage (hematoxylin–eosin stain, magnification × 400). e Glomerular mesangial IgA deposits (magnification × 200). f Electron-dense deposits in mesangial area (arrow) (magnification × 4,000)
After discontinuing dabigatran, PT-INR and APTT were normalized in a week, and the patient’s renal function normalized within four months. Furthermore, urinary protein excretion declined to 0.3 g/gCr, although mild microhematuria persisted.
Discussion
Brodsky et al. first reported that nine patients with chronic kidney disease (CKD) developed inexplicable AKI during warfarin therapy [3]. All patients presented with hematuria and had excessive anticoagulation (PT-INR range 2.0−8.0); renal biopsies were performed in all patients [3]. All specimens exhibited glomerular hemorrhage and severe tubular occlusion by RBC casts, but no active proliferative glomerular lesions were observed; thus, the authors anticipated that excessive anticoagulation with warfarin (PT-INR > 3.0) could cause AKI and named this syndrome warfarin-related nephropathy (WRN) [4]. Perhaps, the primary mechanism for WRN was that the disruption of glomerular filtration barrier could induce glomerular hemorrhage and subsequent tubular RBC casts, causing acute tubular injury because of tubular obstruction and ischemia [3]. In addition, hemoglobin, heme, or iron released from RBC casts, perhaps, contributed to acute tubular injury by increasing oxidative stress [5, 6]. The degree of proteinuria in patients with WRN varied from 0.61 to 5.7 g/day [7]. In general, the proteinuria occurring in tubular diseases is usually less than 2 g/day [8], and the reason for the occurrence of large amount of proteinuria in some patients with WRN remains unclear. Risk factors for WRN comprised CKD, elderly, diabetes mellitus, hypertension, and cardiovascular disease [9]. Reportedly, other oral anticoagulants, including dabigatran, apixaban, and rivaroxaban, could cause similar AKI, and the term “ARN” is now extensively used [7, 10,11,12,13,14,−15].
Our case developed AKI with macrohematuria during anticoagulation therapy with dabigatran. She was over-anticoagulated at the AKI onset, and her renal function enhanced after discontinuing dabigatran. A renal biopsy revealed acute tubular injury with occlusive RBC casts and interstitial hemorrhage, which corroborated the findings of ARN. Glomerular hemorrhage, one of the hallmarks of ARN, was not observed in the specimens, suggesting the recovery phase of ARN because a renal biopsy was performed after her serum creatinine level began decreasing. IgA nephropathy was determined incidentally, but no active glomerular lesions were observed. Overall, she was diagnosed with dabigatran-induced ARN and coexisting nonactive IgA nephropathy.
Dabigatran is a direct thrombin inhibitor and approved for clinical use without needing consistent coagulation monitoring [16]. Nearly 80% of dabigatran is excreted unchanged by the kidneys, and its half-life is 14−17 h in adults with normal renal function [17]. In patients with renal impairment, the half-life of dabigatran is prolonged, and the plasma dabigatran concentration elevates, resulting in the exacerbated risk of serious bleeding adverse events. Thus, dabigatran is contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/min) in Japan. Excessive anticoagulation with dabigatran can be determined by prolonged APTT [18, 19], and PT-INR can also be elevated when dabigatran concentrations are extremely higher than therapeutic levels [17]. Our case developed dabigatran-induced ARN, although her baseline renal function was normal; although its cause remained unknown, in certain conditions that might cause a rapid decline in the renal function such as dehydration, repeat prescriptions of dabigatran could result in drug accumulation and subsequent ARN.
To the best of our knowledge, three case reports of histologically established dabigatran-induced ARN have been published (Table 1) [12,13,14]. All patients, including the one in the present study, were aged greater than 65 years, and none had a severe renal impairment at the baseline. Three of four patients had hypertension, and the period between initiating dabigatran and the AKI onset varied from a few weeks to several years. All patients, except for one, presented with macrohematuria. The amount of proteinuria was 2.09 g/gCr in our patient and less than 1 g/day in the other two patients. At the onset of ARN, APTT was prolonged in three patients and PT-INR was prolonged in all patients. APTT and PT-INR returned to normal rapidly in three and two patients after discontinuing dabigatran, respectively. While one patient needed temporary hemodialysis, all patients had nearly complete recovery to their baseline renal function by discontinuing dabigatran.
Remarkably, all patients of histologically established dabigatran-induced ARN had underlying mild IgA nephropathy. Based on the AKI resolution after discontinuing dabigatran and the absence of active glomerular proliferative lesions, AKI with hematuria in these patients seemingly correlated with not acute worsening of proliferative forms of IgA nephropathy but other mechanisms. In IgA nephropathy, macrohematuria typically occurs after a mucosal (usually upper respiratory) infection [20]. The hematuria usually resolves within a few days; however, in rare cases, AKI occurs during an episode of macrohematuria. The most common pathological findings in AKI due to IgA nephropathy in the presence of macrohematuria are acute tubular necrosis and intratubular RBC casts, which are similar to those of ARN [21]. Excessive anticoagulation with dabigatran may accelerate macrohematuria due to IgA nephropathy and aggravate the tubular injury associated with macrohematuria. In some previous case reports, WRN was also reported in patients with underlying IgA nephropathy [3, 7, 22], and Goblin et al. anticipated that glomerular permeability alterations because of IgA nephropathy might easily initiate glomerular hemorrhage in combination with over-anticoagulation, resulting in ARN [7]. Nevertheless, further studies are warranted to determine whether IgA nephropathy could be a risk factor for ARN.
In conclusion, this report presents the case of dabigatran-induced ARN with underlying IgA nephropathy in a patient with normal renal function. Our case and literature review indicate that underlying IgA nephropathy could be a risk factor for ARN. The most crucial approach to the treatment of ARN is early detection and discontinuing anticoagulant medications. As dabigatran is becoming extensively used in clinical practice, physicians need to determine this clinical entity and closely monitor renal function, urinary findings, and coagulation status, especially in patients with urinalysis abnormalities indicative of chronic glomerulonephritis.
References
Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128(12):1300-5 e2.
Majeed A, Hwang HG, Connolly SJ, Eikelboom JW, Ezekowitz MD, Wallentin L, et al. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation. 2013;128(21):2325–32.
Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54(6):1121–6.
Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80(2):181–9.
Moreno JA, Martin-Cleary C, Gutierrez E, Toldos O, Blanco-Colio LM, Praga M, et al. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clin J Am Soc Nephrol. 2012;7(1):175–84.
Ware K, Qamri Z, Ozcan A, Satoskar AA, Nadasdy G, Rovin BH, et al. N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. Am J Physiol Renal Physiol. 2013;304(12):F1421–7.
Golbin L, Vigneau C, Touchard G, Thervet E, Halimi JM, Sawadogo T, et al. Warfarin-related nephropathy induced by three different vitamin K antagonists: analysis of 13 biopsy-proven cases. Clin Kidney J. 2017;10(3):381–8.
Carroll MF, Temte JL. Proteinuria in adults: a diagnostic approach. Am Fam Physician. 2000;62(6):1333–40.
Narasimha Krishna V, Warnock DG, Saxena N, Rizk DV. Oral anticoagulants and risk of nephropathy. Drug Saf. 2015;38(6):527–33.
Gois M, Azevedo A, Carvalho F, Nolasco F. Anticoagulant-related nephropathy in a patient with IgA nephropathy. BMJ Case Rep. 2017. https://doi.org/10.1136/bcr-2016-218748.
Brodsky SV, Mhaskar NS, Thiruveedi S, Dhingra R, Reuben SC, Calomeni E, et al. Acute kidney injury aggravated by treatment initiation with apixaban: another twist of anticoagulant-related nephropathy. Kidney Res Clin Pract. 2017;36(4):387–92.
Kalaitzidis RG, Duni A, Liapis G, Balafa O, Xiromeriti S, Rapsomanikis PK, et al. Anticoagulant-related nephropathy: a case report and review of the literature of an increasingly recognized entity. Int Urol Nephrol. 2017;49(8):1401–7.
Escoli R, Santos P, Andrade S, Carvalho F. Dabigatran-related nephropathy in a patient with undiagnosed IgA nephropathy. Case Rep Nephrol. 2015;2015:298261.
Moeckel GW, Luciano RL, Brewster UC. Warfarin-related nephropathy in a patient with mild IgA nephropathy on dabigatran and aspirin. Clin Kidney J. 2013;6(5):507–9.
Fujino Y, Takahashi C, Mitsumoto K, Uzu T. Rivaroxaban-related acute kidney injury in a patient with IgA vasculitis. BMJ Case Rep. 2019. https://doi.org/10.1136/bcr-2018-227756.
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–52.
Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292–303.
Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41(1):206–32.
Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory Assessment of the Anticoagulant Activity of Direct Oral Anticoagulants: a systematic Review. Chest. 2017;151(1):127–38.
Kidney disease: improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Chapter 10: immunoglobulin A nephropathy. Kidney Int Suppl. 2012;2:209–17. https://doi.org/10.1038/kisup.2012.23.
Gutierrez E, Gonzalez E, Hernandez E, Morales E, Martinez MA, Usera G, et al. Factors that determine an incomplete recovery of renal function in macrohematuria-induced acute renal failure of IgA nephropathy. Clin J Am Soc Nephrol. 2007;2(1):51–7.
Ishii H, Hirai K, Yanai K, Kitano T, Shindo M, Miyazawa H, et al. Warfarin-related nephropathy with acute kidney injury in a patient with immunoglobulin A nephropathy. CEN Case Rep. 2018;7(2):198–203.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent for publications was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ikeda, M., Tanaka, M., Shimoda, S. et al. Dabigatran-induced anticoagulant-related nephropathy with undiagnosed IgA nephropathy in a patient with normal baseline renal function. CEN Case Rep 8, 292–296 (2019). https://doi.org/10.1007/s13730-019-00410-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-019-00410-7