Abstract
Pullulan is a microbial exopolysaccharide hydrogel biopolymer that is biodegradable, renewable, and environmentally friendly. However, to meet the demands of the utilization, it is still necessary to enhance the yield and molecular characteristics of pullulan formed by different strains. Available in powder form, pullulan enhances the benefits of this natural material when combined with nanoparticles (NPs) and synthesized into pullulan NPs. NPs are gaining attention as a cutting-edge technology in the fields of pharmaceuticals, medicine, food, agriculture processing, and packaging. Pullulan biopolymers provide an environmentally friendly solution that effectively addresses the world's waste disposal issue by removing untreated waste from the agro-food industries and using this waste as a potential substrate for pullulan biosynthesis. Nowadays, pullulan in the form of NPs, nanocomposites, and nanoformulation has become increasingly popular because of their specific application needs with enhanced molecular properties like strength, durability, electrical conductivity, and catalytic activity. This approach offers a valuable product called pullulan-based nanopolymer, which holds promise in various industries. Pullulan with the highest yield capacity to date has the potential to significantly decrease production costs and increase applicability range. This review provides detailed insights into the latest methods for extracting pullulan biopolymers from agricultural and food waste materials in the form of polysaccharides. Moreover, the article covers the synthesis of various types of pullulan-based nanoparticles, nanocomposites, and nanoformulations. Furthermore, it delves into the diverse applications of these pullulan nanopolymers across agriculture, food and medical sectors.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pullulan is a polysaccharide made up of repeating subunits of maltotriose, also called glucan which is synthesized by yeast-like fungus Aureobasidium pullulans by an aerobic synthesis method [1, 2]. It was first observed by Bauer in 1938 as a polysaccharide in A. pullulans after that it was first isolated and characterized by Bernier from A. pullulans broth culture [3, 4]. The industrial market of pullulan is expanding quite quickly. The initial company that commercialized pullulan synthesis was Hayashibara Co. Ltd. in 1976 [5]. Up to the year 2000, the industrialized process of pullulan manufacturing was restricted by patent rights obtained by Hayashibara. However numerous other industries began producing pullulan commercially after Hayashibara's patent expired. The USA's Sigma-Aldrich, Inc. is among the leading manufacturers of pullulan. Pullulan's unique qualities and future uses are driving up its popularity, which is growing daily. According to Singh and coworkers, the market cost of standard pullulan developed by Sigma-Aldrich, Inc. USA was US $ 2000 kg−1 in 2009 [6]. By 2020, that price had risen to US $12,000 kg−1 [1].
It exhibits renewable, odourless, tasteless, non-mutagenic, toxin-free, non-immunogenic, and non-carcinogenic natural polymeric properties [7,8,9]. Therefore, due to its considerable adaptability and flexibility, it plays a wide role in various fields [10,11,12]. Pullulan is typically used as a prebiotic, a filler in beverages and sauces, and as a texturizer in culinary pastes like mayonnaise. In the food sector, pullulan is favoured as a low-calorie food material used to produce low-calorie products including baked pastries, low-calorie noodles, and synthetic rice. Because pullulan has sufficient adhesive properties, it can be used as a binder [13]. In comparison to other polysaccharides, it has a comparatively low viscosity and does not change throughout a broad pH range of 2–11 [14]. It can withstand temperatures as high as 250–280 °C. It begins to break down at temperatures higher than this. It may produce a film with strong oxygen (O2) barrier properties but also is water soluble, non-toxic, biodegradable, and edible. It has been a GRAS product since 2002, and the EU subsequently recognized it for use as a food additive in 2004 [15]. Due to its superb O2 barrier properties, pullulan film is used in most food and agriculture industries as a packaging application. Foods high in unsaturated lipids (such as meat, fish, and nuts) and vitamins (such as fruits and vegetables) are especially well protected by these films [5]. In the 1990s, pullulan coating was used for the first time to protect food, and it quickly gained popularity as an edible thin protective layer [16]. Additionally, because this polymer is non-immunogenic, non-carcinogenic, and non-mutagenic, researchers use it in targeted drug and gene delivery [11, 17]. Pullulan derivatives must possess hydrophobic and cationic moieties to function as an appropriate macromolecule in the nanoparticle production process for drug delivery [18]. Pullulan can easily be chemically modified as it exhibits multiple hydroxyl groups. Pullulan-based NPs also showed remarkable non-immunogenicity so it is useful as a plasma expander [19] and has an inherent affinity for the liver [18, 20]. Despite these advantages, pullulan production is another challenge concerning (i) economical production and its utilization in different industries that completely depend on inexpensive substrates, and (ii) physicochemical similarity with synthetic plastic.
Raw substrates limit the industrial production and commercial application of pullulan. As its production proved to be expensive ($25 kg−1) than other exopolysaccharides [21]. Pullulan production under submerged fermentation containing a medium supplemented with carbon, nitrogen and other essential nutrients makes the upstream process expensive that affects the final cost of pullulan [7]. Since media components are responsible for about 30% of the total production cost, choosing cheaper ones can lower the overall production price [21]. In this regard, many agricultural and agro-derived substrates are utilized as inexpensive substrates for the commercial synthesis of pullulan [7].
Global population growth has raised the need for agro-food production and, consequently, the need for agro-food processing wastes has also been raised. About 1.3 billion tonnes of food are discarded annually, based on the latest assessment from the Food and Agriculture Organisation (FAO) [22]. Food businesses and household wastes are major contributors to agro-food waste, which is harmful to the environment in both prior and after-market sites [23]. Such wastes are frequently dumped in landfills or used to make compost. However, the production of value-added products like pullulan from these agro-food wastes would not only encourage waste recovery but also generate higher benefits [24].
However, the natural physiochemical structure of lignocellulose present in agro-food waste creates a hindrance to the effective valorization of these substrates for pullulan biopolymer production due to their heterogenic and recalcitrance properties. This rigidity of the substrates poses an economic and technical challenge in biomass conversion to biopolymer. To sort out these problems different pretreatment methods are utilized separately and in combination. These treatments alter the physicochemical structure of the agro-food biomass which gives it more accessibility to the substrate for pullulan production [15].
The second drawback related to their physicochemical properties such as poor mechanical property, insolubility in organic solvent, and absence of hydrophobic group, limits the application of pullulan in various fields [11, 25]. These drawbacks can be overcome by the conjunction of pullulan with another nanomaterial. Different studies on pullulan-based nanocomposite showed their possible uses in agro-food, medical, and pharmaceutical industries. The incorporation of some functional ingredients like NPs into pullulan for their application on the agro-foods products surface promotes safety or even nutritional and sensory attributes [26]. Pullulan nanocomposites are an example that is formed by blending a pullulan with NPs that can effectively resolve the problem of biopolymer film/coating. These NPs-incorporated biopolymers exhibit properties such as good antibacterial, antifungal, and antiviral features compared to only pullulan biopolymer film [27]. Whereas, they also have a critical role as a nanoformulation in biomedical and pharmaceutical research and development such as nanogels, nanoparticles (NPs), and microspheres in drug delivery, gene delivery, plasma expander, vaccination and anticoagulants [1, 15, 16]. This review highlights the recent research on the synthesis of pullulan polymer from renewable agro-food waste resources and its transformation into valuable commercial biomaterials as a solution to waste management. Furthermore, it also elaborates on the conjunction of pullulan with other nanoparticle and their relevance in the application of various sectors.
Agro-food waste as a substrate for pullulan production
Pullulans are biological polymers that are produced by microbes using polysaccharide resources like agro-food waste [28]. These wastes are the consequences of the increasing worldwide population and their lifestyle which has increased the requirement for agriculture products and food. These untreated agro-food waste creates severe damage to the environment in the way of pollution [29, 30]. The agro-food waste is tough to manage because it is enriched with many nutrients and its unsafe disposal could create an extensive negative impact on the environment and human health. Traditional waste management such as landfilling, bio-composting, and incineration is previously practiced for agro-food waste management. That proved to be time-consuming and unsafe generating landfill leachate and exposure to toxic gases [31]. Keeping this in consideration, various research on agro-food waste is carried out on merging agro-food management with additional commercial goods like volatile fatty acids (VFA), biohydrogen, bioplastic (PHA), and more [30].
Over the last decades, biopolymers have become more famous among other valuable agro-food wastes-derived products in many different industrial fields including food, agriculture, pharmaceutical, biomedical, and other industrial sectors. This is because of their biodegradability/compostability, biocompatibility, and their sustainable characteristics [23, 24]. Agro-food waste acts as a polysaccharide substrate that can be specifically converted into bio-derived compounds that exhibit various chemical behaviour. These compounds can then be utilized to yield new biopolymers as a substitute for petroleum-derived polymers [32]. Out of agro-food-derived polymers, the extracellular polysaccharides (EPS) derived from fungal polysaccharides like pullulans biopolymers show great interest. Therefore, the transformation of these agro-food waste by-products through different pretreatment methods shows two main benefits: (i) The sustainable degradation of agro-food waste that directly decreases environmental pollution, and (ii) helps a circular bio-economy by the development of value-added biomaterials such as biopolymers that show a broad range of opportunities in different industrial fields.
Furthermore, there remains a need to acquire biomaterials through economically viable methods that can guarantee the complete utilization of agro-food wastes utilizing sustainable practices. Polysaccharides are the least expensive and most readily biodegradable of these biomolecules, making up around 75% of all organic compounds on Earth [33]. These are consequently among the most prevalent polymers that occur naturally, acting as important components of both plants and animals (like cellulose in plants and hyaluronic acid in mammals) or as a method of storing food (like starch or glycogen). All of these biopolymers, composed of algal or microbial polysaccharides like xanthan gum and pullulan as well as exudates from plants like gum arabic, karaya gum, and tragacanthin gum, are the most basic natural carbohydrate molecule building blocks that are made up of repeating units accompanied to one another through glycosidic bonds. They are frequently employed as chemical markers, especially for cell identification [34].
The amount of polysaccharides that can be derived from agro-food waste, which amounts to roughly 300 billion tonnes annually [7], is far greater than the amount of natural raw materials available in nature that are able to produce polysaccharides. Utilization of these wastes will allow for the preservation of natural resources for future generations while also removing some pollutants and lowering greenhouse gas (GHG) pollution [28]. Agro-food waste offers numerous benefits, but it also has drawbacks as it contains a significant amount of lignocellulosic materials, which are difficult to process due to their complex structural makeup. This prevents microorganisms from using them as a source of food. It is regarded as a significant barrier in the way of producing biopolymers by microbial fermentation. Therefore, the pretreatment of the lignocellulosic feedstock turned out to be an extremely significant phase in the fermentation process. The efficiency of these pretreatment procedures controls the availability of free sugars and subsequent conversion towards biopolymers. Enzymatic, physiochemical, chemical and a mix of several processes are some of the pretreatment procedures for breaking lignocellulosic material [30]. According to Kumar and Sharma [35], the choice of pretreatment is based on the characteristics and content of the lignocellulosic material for the hydrolysis process that produces a favourable outcome. This part highlights the pretreatment method selected to synthesize pullulan from food and agricultural waste.
Biosynthesis strategies for pullulan
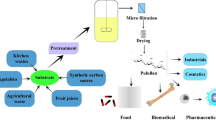
A general method of pullulan production is illustrated in Fig. 1. Biosynthesis of pullulan was highly influenced by substrate type, environmental conditions, and strain modification at the genetic level that is described in this section.
Role of different agro-food waste on pullulan production
Previously, pullulan was produced from A. pullulans using sugars such as sucrose, glucose, fructose, and maltose. However, it can also be alternatively synthesized with various agro-food wastes such as green gram husk [36], cassava starch, corn steep liquor, and soybean meal hydrolysate [37], Sesame seed oil cake [38], potato starch hydrolysate [39], cassava waste [21], sugarcane bagasse hydrolysate [40] and cassava bagasse and Asian palm kernel [41], etc. The composition of saccharified intermediate affects pullulan production from different agro-food wastes. This part covers the pullulan production from the most common agro-food wastes.
The potatoes in the food industry have been investigated most frequently when it comes to developing ready-to-eat items. The processing units for potatoes produce a lot of trash. According to Huang and coworkers [42], potato trash has a rich starch content (2.0%-2.5%) and a low content of reducing sugars (0.08%-0.12%). Potato waste disposal can lead to serious problems for the environment and human health. Different microbial flora may utilize starch to serve as a carbon source for pullulan fermentation. The two molecules that makeup starch are amylose and amylopectin. Because of their complexity in structure, almost all industrially significant microbes find it difficult to use these compounds in their original state. Despite this, starch that has been hydrolyzed serves as simple sugars, making it an excellent substrate for the growth of microorganisms. Two processes are involved in the enzymatic breakdown of starch: first, α-amylase administration, and then partially hydrolyzed starch is exposed to a mixture of pullulanase and glucoamylase. α-Amylase breaks down the α-1,4 glucose bonds in amylose, resulting in liquefied gelatinized starch. To efficiently hydrolyze starch, pullulanase and glucoamylase are combined [43]. Pullulanase hydrolyzed the α-1,6 glucose links of amylopectin, while glucoamylase hydrolyzed the α-1,4 glucose linkages at the same time for the production of high-glucose syrup. The rate of starch liquefaction is increased by the immobilization of glucoamylase and pullulanase in Ca-alginate beads, and the resultant hydrolysate can function as a potential source for pullulan biosynthesis [44]. The hydrolysate that is produced by β-amylase instead of glucoamylase increases pullulan up to two times [45]. Certain strains of A. pullulans can partially undergo hydrolysis of potato starch due to the presence of amylase a starch-degrading enzyme. Pullulan synthesis can be greatly enhanced by this partially hydrolyzed potato starch [46]. A. pullulans has produced more pullulans in a 10-L bioreactor if potato starch hydrolysate is enriched with sucrose [39]. The stimulating effect on the microbial enzyme skilfully raised the pullulan yield in a potato starch hydrolysate-based medium [39].
Grapes constitute a crucial raw substrate used in the production of juice and wine. The grape skin is peeled off and the juice is drawn from the pulp during the processing step. This grape skin and juice-free pulp during processing constitute a waste called grape pomace, while grape juice is primarily utilized in the production of ready-to-drink beverages. According to Israilides and coworkers [47], the composition of grape pomace is as follows: protein (7.8%), total sugars (85.2%), reducing sugars (3.4%), and glucose (1.28%). While grape pomace in its solid state can be challenging to work with, grape skin and pulp extract are more user-friendly. One method for making grape pomace extract is to add hot water to the pomace, agitate for 30 min, and then filter the mixture [48]. The pullulan made up of grape pomace extract exhibited homogeneity and high molecular weight, resulting in increased yield [46].
Whey is a liquid byproduct produced after the coagulation of protein and fat present in the milk during the process of making cheese. According to Yang and coworkers [49], whey comprises lactic acid (0.1%-0.8%), proteins (0.8%), lactose (4.5%), and salts (1.0%). It is an efficient and affordable medium for pullulan biosynthesis because of these components. Furthermore, the proteins in whey might be eliminated by boiling it to 90 °C for 20 min, which will increase its usefulness for fermentation operations. By filtering out the protein precipitates, deproteinized whey is produced, which has around 80% lactose [50]. It has been claimed that A. pullulans may produce pullulan in shake-flask fermentations using deproteinized whey as a possible carbon feedstock [50].
Rice hull is the outer brown covering of rice obtained during rice processing. The primary components of rice hull are lignocellulose including cellulose (50%) and lignin (25%-30%), as well as silica (15%-20%). Rice hull is a promising substrate for fermentation due to these components [51, 52]. The majority of microbes are unable to use lignocellulosic carbohydrates in their natural state. Thus, rice husk hydrolysate is formed by transforming the complex carbohydrates into fermentable sugars through the process of saccharification of the rice hull. The process used to saccharify rice hull determines the quantity of sugars found in the hydrolysate [53]. Wang and coworkers [54], reported that the hydrolysate of rice hulls contains the following constituents: xylose (25.52% ± 0.83%), glucose (5.89% ± 0.18%), arabinose (3.37% ± 0.18%), galactose (0.22% ± 0.20%), and acetic acid (0.35% ± 0.02%). A. pullulans was successfully utilized to produce pullulan using rice hull hydrolysate in a stirred-tank fermentor. The presence of acetic acid in hydrolysate could hinder fungal growth and negatively impact pullulan synthesis. Therefore, to get over this problem, the original strain of A. pullulans can be evolved adaptively from acetic acid to increase the pullulan yield throughout fermentation.
Molasses is a byproduct of sugarcane or sugar beet juice refining to form tiny sugar particles. It is a viscous liquor with a dark brown colour that contains around 45%-55% of all fermentable sugars, along with non-sugar molecules (2%-4%), fructose and glucose (10%-25%), minerals, and moisture [55, 56]. The sugar factory releases substantial quantities of molasses into the closest water supply, which significantly contaminates the environment. Because of these types of sugars, molasses can be readily metabolized by A. pullulans and used as a carbon source for pullulan synthesis [47]. But molasses additionally includes heavy metals (Cu, Fe, Mn, Zn, Ca, Mg, etc.) that prevent microbial growth, deactivate beneficial enzymes, and reduce the production rate of the end product [57]. Therefore, pretreating the molasses is a crucial step in achieving a satisfactory product yield both qualitatively and quantitatively. The easiest way to remove heavy metals from molasses is by treating it with H2SO4. Molasses were pretreated by adding 1 N of H2SO4, letting the mixture rest for 24 h, and then centrifuging the liquid to extract the supernatant [57]. The pullulan obtained from the pretreated molasses was twice as high in the shake-flask fermentation than untreated molasses. In this manner, pretreated molasses proved to be an economical production medium for pullulan synthesis [58]. Pullulan production is increased by 49.0 g/L when pretreatment molasses is used, according to several studies [59], 23.0 g/L [60] in stirred tank reactors and 18.5 g/L in an airlift reactor [61].
Brewery waste is a good substrate for pullulan production. The brewing plants produce a byproduct called spent grain liquor. This spent grain liquor is obtained as liquid waste after the extraction of wort from spent grains. The brewing sector produces a significant volume of discarded grain liquor, which is an incredible source of waste. Both suspended particles and organic compounds can be found in spent grain liquor. According to Xiros and coworkers [62], it is composed of hemicellulose (40%, w/v), cellulose (12%, w/v), starch (2.7%, w/v), proteins (14.2, w/v), lignin (11.5%, w/v), lipids (13%, w/v), and ash (3.3%, w/v). The organic content of spent grain liquor is expressed as biochemical oxygen demand (BOD) and is the main pollutant of the water ecosystem. A. pullulans uptakes this spent grain liquor and is responsible for the pullulan production [50].
Role of physico-chemical/fermentation condition on pullulan biosynthesis
The microbial-assisted approach for the biosynthesis of pullulan by bio-polymerization of agro-food waste is dependent on fermentation [19]. Fermentation is a versatile approach to synthesize products of added value like microbial biopolymers, since fermentation factors influence the feasibility and the economic side of the method [63]. Process optimization plays a crucial role in cutting the expenses of any industrial production [58]. Therefore, optimizing the fermentation process is crucial to advance the formation of microbial EPS. Growth conditions such as pH, temperature, oxygen concentration, agitation, and culture medium composition are important factors to optimize [64, 65]. Microbial type plays an important role in the chemical structure, monomer composition, physical, chemical, and rheological characteristics of polysaccharides and in this way, the process is strain-specific [66]. Consequently, management of the fermentation conditions, such as selecting suitable substrates and exploiting excellent productive strains, makes the industrial production of polysaccharides efficient with the required characteristics possible [67]. Since carbon- and energy-intensive activities play a major role in EPS synthesis, dietary needs, and environmental factors also have an impact on the strains needed to synthesize EPS. As a result, there is controversy over microbial growth, nutrient availability, and other fermentation factors that affect polysaccharide synthesis. Thus, it seems likely that these circumstances have strain-specific impacts on polysaccharide synthesis [65]. Yeast-like cells are the main producers of pullulan in cultures during the late exponential and early stationary phases [5, 68, 69]. Additionally, on the basis of microbial growth, various fermentation circumstances, like batch, batch-fed, or continuous process, may be used for polysaccharide synthesis [70, 71].
pH has the potential to alter the growth profile, which can significantly raise or lower the pullulan yield. It is frequently noted that the ideal temperature and pH conditions for the highest biomass yield and for the synthesis of polysaccharides are distinct. Therefore, it could be necessary to reach the highest biomass within one set of conditions before changing culture conditions to acquire the highest polysaccharides. pH of the medium progressively lowers from initial pH 6.5 throughout the course of the first 24 h fermentation [72]. Pullulan productivity is impacted by structure of A. pullulans, which is influenced by the pH of the medium [73]. According to Lee et al. [74], mycelia are formed at pH 2.5, and yeast-like colonies are formed at pH 4.5. Pullulan synthesis requires a pH of around 5.5 and 7 [73]. There is an optimum pH for polysaccharide synthesis or biomass growth than this [74, 75]. While pullulan biosynthesis is poor at lower pH values, and biomass growth increases [46, 76]. Published data indicate that pullulan synthesis increased when the medium pH was permitted to naturally decline instead of being kept constant [74, 77]. As shown by Lee et al. [74], pullulanase function causes a progressive decline in the fraction of high molecular weight pullulan when the pH is not regulated. But if the pH drops below 2.5, pullulan yield may be negatively impacted by extreme acid exposure [76, 78]. The ideal pH and temperature for pullulan synthesis are not always specified same in the literature; they might range between 5.5 to 7.5 and 25 to 30 °C, based on the physical characteristics of the microbe [58, 79].
The medium's temperature needs to be kept at 30 °C for the cell growth which usually peaks at 75 h. The production of pullulan starts if nutrients start dropping low at the final stage of the logarithmic growth cycle. Pullulan biosynthesis reaches its peak during the stationary phase, which lasts from 70 to 90 h. Pullulan biosynthesis is favoured by nitrogen deprivation. Ammonium ion level reduction inhibits biomass formation, which is a key factor in the redirection of carbon sources to polysaccharide synthesis. But towards the end of the growth cycle, A. pullulans also synthesizes pullulanase, an enzyme that breaks down pullulan. Consequently, pullulan-degrading enzymes cause a reduction in pullulan productivity after the stationary phase [80]. Pullulan yields of roughly 70% and higher are typically attainable [72, 76].
No matter the scale of polysaccharide production, the nitrogen content of the nutritional medium is the limiting factor [81]. Therefore, the fermentation medium is designed to have a high carbon-to-nitrogen ratio. Although the substrates to be used should be inexpensive, the nutrient needs for the industrial production of microbial polysaccharides remain the same [63, 82]. Growth conditions such as on substrate composition (breeding factors), fermentation conditions (pH, temperature), and the improvement of A. pullulans strains also play a key role in the production of microbial EPS including pullulan [83]. Both the cell growth and metabolite production enhancement depend on the culture media contents, mainly proper amount of media nutrients with an optimized carbon/nitrogen concentration [84, 85]. Yeast-like fungus Aureobasidium pullulans exhibited quality to use a multiple type carbon substrate and can produce pullulan by the simple and complex sugars, for example glucose, fructose, mannose, maltose, as well as xylose, ribose, arabinose, sucrose, and lactose [86]. Pullulan is made commercially with starch hydrolysate that has 40%–50% dextrose equivalent (DE). A range of 5% to 15% is kept for the sugar content. Pullulan formation is checked by extreme sugar content [44]. According to reports, too much sugar prevents the biosynthesis of pullulan by inhibiting the enzymes that produce pullulans, such as α-phosphoglucose mutase, UDPG-pyrophosphorylase, and glycotransferase. Pullulan synthesis is therefore more suited for fed-batch reactors [76]. Several carbon substrates, such as mono- or disaccharides, may be employed to produce pullulan. The impact of six distinct sugar supplies (glucose, sucrose, dextrin, fructose, maltose, and xylose) on the pullulan biosynthesis through A. pullulans Y68 was investigated by Duan et al. [86]. According to their findings, pullulan production from glucose (8%) was found the highest possible level, while significantly lowering the yields in sucrose, fructose, xylose, dextrin, and maltose [87].
According to Singh and Saini [88], the capacity to uptake several carbon substrates was likely a unique trait of strains for pullulan biosynthesis. Agricultural waste [72], olive oil and sucrose [89], deproteinized whey [60], beet molasses [90], sweet potato [73], and potato starch hydrolysate [73] were all used as inexpensive substrates in order to effective production of pullulan through fermentation method by employing A. pullulans [58]. This is because the fast growth of the biotechnology sector remains linked to the requirement to broaden the raw material base using new, less expensive carbon substrates [84].
According to reports, pullulan is a secondary metabolite that yeast-like cells synthesize when there is an ammonium shortage [5, 91]. Additionally, each strain is nitrogen specific in order to produce pullulan i.e. present in the growth media [85]. The majority of the strains have been favoured ammonium sulfate as a choice of nitrogen for growth [88]. However, additional nitrogen sources such as soybean hydrolyzate, peptone or tryptone, ammonium acetate, and sodium nitrate, may also be utilized for the biosynthesis and secretion of pullulan in the fermentation medium [84]. Additionally, pullulan yields and the physical characteristics of fungal strains are directly influenced by the pH of the growth media [84]. A polymorphic fungus called Aureobasidium pullulans can produce mycelia, colorless chlamydospores segmented by septa, budding blastoconidia (yeast-like cells), and spores that secrete black melanin. Pullulan is currently only known to be produced by chlamydospores and unicellular blastoconidia [2, 92]. The range of 5.5–6.5 pH is the ideal beginning pH for the synthesis of pullulan [69]. Varying strains of A. pullulans have slightly varying optimal temperatures for the production of pullulan, which have been observed to range from 25 to 28 ˚C [93].
It is generally known that microorganisms including bacteria and fungus produce extracellular metabolites, like polysaccharides [94]. Exopolysaccharides (EPS), intracellular polysaccharides, and structural polysaccharides are all examples of polysaccharides that fall under the category of biopolymers [94]. The extracellular biosynthesis of pullulan occurs at the cell wall membrane as a loose and slimy coating on the outermost layer of the cell [95]. According to recent theories, pullulan biosynthesis may occur in the Aureobasidium melanogenum P16's cell wall and periplasm [2, 96]. Pullulan is the primary linear glucosic polysaccharide derived from fungus Dematium pullulans (de Bary) or yeast-like fungus Aureobasidium pullulans (de Bary) Arnaud (earlier known as Pullularia pullulans de Bary) [88]. However, some species of A. pullulans show limitation to high synthesis of exopolysaccharide pullulan [97]. While various species are identified as pullulan producers, including genus of Aureobasidium, namely A. melanogenum, A. leucospermi, A. proteae, A. thailandense, and A. nambiae [2, 98, 99]. Fungal species also contributed in the pullulan production such as saprophytic fungus Tremella mesenterica [100], parasitic fungal strains Teloschistes flavicans [101] and Cryphonectria parasitica [102]. The pullulan production potential was also observed in the other fungal varieties such as edible mushrooms Cyttaria harioti and Cyttaria darwinii [103, 104], yeasts such as Rhodotorula bacarum and Rhodosporidium paludigenum [105, 106], as well as in bacteria like Micrococcus luteus [107]. The formation of pullulan by A. pullulans results in the unwanted black pigment known as melanin. It develops and is discharged throughout the medium near the finish of the exponential cycle, providing it with a black colour [76]. To overcome this problem, the producer strain of A. pullulans shifts to a colour variant that fails to produce melanin [6, 87].
Role of biosynthetic mechanism on pullulan biosynthesis by A. pullulans
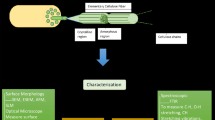
A complex metabolic reaction occurs during the biosynthesis of pullulan. Precursors synthesized inside the microbial system accelerate the rate of pullulan production. According to Simon and coworkers [108], pullulan biosynthesis is facilitated by the deposition of carbohydrate residues within the cell at the early phases of fermentation. Pullulan is biosynthesized in the microbial cell's cytoplasm and subsequently excreted to the cell surface by penetrating the β-glucan layer. This creates a lightly adhered slimy coating over the cell surface which is made up of pullulan biopolymer [109]. Metabolic route for pullulan biosynthesis was described by Duan et al. [86] and Sugumaran and Ponnusami [94]. A. pullulans is able to consume various carbon sources such as mannose, sucrose, maltose, fructose, galactose, xylose and even the agro-industrial waste. It took a while to determine which enzymes and encoding genes play a role in the synthetic pathways of this EPS, and the process of pullulan synthesis was not fully understood before the year 2020 [2]. There are two main phases in the biosynthesis of pullulan. Initially, uridine diphosphate glucose (UDPG), the precursor, is produced [109]. UDP-glucose is a widely recognized precursor of pullulan biosynthesis [110]. However, Shingel also hypothesized that lipid intermediates carrying glucose could potentially have a role in this pathway [111]. Second, pullulan is created by polymerizing the precursor molecules [109]. The transformation of glucose into pullulan compounds depends on the existence of important enzymes like α-phosphoglucose mutase, uridine diphosphoglucose (UDPG) pyrophosphorylase, and glucosyltransferase [86, 88]. α-phospho glucomutase enhances the formation of glucose-1 phosphate from glucose-6 phosphate [86]. Pullulan precursor, UDP-glucose is formed from glucose 1 phosphate by UDPG-phyrophosphorylase [1]. Figure 2 illustrates the detail regarding a general fungal metabolic process for the formation of pullulan and the key enzyme involved.
Pullulan production is dependent on biosynthesis regulation. Pullulan's low productivity, low Mw, and impurities notably black-pigmented melanin [112] have restricted its commercialization currently [113]. Because of the significance of pullulan's characteristics, its production price, bioprocessing, and strain alteration are being extensively researched as ways to increase pullulan's Mw and yield. Genetic engineering is used in numerous attempts to control pullulan production by effective genome editing methods which were developed in 2019 [2]. It helps to control the appropriate genes and enzymes involved in the pullulan production pathway in various strains of Aureobasidium spp. [103].
Chen et al. [92] simultaneously deleted duplicated AMY1 genes encoded α-amylase and duplicated PKS1 genes involved in melanin production in A. melanogenum TN3-1. This resulted in a mutant AMY-PKS-11 that converted 140.0 g/L glucose to yield 103.50 g/L pigment-free pullulan with an Mw of 3.2 à 105 Da [114]. When cultivated in a 10-L fermentor, the triple mutant DT15 produced 58.14 g/L of pullulan with the same Mw of 3.02 × 106 Da. However, its wild-strain P16 yielded 65.5 ± 3.5 g/L of pullulan with a Mw of 0.35 × 106 Da [113].
Therefore, further work needs to be done to increase pullulan yield and to enhance the chemical characteristics of pullulan by altering on molecular level of the producer by synthetic biology techniques. Byproducts such as melanin, glucan, and polymalic acid are typically present in pullulan produced through A. pullulans fermentation [19, 115, 116]. However, the existence of these contaminants makes pullulan purification much more challenging. The A. pullulans strains has a potential to suppress the formation of melanin compounds which is a crucial component of the industrial synthesis of pullulan [85]. Melanin was one of the challenges of pullulan production [93], and this substance was eliminated using an appropriate solvent throughout the fermentation and precipitation of EPS.
The biosynthetic pathways of distinct strains vary, leading to variations in the product molecular weight among strains because of variances in metabolic routes and cell structure [12, 94]. In this reference, Chen and coworkers [12] studied the regulation of the biosynthesis of pullulan in A. pullulans BL06. They discovered that by regulating the key synthesis pathway of pullulan impurities such as deletion of the important genes, the Mw of pullulan products can be changed in addition to the yield and purity of extracellular pullulan. A. pullulans BL06 was isolated from the environment and shown to produce large yields of pullulans. Additionally, three strains with knockout genes were created: A. pullulans BL06 ΔPMAs, A. pullulans BL06 Δmel, and A. pullulans BL06 ΔPMAsΔmel. In addition, a fermentation output of 83.4 g/L pullulan in a 5 L bioreactor with a molecular weight of 3.3 × 106 Da was obtained. The strain BL06 has great prospects for industrialization since it offers the benefits of high production and high Mw. Moreover, a different commercial strain of A. pullulans, BL06 ΔPMAs, was developed by deleting the gene encoding for polymalic acid (PMA) synthase. This strain can produce moderately high yields of pullulan with high purity. After being transformed, the strain was able to produce 1.3 × 105 Da pullulan at a yield of 140.2 g/L in a 5 L bioreactor, without the presence of PMA or melanin. It has been observed that pullulan synthesis is increased by overexpressing the pullulan synthase enzyme [2].
In addition to low Mw, low pullulan yield was another barrier for pullulan industrial application. Usually, high pullulan biosynthesis takes place in a high sucrose medium that avoids glucose. The reason behind this is that pullulan is glucose sensitive and the existence of a high glucose concentration in the culture media represses many genes linked to pullulan biosynthesis and declines the pullulan yield [85, 117]. Hence, depression of glucose is required for the enhancement of pullulan biosynthesis [2].
A previous report indicated that the removal of gene coding for CreA from a glucose-derepressed mutant of A. melanogenum showed a higher pullulan yield. CreA or MIG1 genes are global regulators in fungi, including yeasts that encode glucose repression that takes part in glucose repression. The Mig1 and CreA proteins, Cys2His2 (C2H2) zinc-finger proteins, are able to bind the well-characterized 50-SYGGRG-30-rich sites in the promoters of a variety of glucose-repressed genes, including those responsible for pullulan biosynthesis [117, 118]. Another study showed that a higher yield of pullulan (64.93 g/L) from glucose at a concentration of 120.0 g/L was produced by the glucose-derepressed mutant of A. melanogenum P16 in comparison to the corresponding native strain (52.0 g/L) in the similar growth conditions [117]. The other proteins that play a role in glucose repression and derepression include the transcription factor Mig1, the hexose kinase Hxk2, the protein kinases Reg1-Glc7, Med8, Cyc8-Tup1, Snf1, the protein kinase Adr1, and the catalase Cat8 [118]. Additionally, it is hypothesized that Msn2 and the associated signaling pathway control pullulan production in A. melanogenum P16. The Msn2 protein, a C2H2 Zn finger, has a DNA-binding domain that detects the particular promoter sequences (AGGGG and CCCCT) of the regulated genes, containing the majority of the genes involved in pullulan biosynthesis [2]. The UGP1 gene, which codes for UDPG-pyrophosphorylase (Ugp1), catalyzes the production of UDP-glucose, the sole precursor of pullulan biosynthesis, that is responsible for accelerated pullulan biosynthesis when the Msn2 protein is localised in the nucleus of A. melanogenum P16 [2].
However, hexokinase and isomerase are required for the transformation of various carbon substrates to the pullulan precursor, UDPG [85]. A second gene, UGT1, which codes for a protein, i.e., similar to the UDP glucose, glycoprotein glucosyltransferase (Ugt1), has also been identified as being involved in the production of pullulan [110]. Pullulan was overproduced when this gene was overexpressed in A. melanogenum P16 as produced 63.38 g/L in the natural strain as compared to 80.2 g/L in a modified one. Whereas its deletion greatly lowered the amount of pullulan that was biosynthesized. For incompletely glucosylating folder glycoproteins, the Ugt1 protein was proposed to serve as a "folding sensor" [92]. Currently, it was shown that the PUL1 gene encodes for the pullulan synthetase protein may function as an auxiliary protein for a component necessary for the glucan synthetase 2 (AmAgs2) activity, a crucial enzyme for the production of pullulan. In two mutants of A. pullulans, the PUL1 gene deletion prevented the production of pullulan [15, 92].
Recent methods to extract pullulan from agro-food wastes/downstream process
The most important stage in every biotechnology sector is the planning and establishment of an effective bioprocess. Process development is eventually impacted by the growing requirement to lower the rising product costs [119]. Pullulan undergoes a multi-stage downstream processing step to ensure its security during purity recovery. It must be effective, and reliable, and enhance pullulan extraction productivity while requiring the least amount of manufacturing expenses [120]. Downstream becomes more difficult to extract the pullulan because of interference from contaminants, leftovers from fermentation, and substrate and medium additives employed in the formulation of the fermentation medium.
The choice of an inexpensive, complex substrate typically results in reduced upstream processing costs for pullulan when the fermentation medium is formulated at an industrial level. Additionally, these inexpensive, complex substrate components go unused during fermentation, and isolating them raises the additional steps in the downstream procedure and adds to the price of production. However, the employment of pure substrates in the fermentation medium reduces the number of steps involved in pullulan’s downstream processing, but achieving so comes at a cost. To meet the specifications for pullulan's particular usage, different downstream processing techniques are employed to attain the required degree of purity in the material. Generally, the number of downstream processing steps needs to be kept minimum to minimize production costs and handling times while also preventing pullulan loss at various stages of purification [121].
Liquid–liquid separations are processed after solid–liquid separations in the product recovery stage. Pullulan's downstream processing begins with the separation of solids from liquids. These solids are culture supernatants present in the cellular biomass after fermentation. The efficacy of filtration or sedimentation during solid–liquid separation is influenced by various factors. These factors include the kind of microbe employed in fermentation, as well as its shape and flocculation capabilities [121]. In this stage, cell biomass can be separated by filtering or centrifugation.
Pullulan is processed further by a series of procedures called liquid–liquid separation. Pullulan can be purified using both alcoholic and non-alcoholic organic solvents due to its renowned insolubility in organic solvents. Pullulan has also been processed downstream using standard methods such as chromatography, ultrafiltration, gel filtration, and dialysis. Aqueous two-phase systems have also been employed in a few situations to purify pullulan [121]. Pullulan undergoes a sequence of separate unit activities in its downstream processing that are combined to purify the product. The following steps are used for the extraction of microbial pullulan from the fermentation medium: (1) microbe harvesting; (2) removal of unwanted by-products, like cellular proteins and melanin; (3) polysaccharide precipitation; (4) ultracentrifugation/dialysis; and (5) freeze drying (product polishing step) [94].
Centrifugation
The type of association between the polysaccharide to be extracted and the cell determines the separation as well as purification procedures for capsular polysaccharides. Centrifugation is used to accomplish separation whenever the capsular extracellular polymeric substances (EPS) have a poor association with bacteria [82, 122].
Various techniques are used to extract various exopolysaccharide types from bacteria. Centrifugation is typically used to recover EPS that has slime forms. The kind and viscosity of the polysaccharide determine centrifugation speed and duration. Ultracentrifugation can be used to extract microbial biomass or waste products from the growth culture for improved outcomes in the lab [82]. For the separation of pullulan, the solid-state fermented medium was centrifuged at 10,000 r/min for 25 min. Pullulan present in the resultant supernatant was precipitated by adding double volumes of cold acetone for complete pullulan precipitation. Eventually, the precipitated pullulan was separated by centrifugation at 10,000 r/min for 20 min [21]. Centrifugation and cross-flow filtration are two methods for removing cell biomass [85]. While, activated carbon adsorption or the use of a salt and alcohol mixture are two methods for removing the melanin pigment [5]. Pullulan is soluble in water and insoluble in the majority of organic solvents. Thus, it is possible to extract the product from the purified supernatant through the addition of an adequate volume of organic solvents like ethanol, acetone, and isopropyl alcohol. The polysaccharides start to precipitate when the organic solvent is added. For greater purification, the first two stages must be repeated. Ion exchange and/or ultrafiltration are used to pullulan solutions to get a higher purity of the biopolymer [76].
Pullulan is usually purified by removing the cells from the culture, removing the melanin from the culture, removing the melanin pigment from the broth, precipitating the pullulan by adding the appropriate solvents and resuspending the precipitate. Finally, purifying the resultant mixture through ultrafiltration or chromatography [76]. It is possible to precipitate, dry, and mechanically grind purified pullulan into a powder [76, 85].
Physical treatment
Heat treatment has been utilized to enhance the separation of microbial cells from the broth in the case of thermostable EPS. Heat treatment has no impact on the viscosity of these polysaccharides; however, it does cause pasteurization, which destroys the microbes and inactivates the enzymes of the culture broth [82]. Extraction of capsular polysaccharides requires a distinct process because the capsular EPS must first be separated from the cells [123].
Chemical treatment
Centrifugation is preceded by more intensive treatments (e.g., alkaline treatment, treatment with NaCl, EDTA, and C2H5OH precipitation) for the tightly attached capsular EPS with the cells. In addition to chemical treatments, the microbial cell suspensions can be boiled for 15 min, heated at 60 °C in a saline solution, 65 °C in a mixture of phenol and water, or sonicated [82, 124].
Pullulan nanoparticles/nanocomposites/nanoformulation synthesis
The goal of nanotechnology is to know about and take advantage of the nanoscale processes that nature uses to create its constituent parts. According to Coltelli et al. [28], it is the process of creating and characterizing materials and structures at the nanoscale that has improved physicochemical qualities over bulk materials. To obtain a single material with better physicochemical properties, pullulan must be modified to combine the advantages of natural polymers with other substances [11]. Pullulan's function and application scope can be expanded through derivatization. It is possible to increase pullulan's activity by adding different chemical structures to its framework. It is possible to substitute other chemical groups for the nine hydroxyl groups that are found on Pullulan's repeating units (C6H10O5)n. Chemical processes such as amidification, copolymerization, sulfation, esterification, oxidation, etherification, and others are used in derivatization. Every derivative has distinct physicochemical features as a result of chemical change [125]. All the three types of pullulan derivatives preparation under the specific preparation condition are described in this section. There are various ways to prepare pullulan derivatives with their specific preparation condition.
Pullulan nanoparticles
The primary goal of nanotechnology is to create nanoparticles with predictable shape and size, polymeric film and even distribution. It is commonly known that different metal NPs have a wide range of beneficial uses in many fields, such as biomedicine and health care. As a result of their extensive use, metals like gold, silver, zinc, copper, and so forth are mainly in the spotlight. Nanoparticles can be prepared using a variety of techniques, such as chemical and physical approaches. This can effectively generate pure, well-defined nanoparticles, however, are costly, unstable, lengthy, and possibly hazardous to human welfare and the environment [126]. Green chemistry-based eco-friendly approaches to nanoparticle production could be a substitute for chemical synthesis [127].
Recently, a new "green" idea called the biosynthesis of biopolymers-mediated metal NPs has been developed in light due to their excellent water dispersity, abundance, non-toxicity, stability, biocompatibility, biodegradability, and eco-friendly qualities. So far, biopolymers such as pullulan have been widely employed for the synthesis of NPs [128]. But lately, research has mostly focused on the use of pullulan as a stabilising and reducing agent [129]. Pullulan was utilized for developing compression mouldings, fibres, drug delivery carrier materials, and edible films because of its promising adhesive and film-forming capabilities [13, 130].
Ghaffarlou and coworkers [131] synthesized a simple and green method for pullulan-stabilized silver and gold nanoparticles for the inhibition of quorum sensing. Using this approach, 15 mL of deionized water was stirred at 40 °C until pullulan was fully dissolved. Subsequently, 1 mL of either 0.01 mL of silver nitrate (AgNO3) or 0.01 mL of HAuCl4·3H2O was added to this mixture. The mixture was continuously stirred for 5 h while it was at room temperature. Ag(I) or Au(III) were reduced into zerovalent metallic states present in the metal ion-absorbed pullulan solution by heating it to 70 °C and shaking it for 5 h at 150 rpm. It resulted in the formation of AgNPs/Pull and AuNPs/Pull. Pull-Ag and Pull-Au solutions changed colour after heating to pink and light brown, respectively, signifying the production of metallic Ag and Au NPs. AgNPs/Pull and AuNPs/Pull solutions were employed unfiltered.
Additionally, pullulan-capped Ag NP was effectively created with the use of radiation-induced techniques including gamma irradiation. A reducing agent was not used in the preparation of stable Ag-NP/PL nanocomposites, which had an average size of 3.98 nm. Ag NP production was verified by UV–visible spectroscopy, which found a plasmonic band at 410–420 nm. The XRD pattern demonstrated that the Ag-NPs’ crystalline structure was fcc for every sample. TEM imaging confirmed that the Ag-NPs were evenly distributed throughout the pullulan matrix. Further, the fragmentation was induced by γ in the Ag-NPs. This resulted in a gradual decrease in the particle diameter of silver nanoparticles at higher doses of 50 kGy. Ag-NP/PL was discovered to have a negatively charged zeta potential and to be a stable, well-dispersed particle in a colloidal suspension. This Ag-NP/PL biofilm exhibited potential antibacterial properties against S. aureus. As a result, pullulan-capped Ag NPs are suitable for a wide range of applications such as antimicrobial biofilm packaging [132].
Hong and coworkers [133], synthesized phthalyl pullulan NP. They dissolved 1 g of pullulan in 10 mL of dimethyl formamide (DMF) and introduced 0.1% (by mole) of dimethylaminopyridine per pullulan sugar residue to the mixture as a catalyst. To the aforementioned solution, more phthalic anhydride was added at various molar ratios per pullulan: 6:1 (phthalic anhydride: pullulan; PPN1), 9:1 (phthalic anhydride: pullulan; PPN2), and 12:1 (phthalic anhydride: pullulan) (PPN3). This resulted in PPNs with varying levels of phthalic group substituted. The reaction was run in nitrogen for 48 h at 54 °C. To create self-assembled phthalyl pullulan nanoparticles, the resulting PPNs were dialyzed twice: once in DMF to eliminate any unreacted phthalic anhydride, and again in distilled water (D.W) for 1 day at 4 °C. Following ultra-centrifugation of the produced PPNs, the unreacted pullulan was extracted [133].
In support of this, Jayeoye and coworkers [134] demonstrated that pullulan (PUL) not only acted as a stabilizing agent but also took part in the production of the NP by adjusting the reaction environment's surface energy. They used tannic acid (TA) to synthesize pullulan-stabilized Au NP and then explored hazardous Ag+ detection based on Au/Ag core–shell nanostructure creation. Initially, 8 g of pullulan was dissolved in 100 mL of water and agitated at room temperature to create an 8% pullulan solution. The solution was held for an hour after being heated to 60 °C. The solution was kept swirling while the heat supply was cut off. After that, it was kept for an entire night at 4 °C to guarantee the polymer's total dissolution. The fabrication of TA/PUL-AuNPs was done as follows. 40 mL of 2.5% PUL (optimal amount of biopolymer) was poured into an aluminium foil-shielded beaker while shaking. Next, 0.8 mL of tannic acid (18 mM) was introduced, followed by 1.2 mL of 0.1 M NaOH, and lastly, 1 mL of Au (III) chloride trihydrate (78 mM) was quickly introduced. Before being utilized, the combination was kept at room temperature for an hour while being vigorously stirred. Finally, it was then kept in an amber bottle at 4 °C [134].
In another study, a polymeric NP carrying valsartan was produced [135]. Valsartan is a cardiovascular drug function as an angiotensin II receptor blocker. Pullulan acetate served as a degradable polymeric structure, while Pluronic F127 was used as a stabilizer to create pullulan NP by the nanoprecipitation process. In short, 5 mL of acetone was used for dissolving pullulan acetate. A pullulan/acetone solution was used to precisely weigh and dissolve valsartan. 15 mL of D.W was used to dissolve 10 mg of pluronic-F127. The aqueous phase solution was mixed with the organic phase dropwise until the organic solvent completely evaporated. This was done while the mixture was magnetically agitated at 1000 rpm and room temperature (25 °C). To extract the unbound drug from the created NP, the final nanosuspension was centrifuged for 30 min at 30 °C and 10,000 rpm. According to Pavaliou and coworkers [135], the precipitate-carrying NP was resuspended in D.W and screened through a 0.22 μm Millex filter membrane after the supernatant was thoroughly drained. The NP-carrying valsartan exhibited a limited dispersity (polydispersity index < 0.2), nanometric diameters (below 200 nm), and high trapping capacity of valsartan. According to this study, pullulan and its derivatives have a lot of possibilities for creating NPs that could be used to carry drugs for the cardiovascular diseases.
According to reports, pullulan-based NPs are effective against a range of microbes with their antibacterial properties. Pullulan was utilized as the reducing and stabilizing agent which shows the fast technique for the biosynthesis of silver nanoparticles (AgNPs) stabilized by pullulan [128]. The resulting NPs have been discovered to be in spherical shape and range in size from 10 to 55 nm on average. Additionally, the antimicrobial activity of these pullulan stabilized AgNPs was assessed by employing the agar well diffusion technique against two Gram-positive (Bacillus subtilis and Staphylococcus aureus) and two Gram-negative (Escherichia coli and Serratia marcescens) bacteria. According to the results obtained by this experiment, it was clearly indicated that all pathogenic bacteria were suppressed in a dosage-dependent way. Nevertheless, the examined strains of B. subtilis, S. aureus, S. marescenes, and E. coli showed a reduction in the inhibitory impact of pullulan-stabilized NPs [128].
Pullulan-mediated NPs showed notable effectiveness against foodborne and multidrug-resistant bacteria along with common infectious microorganism. Pullulan biopolymer was used with AgNPs, which then tested for antibacterial, antifungal, and antibiofilm properties in vitro [136]. These NPs, which ranged in size from 2 to 40 nm, possessed a rod-like shape and a hexagonal shape. Their antibacterial activity was tested against infectious agents, i.e., responsible for foodborne illnesses and are drug-resistant, including E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and L. monocytogenes. They were also tested for their antifungal and antibiofilm properties against pathogenic organisms that form biofilms, including E. coli, Bacillus cereus, L. monocytogenes, and P. aeruginosa.
According to the findings, P. aeruginosa, K. pneumoniae, and E. coli are the three pathogenic bacteria that are most sensitive to AgNPs. Contrarily, L. monocytogenes, a pathogen found in food has been shown to be less sensitive to biogenic AgNPs. Both fungal pathogens were discovered to be sensitive to AgNPs in a way, i.e., dose-dependent with regard to antifungal action. Additionally, beneficial behaviors against all biofilm microorganisms have been observed [136]. The formation of AgNPs has also been explored with pullulan produced from the fungus Aureobasidium mangrovei, which was isolated from Oman. Additionally, the antibacterial and antifungal properties of the resulting pullulan-AgNPs were also assessed towards bacteria including E. coli, S. aureus, B. cereus, and P. aeruginosa as well as fungi including Curvularia lunata and Fusarium incarnatum.
All investigated bacteria and fungi responded well to the ability of pullulan-mediated silver nanoparticles to limit their growth [137]. The previous findings of many authors revealed that Gram-negative bacteria are more susceptible to pullulan-capped AgNPs than Gram-positive bacteria, i.e., generally described in terms of distinctions between the cell wall compositions of both bacteria [136, 138]. As Gram-positive bacteria are made up of a thicker three-dimensional peptidoglycan layer than Gram-negative bacteria. In this way, the peptidoglycan layer prevents AgNPs from penetrating Gram-positive bacteria [139].
The previous studies regarding the antibacterial activity of pullulan-stabilized NPs are in conflict [128, 136]. The physical and chemical characteristics of NPs, such as size, shape, and surface charge, may represent the primary cause of the variation in AgNPs' action on the two types of bacteria [140, 141]. When comparing three different shapes of AgNPs such as spherical, rod-shaped, and truncated triangular, it was found that the latter truncated triangular had the strongest antibacterial properties [142]. But most recently, it was revealed that spherical AgNPs had the highest antibacterial activity in the inhibitory zone, followed by disk AgNPs and triangular plate AgNPs [143]. The pullulan-stabilized AgNPs produced in the two investigations mentioned above have also multiple forms [144].
AgNPs' antibacterial properties result in the release of Ag ions [Ag +] from the NPs [Ag°]. Because of this, the surface area of NPs which is determined by their shape depends on the number of Ag ions they discharge [143]. Pullulan is regarded as a biopolymer substance for the production of Ag NPs to enhance the reduction mechanism excluding the use of accelerating, reducing, or complexing agents. Pullulans polysaccharide structure is also thought to increase its antibacterial properties by surrounding Ag-NPs, resulting in more stable, uniform, and monodisperse NPs. Furthermore, the particle size can be adjusted to the appropriate size by adding capping agents like polymers, which include pullulan [132].
It has been noted that the pullulan polymer works as a capping and stabilising agent, causing the synthesis of stabilized pullulan-capped AgNPs after gamma irradiation, which first decreased the Ag ions [132]. AgNPs are stabilized and protected from agglomeration by the capping process. Additionally, after capping, surface-capped AgNPs exhibit improved antibacterial properties [15, 145]. These pullulan-stabilized AgNPs have a surface charge of -72 mV, making them extremely stable with little tendency to aggregate. The significant antibacterial action of these NPs for S. aureus was also mentioned in the reports [132]. Similar to this, a nanocomposite thin film synthesized from transparent pullulan and AgNPs was assessed for its effectiveness against A. niger [146]. On observation, disruptive effects on conidia following this interaction were clearly seen in the scanning electron microscopic analysis of A. niger conidia after treatment with this pullulan nanocomposite film. These results imply that conidial damages may be linked to cell wall breakdown and eventual cytoplasmic leakage, which results in cell death [146].
Additionally, it has been noticed that the cell disruption process is dependent on the AgNP concentration in the film; the greater the concentration, the greater the cell disruption observed.
The aforementioned research demonstrates that pullulan-based NPs have significant antibacterial capability against a variety of microbial diseases, among which multidrug-resistant and biofilm-forming organisms [15].
Pullulan nanocomposites
Pullulan showed a potential application in food packaging and biomedical applications. However, pure pullulan films have significant disadvantages regarding their low physical and chemical properties such as brittleness and antimicrobial properties. In the food sector, designing pullulan-derived blends and composite films is the best way to get beyond these inherent restrictions. Thus, it acquires multipurpose packaging solutions that enhance the safety of food, preservation time, and quality. Moreover, combining nanoparticles with biopolymer has drawn interest as a way to modify biopolymers [147, 148]. When compared to traditional micro composites, the high surface area and uniform dispersion of the nanoparticles in the polymeric matrix produce exceptional qualities [148, 149]. However, because of their strong antibacterial and antioxidant properties, natural essential oils are a viable option for creating innovative packaging materials that will extend the period of storage of food items [150]. A derivative of pullulan showed enhanced mechanical properties.
Pullulan’s mechanical properties can be improved by carboxymethylation and periodate oxidation, which in turn improve the efficiency of nanocomposite materials. The process of carboxymethylation requires adding carboxylate or carboxymethyl chitosan to the chemical makeup of pullulan. As illustrated in Fig. 3, carboxymethylated pullulan can be made by reacting pullulan with isopropyl alcohol and sodium chloroacetate. As an outcome, pullulan's structure acquires carboxylate groups [11, 151].
The mechanical durability of pullulan is also improved by periodic oxidation. Pullulan's compatibility with blood and biodegradation properties have led to its selection as a carrier. Nevertheless, there is no functional group in the pullulan structure that can transport the macromolecular product. Pullulan's interaction with sodium periodate that adds an aldehyde group is known as "periodate oxidation of pullulan." The polysaccharide directly couples with the drug upon the addition of the aldehyde group, making it an appropriate transporter [25].
Pullulan has been combined with different functional agents like sakacin-A [152], thymol [153, 154], silver nanoparticles [131, 155], essential oils and nanoparticles [150, 155, 156], lysozyme [157], cholinium carboxylate ionic liquids [158], graphene oxide [159], bacterial cellulose [160, 161], nanofibrillated cellulose [162, 163], and gelatine [103]. Due to the interaction of the different individual components, either chemically or physically, it enhances the films' mechanical properties [148] or provides their bioactivity, especially antibacterial properties [158].
Additionally, silicon oxide was used to create nano-SiO2 (NS), an amorphous powder that exhibits a tridimensional structure, using the SiO(2-x) formulation, where x ranges between 0.4 and 0.8 [148]. Because of its remarkable properties such as its small size, high surface energy, wide specific surface area, unsaturated chemical bonds, and hydroxyl group on the surface, NS is easily dispersed across polymer matrices and is increasingly being used as a nanofiller in nanocomposites [148]. SiO2 is the primary component of sand in various world regions [164]. Nearly every NP exhibits a few negative impacts on the human body. However, there is little worry regarding NS's eco-pollution [148, 164].
Pullulan was utilized to create a nanocomposite bone scaffold. Ag-silica Janus particles (Ag-silica JPs) are a nano-platform, exhibiting a ball-stick morphology. Ag-silica JPs and pullulan were combined to create a nanocomposite scaffold that had improved mechanical and biological characteristics. The synergistic capabilities of Ag and silica’s antibacterial and bioactive activities were obtained by synthesizing Ag-silica JPs using a one-step sol–gel technique [165].
Utilizing polysaccharide-based nanofillers is a good way to create bio-nanocomposite films. These films are far better suited to applications such as surface hydrophobicity, mechanical strength, and barrier than pure biopolymer coatings. This study examined the effects of starch nanocrystals derived from waxy rice starch (WSNC) and native starch (NSNC) on the physical–chemical characteristics of pullulan-based nanocomposite films and the successful maintenance of fresh beef [166].
Chitosan-pullulan-silver-nanocomposite (CSPN) films is a novel model drug moxifloxacin (Mox) loaded ternary nanocomposite. This was effectively created employing traditional solvent casting of an aqueous composite solution. Pullulan and chitosan, two polysaccharides, were used as reducing, capping, or stabilizing agents in ternary nanocomposites. Both were shown to be a potential carrier system for Mox and AgNPs. The produced films known as ternary nanocomposite (CSPN) showed antibacterial effectiveness. Thus, it may be used successfully in tissue engineering, drug delivery, scaffolding for skin conditions, wound healing, and other medical uses [167].
Pullulan nanoformulation
Drugs are delivered at a predefined rate through sustained-release drug delivery nanoplatforms. This keeps the delivery of drug dosage steady for a predetermined rate of time while minimizing adverse reactions. Sustained-release drug delivery nanomaterials can be classified into several types. First is the lipid-based nanoplatforms (including nanostructured lipid carriers, solid lipid nanoparticles, cubosomes, microemulsion, and liposomes). Second is the polymer-based nanoplatforms (dendrimers, polymeric nanocarriers micelles, hydrogels); metallic nanocarriers (superparamagnetic nanocarriers, silver, and gold-based nanocarriers); carbon-based nanoplatforms (fullerenes, carbon nanotubes, nanodiamond); nanoplatforms derived from cells or biomimetic ones (exosomes, virus-based nanocarriers). Third is the protein-based nanoplatforms (zein and albumin nanoparticles) [125].
Biodegradable polymeric nanosystems are now becoming one of the most interesting drug/gene delivery systems and anticoagulants. Pullulan is a type of biopolymer known to be both extremely biocompatible and biodegradable. Due to their special ability to self-assemble into core–shell structured NPs, they are, therefore, frequently employed in these nanoformulations [168]. Since NPs can greatly minimize drug adverse reactions and increase the efficiency of drugs, targeted therapy using NPs has gained attention in the treatment of cancers [169]. Its blood compatibility and biodegradation features make it suitable to be utilized as a carrier for macromolecules. However, there is no functional group in the pullulan structure that can transport the macromolecular product. Moreover, it is a hydrophilic polymer that dissolves in water, which makes it challenging to encapsulate charged and hydrophobic proteins [170]. Thus, its low stability, hydrophilic nature, and lack of functional groups are the major challenges limiting the application of the delivery system. These three bottlenecks remarkably affect the in vivo release and bioavailability of the drugs [168].
Pullulan stability and hydrophilicity can be modified by means of succinylation, cholesterol integration, and urethane derivatization and employed as a stabilizing agent. To address this problem, pullulan was modified to carry hydrophobic or charged regions. Through a reaction with negatively charged succinic anhydride (acetic, propionic, and butyric anhydrides), succinylation triggers the integration of the carboxylic group into pullulan. It results in the formation of pullulan acetate (PLAc), pullulan propionate (PLPr), and pullulan butylate (PLBu). This renders pullulan suitable for the transport of positively charged proteins in drugs [11, 171]. As illustrated in Fig. 4, when 4-dimethylaminopyridine (DMSO) is present as a catalyst, succinylation takes place for 24 h at 40 °C.
In pullulan, C-6 acts as the chosen location for succinic anhydride. It is necessary for N, N′-carbonyldiimidazol to activate the COOH group in succinylated pullulan. The amine and the resultant derivative can be linked [170]. SPA (succinylated pullulan acetate) was used to create a microsphere for the transportation of protein. The double emulsion approach was used for loading lysozyme (Lys) as a model protein drug into the microsphere, replacing PLGA [poly (dl-lactic acid-co-glycolic acid)] with SPA. As a result, extended stability of proteins and three times greater protein loading capacity were observed.
Cholesterol content modification is another method to improve the long-term viability of the pullulan nanoformulation. Pullulan integrated cholesterol as a hydrophobic group. Pullulan which had been treated with cholesterol was used to create an epirubicin self-assembled nanoparticle. To do this, a hydrophobic component, like long carbon chains or cholesterol groups, must modify pullulan [172]. An amphiphilic polymer will be produced as a result of this. This can be used to create the NP that self-assembles. Pullulan with a hydrophobic group is made by attaching the cholesterol group to pullulan using alkylenediamine and monochloroacetate as depicted in Fig. 5.
Cholesterol-modified nanoparticles demonstrated improved drug stability with an extended half-life, increased blood plasma concentration, and relatively low toxicological effects for drugs [172]. To load the drug mitoxantrone (MTO), an additional NP was developed utilizing pullulan conjugated with cholesterol. The chosen drug has a lot of negative consequences because it is not specific to cancers. Targeted administration of the drug improved stability, permeability, retention impact, effectiveness, and decreased drug update by normal tissues. These were the outcomes of loading MTO in cholesterol-modified pullulan NP. Human serum albumin (HAS) was added again to the MTO-CHP NP, which stabilized the release of the drug by decreasing the rate of drug distribution in acidic environment [173].
Yuan and coworkers [174] worked on cholesterol content modification of pullulan and synthesized three kinds of pullulan NP polymers by the dialysis method. These three pullulans were called CH-modified animated pullulan (CHAP), CH-modified carboxylated pullulan (CHSP), and cholesteric hydrophobically (CH)-modified pullulan (CHP). This demonstrated the interaction between HSA protein and the various surface elements of NPs. When polymers have the same degree of cholesterol substitution, the number of charge groups changes the size of NPs. The quantity and kind of charge of nanomaterials were strongly correlated with their size, charge, drug loading characteristics, interaction with HSA, and drug release. With greater amino substitutions, CHAP NPs comprised the largest, followed by CHSP NPs in the next place, and CHP NPs in the last place, all having a similar level of hydrophobicity alteration. The properties like the binding constant, the delayed drug release, and the HSA coverage all depended on the size and surface charge of the NPs. CHP NPs exhibited the maximum coverage, while the positively charged CHAP binding constant remained the strongest, indicating the quickest drug release. The drug release of NPs was further delayed by the addition of HSA. The slowest rate of drug release was observed when CHAP NPs adsorbed HSA.
In addition to the first two pullulan derivatives, the pullulan urethane derivative seeks to improve the nanoformulations' thermostability [1, 175]. The synthesis of urethane derivative improves pullulan's solubility in a wide range of organic solvents, water resistance, and thermostability [176]. The preparation involves reacting pullulan with either hexyl or phenyl isocyanate (HIC), which introduces N-phenyl urethane or N-hexyl urethane groups into the pullulan structure. The insertion of PIC and HIC modifies pullulan’s features in different ways. For example, adding more PIC causes pullulan’s tensile strength and glass transition temperature to drop. Pullulan becomes more soluble in ethanol when PI is added, whereas it becomes more soluble in acetone and H2O when HI is added [11, 175].
Opportunities of pullulan nanopolymer
The pullulan production from agro-food processing waste has been one of the exciting approaches to managing piles of an organic-rich substrate with the production of value-added products such as nanoparticles/composites/hydrogels [177]. Pullulan nanopolymer films are superior to conventional food packaging with increased antimicrobial activity, physico-chemical properties that increase their durability.
Furthermore, integration of other NPs with pullulan polymer would be a ground-breaking technique by shrinking the edible film particles' size to the nanometre (nm) range [178]. It enhanced the film's physicochemical strength, food bioavailability, taste, texture, and consistency, achieved through modification of particle size, possible cluster formation, and surface charge of food materials [179]. This section summarizes the application of pullulan nanopolymer in the form of nanoparticle/nanocomposites/nanoformulation in different sector.
Pullulan nanocomposite in agriculture and food
As a packaging agent
Packaging is described as a vital tool for storing food products for a prolonged duration of time without spoiling while maintaining their original, chemical, and sensory attributes [180]. Agricultural products (seeds and crops) and food products (vegetables and foods) are protected by the packaging to avoid impurities, dirt, microbes, and chemicals. The application of non-biodegradable petroleum-derived packaging for post-harvested horticulture products is extremely hazardous to the health of humans and the environment. Thus, the use of natural biodegradable packaging materials for agro-food packaging has received particular attention. Due to its water-soluble, non-toxic, and non-mutagenic edible properties, experts think pullulan may prove a viable polymer for their application in this scenario. Primarily because of its superior film-forming and adhesion qualities, this biopolymer can create edible films or coatings for a variety of foodstuffs [181].
A lot of emphasis has recently been focused on the production of edible films carrying antimicrobial chemicals as a successful method to increase the period of storage of fruits and vegetables and limit the possibility of spoilage by pathogens. Approximately one-third of conventionally grown crops suffer destruction, mostly as a result of infestations of insects, harm from microbes, extreme weather, poor soil performance, and a lack of available nutrients. To solve these problems, we urgently need more advanced technology. In this way, the agro-technological revolution, i.e., about to change the current agricultural system while ensuring food security has been made possible in part by nanotechnology. As a result, NPs are evolving into cutting-edge materials, so they will change the way of contemporary agriculture. The United States Department of Agriculture-USDA first placed up a "roadmap" for the use of nanotechnology utilization in the areas of agriculture and food in December 2002 [182].
Considering the potential for the creation of biodegradable polymers, nanocomposites are opening up new avenues for investigation. Polymer-silicate nanocomposites were found to have better properties, including high thermal stability, durability, and enhanced gas barrier capabilities [183]. AgNPs were combined with petroleum-derived polymers like PVC (polyvinyl chloride), PE (polyethylene), and PET (polyethylene terephthalate) to provide a more opulent material for packaging. AgNPs are extremely effective against a variety of microorganisms, such as bacteria, viruses, and fungi, along with enhancing packaging protection [184,185,186].
In a single investigation, the authors showed the antibacterial effectiveness of pullulan coatings integrating with AgNPs, zinc oxide NPs (ZnONPs), oregano oil (2%), and rosemary oil (2%) against L. monocytogenes and S. aureus. These bacteria cause degradation of the nutritional value of Turkey deli meat [187]. These findings also revealed that S. aureus was less resistant to the edible pullulan coatings including the aforementioned NPs and essential oils than L. monocytogenes. In a different investigation, pullulan coatings were synthesised with a combination of antibacterial and anti-browning chemicals [188].
Pullulan was utilized as a thickener that may produce semipermeable films, chito-oligosaccharide served as an antibacterial agent, and glutathione proved as a powerful reducing agent. Additionally, the effectiveness of these edible coatings was assessed on apple slices stored at a low temperature. When compared to the control, it proved that pullulan coatings successfully prevented microbial growth and the respiration rate of apple slices. Additionally, it was revealed that these pullulan-based coatings prevented enzymatic browning, maintained firmness, and reduced weight loss. These results implied that the use of pullulan-based coatings along with glutathione and chito-oligosaccharides proved to be beneficial to increase the storage period of apple slices [129]. The pullulan-based edible coating can be applied to extend the storage period of eggs as well as fruits. An experiment conducted in which fresh eggs were coated with pullulan and pullulan-carrying nisin while kept uncoated eggs as a control [189]. According to the findings, pullulan-coated eggs are healthier as compared to uncoated and pullulan-carrying nisin-coated eggs because they lose less weight and yolk index.
It was observed from these investigations that pullulan coatings may maintain fresh eggs' internal integrity intact, increase their storage period, and minimize weight reduction [189]. Additionally, other pullulan-based composites that are combined with distinct polymers, like pectin and chitosan, to synthesize films/coatings for food play an important role. Edible films were developed by combining pectin and pullulan in a variety of ratios and their effectiveness were assessed in food packaging [190]. Following to FTIR assessment, an intermolecular H-bond was seen to develop between the carboxyl group of pectin and the hydroxyl group of pullulan, which strengthen the complex. Furthermore, the combination of pullulan and pectin used to make the film that exhibits the maximum heat resistance and surface hydrophobicity. Compared to the individual films, this combination boosted strength while maintaining flexibility and stiffness.
In a different work, researchers made pullulan-chitosan and pullulan-carboxymethyl chitosan (CMCH) integrated films and examined their viscosity, mechanical capabilities, barrier qualities against H2O and O2, water solubility, and additional features. According to reports, the mechanical and O2 barrier qualities of the film were considerably altered by the incorporation of chitosan or CMCH into pullulan. Pullulan and chitosan should be blended 1:1 to produce films with the above-mentioned properties. The use of such films in food packaging has the potential to extend the storage period of food items [129]. Comparable to this, Li and coworkers [191] investigated the pH impact on the performance and characteristics of pullulan-chitosan blended films. It has been noted that the extra conformation of chitosan in a pH 4.0 solution raised the intermolecular interactions with pullulan when compared to the more compact coiled form. This provides higher tensile strength and barrier capacity of the pullulan-chitosan film and higher viscosity of the film-forming solution. All of this research showed that the inclusion of edible coating/ films made of pullulan considerably aids in maintaining the nutritional value of fruits and food items by preventing their spoiling from pathogenic microbes.
Pullulan-based smart packaging that is coated with NPs and essential oils is becoming more popular these days because of its potential for antibacterial use in a variety of industries. A report addressing the use of pullulan active packaging combined with AgNPs and essential oils to lessen the perishability of meat recently came out [192]. This study examined the intriguing potential of pullulan active packaging combined with various NPs and essential oils. These active coatings are particularly potent in the growth inhibition of Salmonella typhimurium, S. aureus, E. coli, and Clostridium perfringens. This has been causing outbreaks in meat by the elimination of cholesterol oxidation products that are hypothesized as the mechanism. All these infections may be efficiently controlled by pullulan active packaging, which also significantly lengthens the storage period and increases the popularity among consumers of meat. Previously, the antimicrobial activity of pullulan films incorporating essential oils and NPs against four foodborne pathogens was investigated [193]. According to preliminary research, 2% oregano essential oil proved efficient against S. aureus and S. typhimurium but poorly efficient against L. monocytogenes and E. coli O157:H7. Although, in contrast to 1% of rosemary essential oil, 2% proved to be efficient over all four of the aforementioned microbes. They were also discovered to be significantly more effective when combined with zinc oxide and silver NPs against the tested microbes. The findings showed that pullulan-based edible films in conjunction with NPs or essential oils increased the quality of fresh, chilled, or further processed meat and poultry items.
Comparable to this, a pullulan film incorporating caraway essential oil was created and assessed the film's impact on the nutritional content of fresh baby carrots as well as its antibacterial effectiveness against Salmonella enteritidis, S. aureus, Saccharomyces cerevisiae, and A. niger [194]. All of the examined microorganisms were found to have their growth severely hindered by caraway essential oil. All of these food-borne pathogens were discovered to be more susceptible to pullulan films carrying 8% to 10% caraway essential oil. Additionally, these films were also seen to maintain a higher visual appearance on fresh baby carrots than untreated samples [15]. The various pullulan-based edible films/coatings and their possible application in the agro-food sector are summarized in Table 1.
Pullulan nanoformulation in biomedical research and development
Pullulan has GRAS certification for safety by the FDA in the United States due to its physicochemical properties and eco-friendly approach [199]. Additionally, it is recognized as a safe material under Japanese standards for ingredients. It offers a wide range of possible uses in biomedical research and development as highlighted in Table 2. The diverse role of pullulan in drug delivery, gene delivery, tissue engineering, anticoagulating agent, and vaccination is indicated in Fig. 6 and discussed below in detail.
Pullulan and its derivatives in drug delivery
The exceptional permeability exhibited by pullulan and its derivatives plays a crucial role in ensuring the optimal preservation of pharmaceuticals. This substance is versatile and can be utilized in a range of nanoformulations, such as nano gels, NPs, and microspheres. When combined with drugs, pullulan formulations exhibit reduced toxicity toward healthy cells and have a greater ability to target cancerous cells or tissues [17]. Sick cells use integrins of cell adhesion receptors to internally incorporate formulations, as explained by Haas and Plow in 1994. Drugs contained within cholesterol-modified pullulan nanoparticles (NPs) have a short half-life and a tendency to concentrate on liver cells [200].
Research has suggested that incorporating cyclodextrins into pullulan microspheres can greatly enhance the effectiveness and speed of drug dissolution [201]. Furthermore, it has been reported that CHP has the potential to help transport drugs through the blood–brain barrier and treat neurological conditions such as Alzheimer's disease [202]. Research has shown that modifying cholesterol can enhance the biological activity of insulin and provide long-term circulation stabilization [203]. Pullulan formulations have proven to be effective drug carriers for targeting multiple diseases such as hepatitis C virus [204], autoimmune diseases [205], atherosclerosis, graft rejection, ischemia, asthma [206], liver cancer [207], and intestinal cancer [208]. According to Laha and Maiti, a stearyl pullulan nanostructure with a core–shell composition containing a substantial quantity of glipizide in its hydrophobic core displayed consistent drug release in simulated gastrointestinal conditions [209].
Carboxymethyl pullulan (CMP) NPs have the capability to form hydrazone bonds with drugs that possess antioxidant properties. This interaction is crucial in the safe delivery of drugs to cancer cells and provides a significant increase in binding affinity for lymph nodes, spleen, and other tissues [210]. Formulations that include pullulan acetate (PA) could coat hydrophobic drugs. This allows for targeted delivery to tumor cells and prolonged circulation times in the bloodstream. [211]. A glucose-sensitive CMP hydrogel has been described as a means of achieving regulated and intelligent insulin release [212]. The sensitivity of PA nanoparticles to ionic strength makes them ideal for delivering radioisotopes to tumor cells [213]. The modification of pullulans using folate particles has been shown to improve its ability to target cancer cells in various areas of the body such as the kidney, brain, lung, breast, and ovary [214].
Several additional pullulan derivatives have proven to be highly effective in delivering drugs to specific targets. It includes succinylated pullulan [215], diethylenetriamine pentaacetic acid pullulan [204], pullulan-deoxycholic acid [216], and polyethyleneimine pullulan [97, 217]. A poly(acrylamide) graft pullulan derivative that responds to electricity has been identified as a transdermal delivery technique [218]. Some pullulan formulations are sensitive to changes in pH and temperature. These formulations have lower toxicity to normal cells and better retention in tumor cells, resulting in improved drug effectiveness.
Pullulan and its derivatives in gene delivery
Pullulan has been found to be an excellent carrier for DNA or genes due to its exceptional potency. When transformed into hydrogel nanoparticles with a hydrophilic core, it becomes a safe and secure option for delivering genes to specific targets. These nanoparticles can be further improved by attaching a ligand to their surface, allowing for even greater accuracy and efficiency in gene delivery. Modified pullulan formulations show great promise in targeted gene delivery, protecting the genes from degradation. New research findings have revealed that pullulan could exhibit self-aggregation features when it is joined together with a cholesterol group [17], forming a hydrophobic core within the formulation. Such characteristics allow the specific delivery of diverse hydrophobic proteins or genes using CHP [241]. Proteins for immune cell treatment, like truncated HER2-147, have been successfully delivered using this method [242]. Pullulan hydrogels have the remarkable ability to deliver DNA to cancer cells for an extended period, while also having the capacity to effectively load plasmid DNA [243].
Utilizing cationic pullulan formulations like polyethyleneimine pullulan (PEIP) can confidently target the liver with specific genes [244]. PEIP is often used to target cancerous cells because it reduces the negative effects on DNA or genes [245, 246]. Pullulan made of diethylaminoethylamine can be formed into tubular or three-dimensional matrices to deliver genes to nearby arteries or muscle cells while also shielding them from DNase destruction [247]. The effectiveness of gene transfection is increased by modification with folate [54], which also improves gene silencing. According to reports, pullulan spermine supports the release of dopamine and is said to supply the notch intracellular gene for the treatment of Parkinson's disease [248]. Additionally, it is employed to transport genes for neurons [249] and target genes for human bladder tumor cells [250]. Formulations of pullulan derivatives, such as pullulan-g-poly(L-lysine) [251], pullulan-protamine [221, 252], and succinylated pullulan [253] are effective for the delivery of specific genes with minimal cytotoxicity.
Pullulan and its derivatives in tissue engineering
Pullulan derivatives have proved to be highly effective in tissue engineering and wound healing. Various pullulan derivatives were utilized to develop three-dimensional scaffolds with the aim of improving the self-healing process of damaged tissues or organs [17, 254, 255]. The formulations exhibit outstanding mechanical properties, high hydration capacity, and exceptional biocompatibility [256].
The scaffolds made from pullulan exhibited macro-porosity which facilitated the controlled release of nutrients and metabolites for tissue engineering purposes [257]. In tissue engineering and wound healing, pullulan derivatives have found extensive use. These derivatives have been employed as scaffolds for bone, vascular endothelial, and skin cell regeneration. Additionally, they possess anti-adhesion properties, which help to reduce the risk of intestinal blockage and postoperative discomfort [258] as well as avoid infection of the wound [259, 260]. These hydrogels have a crucial role in the growth of osteoid tissue, orthopedic procedures, and recovery of maxillofacial injuries. By promoting the migration of osteoprogenitor cells, pullulan hydrogels accelerate the healing process of bone deformities in the skull [261, 262] and aid in cartilage tissue regeneration [263]. Pullulan scaffolds can be utilized to replicate skin architecture for effective wound recovery [264]. Nanogels from CHP are beneficial for wound healing as they promote controlled release of prostaglandin E1, leading to neovascularization and neoepithelialization [265].
Pullulan derivatives in the form of phosphorylated pullulan, carboxylated pullulan [266], pullulan-cellulose acetate [267], and others have been employed for tissue engineering purposes. The antioxidant capabilities of the salt-induced pullulan hydrogels shield cells from oxidative damage and promote wound healing [268]. Small to massive bone abnormalities are repaired with pullulan microspheres [263]. Pullulan-based films have great significance in skin tissue engineering since they are extremely biodegradable and biocompatible [1]. Research has shown that pullulan membranes can enhance the growth and division of fibroblasts, ultimately aiding in the process of wound healing by allowing for the necessary movement of cells [269, 270].
Pullulan derivative for improved anticoagulant property
Anticoagulants are used to prevent the development of blood clots and thickening. Heparin is a commonly used anticoagulant, but sulfated pullulan has been developed as an effective substitute. To produce sulfated pullulan, pullulan is reacted with a sulfur trioxide-pyridine complex in DMF at 75 and 95 °C for 3 to 8 h. Alternatively, pullulan reactions using N, N-dimethylformamide (DMF) complex can also provide sulfated pullulan, but this may result in highly reactive and less viscous pullulan. Therefore, SO3-Py (pyridine) complexes are preferred to produce stable and viscous derivatives. Sulfation at C-6 is the most favourable location for sulphated pullulan, followed by C-3, while C-4 is primarily left unsulfated. Pullulan sulfate has been found to act as an anticoagulant by interacting with various coagulation phases [11, 271].
Pullulan and its derivatives in vaccination
Treatment of cancer cells requires an appropriate immune response to antigen peptides, which can be accomplished with peptide and protein vaccines. According to reports, CHP has been responsible for cancer immunotherapy by utilizing the protooncogene for human epidermal growth factor receptor 2 to treat carcinomas of the stomach, breast, bladder, ovary, and dendritic cells. CHP has been coupled with additional cancer-related antigens, such as NY-ESO-l or MAGE A4, to develop a cancer vaccine [272]. The killer and helper T immune cells that deliver antigens can be stimulated by this pullulan complex. The CHP-based NY-ESO-1 vaccine stimulates an efficient immune response for the therapy of esophageal cancer [273]. As a mucosal vaccination, the cationized version of CHP is utilized to defend against Pneumococcus-related respiratory infections and colonization [274, 275].
An effective vaccination that first adheres to the nasal epithelium and then affects the mucosal dendritic cells is made up of cationic CHP and Clostridium botulinum type-A neurotoxin [276]. According to reports, the cationic version of CHP can combine with tetanus toxoid and aids in the development of potent mucosal and systemic immune responses. Because it contains fluorescent quantum dots for labelling inner body cells and creating visual representations of the inside of the body, the amine-modified CHP has prospective for medical imaging [277]. Infrared dye 900 is more effectively retained in sentinel nodes and has a smaller dispersion when combined with CHP NPs [278].
In addition, diethylenetriamine pentaacetic acid pullulan has drawn interest in cancer treatment using magnetic resonance imaging [279]. Pullulan derivatives are highly effective in enhancing insulinotropic action. Pullulan sulfonyl urea was previously mentioned in the production of an artificial pancreas to activate the insulinotropic function of pancreatic islets to re-establish an ordinary insulin secretion pattern [280]. It encourages the release of insulin both at low and high blood glucose concentrations.
Pullulan and its derivatives in expanding plasma
Patients who have experienced a medical condition or injury experience severe bleeding, which lowers their osmotic pressure and causes their blood vessels to burst. Shallow blood circulation might result in immediate death or chronic problems for the patients. Pullulan and its derivatives are employed in place of blood plasma to resolve these issues [281]. Pullulan is known to be entirely non-toxic and very compatible with the human body. Thus, it can be utilized to support normal blood flow by preserving the osmotic pressure inside blood vessels. After the intended therapeutic effect, it can be readily removed and metabolized to balance the loss of blood [1, 282]. It was observed that a γ-irradiated pullulan with low molecular weight and viscosity may expand blood plasma [281]. Chemical changes cause pullulan to develop a resistance against the amylase and give it the capacity to survive in blood vessels [283]. By substituting isovolumetric blood, pullulan aids in the regulation of blood microcirculation to a healthy condition, cardiac contraction rate, cardiac output, and blood circulation index [88].
Conclusion
The current review provides useful information regarding the production of pullulan from agro-food waste and their agro-food and biomedical applications. Pullulan, in particular, offers important advantages like non-mutagenic, toxin-free, non-immunogenic, and non-carcinogenic natural polymeric properties. Unfortunately, its high price restricts its successful applications at commercial and industrial level. The agro-food wastes are rich in nutrients, organic and inorganic matter. These wastes proved to be a good feedstock for pullulan production. Agro-food wastes showed recalcitrance to degradation that makes the utilization of them as substrate difficult by microbes during fermentation for pullulan production. This drawback could be overcome by applying various valorization methods. Thus, exploitation of these agro-food waste proved to be a cost-effective feedstock for pullulan production. Another drawback of this polymer is its poor mechanical property and the unavailability of functional groups to carry macromolecules, which restricts its application in various fields. Reinforcement of biodegradable pullulan with any active substance such as nanoparticles, nanogels, microspheres, and essential oils may be selected as the alternative technology. Additionally, the nanoparticles of pullulan derivatives created their prospects in serving as gene/drug vehicles or carrier in the biomedical field. To increase the biocompatibility and stability of pullulan for biomedical and tissue-engineering applications, novel methodologies for the preparation of pullulan nanoformulations are preferred. These nanoformulations improved its multifunctionality and broke the barrier between its pilot-scale and large-scale applications.
Future prospective
Pullulan is approved for usage in agro-food, pharmaceutical, and medical devices due to its distinct physicochemical characteristics. Chemical derivatization has been carried out to broaden its application. This has increased pullulan’s solubility in organic solvents, as well as its mechanical, pH-sensitive, anticoagulant, and antibacterial properties. Although the potential uses of pullulan nanopolymer in food products are largely known and accepted, they have not been thoroughly investigated on an industrial basis. Furthermore, pullulan is present in many commercialized formulations and is the subject of much research; pullulan derivatives, however, are currently being studied and have not yet received commercial approval. The future perspective is to describe pullulan’s chemical and physical characteristics, which are consistently enhanced to produce a derivative that is more suited for the pharmaceutical, medicinal, and agro-food industries.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AgNp:
-
Silver nanoparticle
- CAPL:
-
Charge reversible pullulan-based shell
- CFC:
-
Trichlorotrifluoroethane
- CHP:
-
Cholesterol-modified pullulan
- CMP:
-
Carboxymethyl pullulan
- CNC:
-
Cellulose nanocomposite
- CNFs:
-
Cellulose nanofibril
- CQD:
-
Carbon quantum dots
- CuO:
-
Copper oxide
- DCW:
-
Dry cell weight
- DOX:
-
Doxorubicin
- EPS:
-
Exo-polysaccharide
- GRAS:
-
Generally regarded as safe
- HeLa:
-
Henrietta Lacks
- HEK293:
-
Human embryonic kidney 293 cells
- H2O2 :
-
Hydrogen peroxide
- HepG2:
-
Human hepatocellular carcinoma cells
- h:
-
Hour
- MCF-7:
-
Michigan Cancer Foundation-7
- L929:
-
Mouse fibroblast cells
- mcl-PHA:
-
Medium chain length polyhydroxyalkanoate
- MHCC:
-
Human hepatocellular carcinoma cell line
- PBAE:
-
Poly(β-amino ester)
- PBS:
-
Poly(butylene succinate)
- PLAG:
-
Poly(lactic-co-glycolic acid) core
- PLV:
-
Pullulan-encapsulated lovastatin
- PNCs:
-
Polymer nanocomposites
- PTX:
-
Paclitaxel
- SiO2 :
-
Silicon dioxide
- TNBC:
-
Triple-negative breast cancer
- TiO2 :
-
Titanium dioxide
- ZnO:
-
Zinc oxide
References
Singh RS, Kaur N, Hassan M, Kennedy JF (2021) Pullulan in biomedical research and development-a review. Int J Biol Macromol 166:694–706. https://doi.org/10.1016/j.ijbiomac.2020.10.227
Wei X, Liu GL, Jia SL, Chi Z, Hu Z, Chi ZM (2021) Pullulan biosynthesis and its regulation in Aureobasidium spp. Carbohydr Polym 251:117076. https://doi.org/10.1016/j.carbpol.2020.117076
Bauer R (1938) Zentralbl Bacteriol Parasitenkd Infektionskr Hyg Abt 2(98):133
Bernier B (1958) The production of polysaccharides by fungi active in the decomposition of wood and forest litter. Can J Microbiol 4:195–204. https://doi.org/10.1139/m58-020
Singh RS, Saini GK, Kennedy JF (2008) Pullulan: microbial sources production and applications. Carbohydr Polym 73:515–531. https://doi.org/10.1016/j.carbpol.2008.01.003
Singh RS, Singh H, Saini GK (2009) Response surface optimization of the critical medium components for pullulan production by Aureobasidium pullulans FB-1. Appl Biochem Biotechnol 152:42–53. https://doi.org/10.1007/s12010-008-8180-9
Singh RS, Kaur N, Kennedy JF (2019) Pullulan production from agroindustrial waste and its applications in food industry: a review. Carbohydr Polym 217:46–57. https://doi.org/10.1016/j.carbpol.2019.04.050
Nishimura T, Shishi S, Sasaki Y, Akiyoshi K (2020) Thermoresponsive polysaccharide graft polymer vesicles with tunable size and structural memory. J Am Chem Soc 142:11784–11790. https://doi.org/10.1021/jacs.0c02290
An JM, Shahriar SMS, Hasan MN, Cho S, Lee YK (2021) Carboxymethyl cellulose pluronic and pullulan-based compositions efficiently enhance antiadhesion and tissue regeneration properties without using any drug molecules. ACS Appl Mater Interfaces 13:15992–16006. https://doi.org/10.1021/acsami.0c21938
Feng Z, Chen S, Ahmad A, Chen L, Bai W (2022) Ultra-highmolecular weight pullulan-based material with high deformability and shape-memory properties. Carbohydr Polym 295:119836 https://doi.org/10.1016/j.carbpol.2022.119836
Agrawal S, Budhwani D, Gurjar P, Telange D, Lambole V (2022) Pullulan based derivatives: synthesis enhanced physicochemical properties and applications. Drug Deliv 29:3328–3339. https://doi.org/10.1080/10717544.2022.2144544
Chen S, Zheng H, Gao J, Song H, Bai W (2023) High-level production of pullulan and its biosynthesis regulation in Aureobasidium pullulans BL06. Front Bioeng Biotechnol 11:1131875. https://doi.org/10.3389/fbioe.2023.1131875
Yang Y, Xie B, Liu Q, Kong B, Wang H (2020) Fabrication and characterization of a novel polysaccharide based composite nanofiber films with tunable physical properties. Carbohydr Polym 236:116054. https://doi.org/10.1016/j.carbpol.2020.116054
Prajapati VD, Jani GK, Khanda SM (2013) Pullulan: an exopolysaccharide and its various applications. Carbohydr Polym 95:540–549. https://doi.org/10.1016/j.carbpol.2013.02.082
Rai M, Wypij M, Ingle AP, Trzcińska-Wencel J, Golińska P (2021) Emerging trends in pullulan-based antimicrobial systems for various applications. Int J Mol Sci 22:13596. https://doi.org/10.3390/ijms222413596
Farris S, Unalan IU, Introzzi L, Fuentes‐Alventosa JM, Cozzolino CA (2014) Pullulan‐based films and coatings for food packaging: present applications emerging opportunities and future challenges. J Appl Polym Sci 131:40539 https://doi.org/10.1002/app.40539
Singh RS, Kaur N, Rana V, Kennedy JF (2017) Pullulan: a novel molecule for biomedical applications. Carbohydr Polym 171:102–121. https://doi.org/10.1016/j.carbpol.2017.04.089
Constantin M, Bucatariu S, Sacarescu L, Daraba OM, Anghelache M, Fundueanu G (2020) Pullulan derivative with cationic and hydrophobic moieties as an appropriate macromolecule in the synthesis of nanoparticles for drug delivery. Int J Biol Macromol 164:4487-4498 https://doi.org/10.1016/j.ijbiomac.2020.09.064
Liu H, Kumar V, Jia L, Sarsaiya S, Kumar D, Juneja A, Awasthi MK (2021) Biopolymer poly-hydroxyalkanoates (PHA) production from apple industrial waste residues: a review. Chemosphere 284:131427. https://doi.org/10.1016/j.chemosphere.2021.131427
Kaneo Y, Tanaka T, Nakano T, Yamaguchi Y (2001) Evidence for receptor-mediated hepatic uptake of pullulan in rats. J Control Release 70:365–373. https://doi.org/10.1016/s0168-3659(00)00368-0
Viveka R, Varjani S, Ekambaram N (2021) Valorization of cassava waste for pullulan production by Aureobasidium pullulans MTCC 1991. Energy Environ 32:1086–1102. https://doi.org/10.1177/0958305X20908065
Blakeney M (2019) Food loss and food waste: causes and solutions. Edward Elgar Publishing. https://doi.org/10.1111/1467-8489.12344
Ranganathan S, Dutta S, Moses JA, Anandharamakrishnan C (2020) Utilization of food waste streams for the production of biopolymers. Heliyon 6:e04891. https://doi.org/10.1016/j.heliyon.2020.e04891
Theagarajan R, Malur Narayanaswamy L, Dutta S, Moses JA, Chinnaswamy A (2019) Valorisation of grape pomace (cv. Muscat) for development of functional cookies. Int J Food Sci Technol 54:1299–1305. https://doi.org/10.1111/ijfs.14119
Bruneel D, Schacht E (1993) Chemical modification of pullulan: 1) periodate oxidation. Polymer 34:2628–2632. https://doi.org/10.1016/0032-3861(93)90600-F
Miteluț AC, Popa EE, Drăghici MC, Popescu PA, Popa VI, Bujor OC, Popa ME (2021) Latest developments in edible coatings on minimally processed fruits and vegetables: a review. Foods 10:2821. https://doi.org/10.3390/foods10112821
Odetayo T, Tesfay S, Ngobese NZ (2022) Nanotechnology-enhanced edible coating application on climacteric fruits. Food Sci Nutr 10:2149–2167. https://doi.org/10.1002/fsn3.2557
Coltelli MB, Danti S, De Clerck K, Lazzeri A, Morganti P (2020) Pullulan for advanced sustainable body-and skin-contact applications. J Funct Biomater 11:20. https://doi.org/10.3390/jfb11010020
Sindhu R, Manju A, Mohan P, Rajesh RO, Madhavan A, Arun KB, Reshmy R (2020) Valorization of food and kitchen waste: an integrated strategy adopted for the production of poly-3-hydroxybutyrate, bioethanol, pectinase and 2, 3-butanediol. Bioresour Technol 310:123515. https://doi.org/10.1016/j.biortech.2020.123515
Bhatia SK, Otari SV, Jeon JM, Gurav R, Choi YK, Bhatia RK, Yang YH (2021) Biowaste-to-bioplastic (polyhydroxyalkanoates): conversion technologies, strategies, challenges, and perspective. Bioresour Technol 326:124733. https://doi.org/10.1016/j.biortech.2021.124733
Sharma P, Gaur VK, Kim SH, Pandey A (2020) Microbial strategies for bio-transforming food waste into resources. Bioresour Technol 299:122580. https://doi.org/10.1016/j.biortech.2019.122580
Cywar RM, Rorrer NA, Hoyt CB, Beckham GT, Chen EYX (2022) Bio-based polymers with performance-advantaged properties. Nat Rev Mater 7:83–103. https://doi.org/10.1038/s41578-021-00363-3
Barsett H, Ebringerová A, Harding SE, Heinze T, Hromádková Z, Muzzarelli C, El Seoud OA (2005) In: Abe A, Albertsson AC, Coates GW, Genzer J, Kobayashi S, Lee KS, Leibler L, Long TE, Möller M, Okay O, Percec V, Tang BZ, Terentjev EM, Theato P, Voit B, Wiesner U, Zhang X (eds) Advances in Polymer Science. Springer https://doi.org/10.1007/b136812
Hou Y, Ding X, Hou W (2015) Composition and antioxidant activity of water-soluble oligosaccharides from Hericium erinaceus. Mol Med Rep 11:3794–3799. https://doi.org/10.3892/mmr.2014.3121
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feed stocks: a review. Bioresour Bioprocess 4:1–19. https://doi.org/10.1186/s40643-017-0137-9
Kumar V, Naik B, Choudhary M, Kumar A, Khanduri N (2022) Agro-waste as a substrate for the production of pullulanase by Penicillium viridicatum under solid-state fermentation. Sci Rep 12:12661. https://doi.org/10.1038/s41598-022-16854-4
He C, Zhang Z, Zhang Y, Wang G, Wang C, Wang D, Wei G (2021) Efficient pullulan production by Aureobasidium pullulans using cost-effective substrates. Int J Biol Macromol 186:544–553. https://doi.org/10.1016/j.ijbiomac.2021.07.068
Mirzaee H, Khodaiyan F, Kennedy JF, Hosseini SS (2020) Production, optimization and characterization of pullulan from sesame seed oil cake as a new substrate by Aureobasidium pullulans. Carbohydr Polym Technol Appl 1:100004. https://doi.org/10.1016/j.carpta.2020.100004
An C, Ma SJ, Chang F, Xue WJ (2017) Efficient production of pullulan by Aureobasidium pullulans grown on mixtures of potato starch hydrolysate and sucrose. Braz J Microbiol 48:180–185. https://doi.org/10.1016/j.bjm.2016.11.001
Hilares RT, Resende J, Orsi CA, Ahmed MA, Lacerda TM, Da Silva SS, Santos JC (2019) Exopolysaccharide (pullulan) production from sugarcane bagasse hydrolysate aiming to favour the development of biorefineries. Int J Biol Macromol 127:169–177. https://doi.org/10.1016/j.ijbiomac.2019.01.038
Sugumaran KR, Ponnusami V (2017) Conventional optimization of aqueous extraction of pullulan in solid-state fermentation of cassava bagasse and Asian palm kernel. Biocatal Agric Biotechnol 10:204–208. https://doi.org/10.1016/j.bcab.2017.03.010
Huang LP, Jin B, Lant P, Zhou J (2003) Biotechnological production of lactic acid integrated with potato wastewater treatment by Rhizopus arrhizus. J Chem Technol Biotechnol 78:899–906. https://doi.org/10.1002/jctb.877
Roy I, Gupta MN (2004) Hydrolysis of starch by a mixture of glucoamylase and pullulanase entrapped individually in calcium alginate beads. Enzyme Microb Technol 34:26–32. https://doi.org/10.1016/j.enzmictec.2003.07.001
Göksungur Y, Uzunoğulları P, Dağbağlı S (2011) Optimization of pullulan production from hydrolysed potato starch waste by response surface methodology. Carbohydr Polym 83:1330–1337. https://doi.org/10.1016/j.carbpol.2010.09.047
Barnett C, Smith A, Scanlon B, Israilides CJ (1999) Pullulan production by Aureobasidium pullulans growing on hydrolysed potato starch waste. Carbohydr Polym 38:203–209. https://doi.org/10.1016/S0144-8617(98)00092-7
Israilides C, Smith A, Scanlon B, Barnett C (1999) Pullulan from agro-industrial wastes. Biotechnol Genet Eng Rev 16:309–324. https://doi.org/10.1080/02648725.1999.10647981
Israilides C, Scanlon B, Smith A, Harding SE, Jumel K (1994) Characterization of pullulans produced from agro-industrial wastes. Carbohydr Polym 25:203–209. https://doi.org/10.1016/0144-8617(94)90205-4
Israilides CJ, Smith A, Harthill JE, Barnett C, Bambalov G, Scanlon B (1998) Pullulan content of the ethanol precipitate from fermented agro-industrial wastes. Appl Microbiol Biotechnol 49:613–617. https://doi.org/10.1007/s002530051222
Yang ST, Zhu H, Li Y, Hong G (1994) Continuous propionate production from whey permeate using a novel fibrous bed bioreactor. Biotechnol Bioeng 43:1124–1130. https://doi.org/10.1002/bit.260431117
Roukas T (1999) Pullulan production from deproteinized whey by Aureobasidium pullulans. J Ind Microbiol Biotechnol 22:617–621. https://doi.org/10.1038/sj.jim
Ummah H, Suriamihardja DA, Selintung M, Wahab AW (2015) Analysis of chemical composition of rice husk used as absorber plates sea water into clean water. ARPN J Eng Appl Sci 10:6046–6050
Le Guen MJ, Hill S, Smith D, Theobald B, Gaugler E, Barakat A, Mayer-Laigle C (2019) Influence of rice husk and wood biomass properties on the manufacture of filaments for fused deposition modeling. Front Chem 7:735. https://doi.org/10.3389/fchem.2019.00735
Zemnukhova LA, Tomshich SV, Mamontova VA, Komandrova NA, Fedorishcheva GA, Sergienko VI (2004) Composition and properties of polysaccharides from rice husk. Russ J Appl Chem 77:1883–1887. https://doi.org/10.1007/s11167-005-0181-7
Wang D, Ju X, Zhou D, Wei G (2014) Efficient production of pullulan using rice hull hydrolysate by adaptive laboratory evolution of Aureobasidium pullulans. Bioresour Technol 164:12–19. https://doi.org/10.1016/j.biortech.2014.04.036
Solomon S (2011) Sugarcane by-products based industries in India. Sugar Tech 13:408–416. https://doi.org/10.1007/s12355-011-0114-0
Jamir L, Kumar V, Kaur J, Kumar S, Singh H (2021) Composition, valorization and therapeutical potential of molasses: a critical review. Environ Technol Rev 10:131–142. https://doi.org/10.1080/21622515.2021.1892203
Roukas T (1998) Pretreatment of beet molasses to increase pullulan production. Process Biochem 33:805–810. https://doi.org/10.1016/S0032-9592(98)00048-X
Srikanth S, Swathi M, Tejaswini M, Sharmila G, Muthukumaran C, Jaganathan MK, Tamilarasan K (2014) Statistical optimization of molasses based exopolysaccharide and biomass production by Aureobasidium pullulans MTCC 2195. Biocatal Agric Biotechnol 3:7–12. https://doi.org/10.1016/j.bcab.2013.11.011
Lazaridou A, Biliaderis CG, Roukas T, Izydorczyk M (2002) Production and characterization of pullulan from beet molasses using a nonpigmented strain of Aureobasidium pullulans in batch culture. Appl Biochem Biotechnol 97:1–22. https://doi.org/10.1385/ABAB:97:1:01
Roukas T, Liakopoulou-Kyriakides M (1999) Production of pullulan from beet molasses by Aureobasidium pullulans in a stirred tank fermentor. J Food Eng 40:89–94. https://doi.org/10.1016/S0260-8774(99)00043-6
Roukas T, Serris G (1999) Effect of the shear rate on pullulan production from beet molasses by Aureobasidium pullulans in an airlift reactor. Appl Biochem Biotechnol 80:77–89. https://doi.org/10.1385/ABAB:80:1:77
Xiros C, Topakas E, Katapodis P, Christakopoulos P (2008) Hydrolysis and fermentation of brewer’s spent grain by Neurospora crassa. Bioresour Technol 99:5427–5435. https://doi.org/10.1016/j.biortech.2007.11.0102900675
Oner ET (2013) Microbial production of extracellular polysaccharides from biomass. Pretreatment Techniques for Biofuels and Biorefineries 2013:35–56. https://doi.org/10.1007/978-3-642-32735-3_2
Mahato RP, Kumar S, Singh P (2023) Production of polyhydroxyalkanoates from renewable resources: a review on prospects, challenges and applications. Arch Microbiol 205:172. https://doi.org/10.1007/s00203-023-03499-8
Nicolaus B, Kambourova M, Oner ET (2010) Exopolysaccharides from extremophiles: from fundamentals to biotechnology. Environ Technol 31:1145–1158. https://doi.org/10.1080/09593330903552094
Lembre P, Lorentz C, Di Martino P (2012) Exopolysaccharides of the biofilm matrix: a complex biophysical world. Complex World Polysaccharides 2012:371–392. https://doi.org/10.5772/51213
Mahato RP, Kumar S, Singh P (2021) Optimization of growth conditions to produce sustainable polyhydroxyalkanoate (PHA) bioplastic by Pseudomonas aeruginosa EO1. Front Microbiol 12:1–9. https://doi.org/10.3389/fmicb.2021.711588
Catley B (1973) The rate of elaboration of the extracellular polysaccharide, pullulan, during growth of Pullularia pullulans. J Gen Microbiol 78:33–38. https://doi.org/10.1099/00221287-78-1-33
Ono K, Yasuda N, Ueda S (1977) Effect of pH on pullulan elaboration by Aureobasidium pullulans S-1. Agric Biol Chem 41:2113–2118. https://doi.org/10.1080/00021369.1977.10862824
Castillo NA, Valdez AL, Fariña JI (2015) Microbial production of scleroglucan and downstream processing. Front Microbiol 6:1106. https://doi.org/10.3389/fmicb.2015.01106
Koller M, Braunegg G (2015) Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering 2:94–121. https://doi.org/10.3390/bioengineering2020094
Leathers TD (2003) Biotechnological production and applications of pullulan. Appl Microbiol Biotechnol 62:468–473. https://doi.org/10.1007/s00253-003-1386-4
Wu S, Jin Z, Tong Q, Chen H (2009) Sweet potato: a novel substrate for pullulan production by Aureobasidium pullulans. Carbohydr Polym 76:645–649. https://doi.org/10.1016/j.carbpol.2008.11.034
Lee JH, Kim JH, Lim SM, Nam SW, Lee JW, Kim SK (2002) Effect of dissolved oxygen concentration and pH on the mass production of high molecular weight pullulan by Aureobasidium pullulans. J Microbiol Biotechnol 12:1–7
Cheng KC, Demirci A, Catchmark JM (2010) Effects of plastic composite support and pH profiles on pullulan production in a biofilm reactor. Appl Microbiol Biotechnol 86:853–861. https://doi.org/10.1007/s00253-009-2332-x
Ponnusami V, Gunasekar V (2015) Production of pullulan by microbial fermentation. Polysaccharides 2015:581–596. https://doi.org/10.1007/978-3-319-16298-0_58
Seviour RJ, Stasinopoulos SJ, Auer DPF, Gibbs PA (1992) Production of pullulan and other exopolysaccharides by filamentous fungi. Crit Rev Biotechnol 12:279–298. https://doi.org/10.3109/07388559209069196
Wang D, Yu X, Gongyuan W (2013) Pullulan production and physiological characteristics of Aureobasidium pullulans under acid stress. Appl Microbiol Biotechnol 97:8069–8077. https://doi.org/10.1007/s00253-013-5094-4
Wu S, Lu M, Chen J, Fang Y, Wu L, Xu Y, Wang S (2016) Production of pullulan from raw potato starch hydrolysates by a new strain of Aureobasidium pullulans. Int J Biol Macromol 82:740–743. https://doi.org/10.1016/j.ijbiomac.2015.09.075
Campbell BS, McDougall BM, Seviour RJ (2003) Why do exopolysaccharide yields from the fungus Aureobasidium pullulans fall during batch culture fermentation? Enzyme Microb Technol 33:104–112. https://doi.org/10.1016/S0141-0229(03)00089-9
Delbarre-Ladrat C, Sinquin C, Lebellenger L, Zykwinska A, Colliec-Jouault S (2014) Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front Chem 2:85. https://doi.org/10.3389/fchem.2014.00085
Jindal N, Khattar JS (2018) Microbial polysaccharides in food industry. Biopolym Food Des 2018:95–123. https://doi.org/10.1016/B978-0-12-811449-0.00004-9
Rühmann B, Schmid J, Sieber V (2015) High throughput exopolysaccharide screening platform: from strain cultivation to monosaccharide composition and carbohydrate fingerprinting in one day. Carbohydr Polym 122:212–220. https://doi.org/10.1016/j.carbpol.2014.12.021
Gniewosz M, Sobczak E (1999) Utility of Aureobasidium pullulans and pullulan in food biotechnology. Biotechnologia 2:81–93
Cheng KC, Demirci A, Catchmark JM (2011) Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol 92:29–44. https://doi.org/10.1007/s00253-011-3477-y
Duan X, Chi Z, Wang L, Wang X (2008) Influence of different sugars on pullulan production and activities of α-phosphoglucose mutase, UDPG-pyrophosphorylase and glucosyltransferase involved in pullulan synthesis in Aureobasidium pullulans Y68. Carbohydr Polym 73:587–593. https://doi.org/10.1016/j.carbpol.2007.12.028
Kırtel O, Avşar G, Erkorkmaz BA, Öner ET (2017) Microbial polysaccharides as food ingredients. Microb Prod Food Ingred Addit 2017:347–383. https://doi.org/10.1016/B978-0-12-811520-6.00012-X
Singh RS, Saini GK (2012) Biosynthesis of pullulan and its applications in food and pharmaceutical industry. Microorgan Sustain Agricul Biotechnol 2012:509–553. https://doi.org/10.1007/978-94-007-2214-9_24
Youssef F, Roukas T, Biliaderis CG (1999) Pullulan production by a non-pigmented strain of Aureobasidium pullulans using batch and fed-batch culture. Process Biochem 34:355–366. https://doi.org/10.1016/S0032-9592(98)00106-X
Göksungur Y, Uçan A, GÜvenÇ U (2004) Production of pullulan from beet molasses and synthetic medium by Aureobasidium pullulans. Turk J Biol 28:23–30 https://journals.tubitak.gov.tr/biology/vol28/iss1/4
Bulmer MA, Catley BJ, Kelly PJ (1987) The effect of ammonium ions and pH on the elaboration of the fungal extracellular polysaccharide, pullulan, by Aureobasidium pullulans. Appl Microbiol Biotechnol 25:362–365. https://doi.org/10.1007/BF00252548
Chen L, Chi Z, Liu GL, Xue SJ, Wang ZP, Hu Z, Chi ZM (2019) Improved pullulan production by a mutant of Aureobasidium melanogenum TN3-1 from a natural honey and capsule shell preparation. Int J Biol Macromol 141:268–277. https://doi.org/10.1016/j.ijbiomac.2019.08.264
Li BX, Zhang N, Peng Q, Yin T, Guan FF, Wang GL, Li Y (2009) Production of pigment-free pullulan by swollen cell in Aureobasidium pullulans NG which cell differentiation was affected by pH and nutrition. Appl Microbiol Biotechnol 84:293–300. https://doi.org/10.1007/s00253-009-1955-2
Sugumaran KR, Ponnusami V (2017) Review on production, downstream processing and characterization of microbial pullulan. Carbohydr Polym 173:573–591. https://doi.org/10.1016/j.carbpol.2017.06.022
Simon L, Caye-Vaugien C, Bouchonneau M (1993) Relation between pullulan production, morphological state and growth conditions in Aureobasidium pullulans: new observations. J Gen Microbiol 1993:139
Chen TJ, Chi Z, Jiang H, Liu GL, Hu Z, Chi ZM (2018b) Cell wall integrity is required for pullulan biosynthesis and glycogen accumulation in Aureobasidium melanogenum P16. Biochimica et Biophysica Acta (BBA)-General Subjects 1862:1516–1526 https://doi.org/10.1016/j.bbagen.2018.03.017
Chen L, Ji F, Bao Y, Xia J, Guo L, Wang J, Li Y (2017) Biocompatible cationic pullulan-g-desoxycholic acid-g-PEI micelles used to co-deliver drug and gene for cancer therapy. Mater Sci Eng C 70:418–429. https://doi.org/10.1016/j.msec.2016.09.019
Prasongsuk S, Lotrakul P, Ali I, Bankeeree W, Punnapayak H (2018) The current status of Aureobasidium pullulans in biotechnology. Folia Microbiol 63:129–140. https://doi.org/10.1007/s12223-017-0561-4
Hamidi M, Kennedy JF, Khodaiyan F, Mousavi Z, Hosseini SS (2019) Production optimization, characterization and gene expression of pullulan from a new strain of Aureobasidium pullulans. Int J Biol Macromol 138:725–735. https://doi.org/10.1016/j.ijbiomac.2019.07.123
Fraser CG, Jennings HJ (1971) A glucan from Tremella mesenterica NRRL-Y6158. Can J Chem 49:1804–1807. https://doi.org/10.1139/v71-297
Reis RA, Tischer CA, Gorin PA, Iacomini M (2002) A new pullulan and a branched (1→ 3)-, (1→ 6)-linked β-glucan from the lichenised ascomycete Teloschistes flavicans. FEMS Microbiol Lett 210:1–5. https://doi.org/10.1111/j.1574-6968.2002.tb11152.x
Forabosco A, Bruno G, Sparapano L, Liut G, Marino D, Delben FJCP (2006) Pullulans produced by strains of Cryphonectria parasitica: I) production and characterisation of the exopolysaccharides. Carbohydr Polym 63:535–544. https://doi.org/10.1016/j.carbpol.2005.10.005
Waksman N, de Lederkremer RM, Cerezo AS (1977) The structure of an α-D-glucan from Cyttaria harioti Fischer. Carbohydr Res 59:505–515. https://doi.org/10.1016/S0008-6215(00)83187-0
Oliva EM, Cirelli AF, de Lederkremer RM (1986) Characterization of a pullulan in Cyttaria darwinii. Carbohydr Res 158:262–267. https://doi.org/10.1016/0008-6215(86)84025-3
Money NP (2016) In: Watkinson SC, Boddy L, Money NP (eds) The Fungi (Chap. 12). Academic Press https://doi.org/10.1016/B978-0-12-382034-1.00012-8
Mina M, Tsaltas D (2017) Contribution of yeast in wine aroma and flavour. Yeast Ind Appl 2017:117–134 intechopen.70656
Mishra B, Varjani S (2019) Evaluation of pullulan production by a newly isolated Micrococcus luteus. Indian J Exp Biol 57:813–820 https://scholars.cityu.edu.hk/en/publications/publication(ff053028-8309-448f-9f8a-515f45b95460).html
Simon L, Bouchet B, Caye-Vaugien C, Gallant DJ (1995) Pullulan elaboration and differentiation of the resting forms in Aureobasidium pullulans. Can J Microbiol 41:35–45. https://doi.org/10.1139/m95-005
Ullah MW, Ul-Islam M, Khan T, Park JK (2021) In: Phillips GO, Williams PA (eds) Handbook of Hydrocolloids, 2th end. CRC Press, New York
Chen X, Wang QQ, Liu NN, Liu GL, Chi Z, Chi ZM (2017) A glycosyltransferase gene responsible for pullulan biosynthesis in Aureobasidium melanogenum P16. Int J Biol Macromol 95:539–549. https://doi.org/10.1016/j.ijbiomac.2016.11.081
Shingel KI (2004) Current knowledge on biosynthesis, biological activity, and chemical modification of the exopolysaccharide, pullulan. Carbohydr Res 339:447–460. https://doi.org/10.1016/j.carres.2003.10.034
Hilares RT, Orsi CA, Ahmed MA, Marcelino PF, Menegatti CR, da Silva SS, Dos Santos JC (2017) Low-melanin containing pullulan production from sugarcane bagasse hydrolysate by Aureobasidium pullulans in fermentations assisted by light-emitting diode. Bioresour Technol 230:76–81. https://doi.org/10.1016/j.biortech.2017.01.052
Liu NN, Chi Z, Liu GL, Chen TJ, Jiang H, Hu Z, Chi ZM (2018) α-Amylase, glucoamylase and isopullulanase determine molecular weight of pullulan produced by Aureobasidium melanogenum P16. Int J Biol Macromol 117:727–734. https://doi.org/10.1016/j.ijbiomac.2018.05.235
Xue SJ, Chen L, Jiang H, Liu GL, Chi ZM, Hu Z, Chu Z (2019) High pullulan biosynthesis from high concentration of glucose by a hyperosmotic resistant, yeast-like fungal strain isolated from a natural comb-honey. Food Chem 286:123–128. https://doi.org/10.1016/j.foodchem.2019.01.206
Zeng W, Zhang B, Jiang L, Liu Y, Ding S, Chen G, Liang Z (2020) Poly(malic acid) production from liquefied corn starch by simultaneous saccharification and fermentation with a novel isolated Aureobasidium pullulans GXL-1 strain and its techno-economic analysis. Bioresour Technol 304:122990. https://doi.org/10.1016/j.biortech.2020.122990
Chen X, Wang Y, He CY, Wang GL, Zhang GC, Wang CL, Wei GY (2021) Improved production of β-glucan by a T-DNA–based mutant of Aureobasidium pullulans. Appl Microbiol Biotechnol 105:6887–6898. https://doi.org/10.1007/s00253-021-11538-x
Wang QQ, Lu Y, Ren ZY, Chi Z, Liu GL, Chi ZM (2017) CreA is directly involved in pullulan biosynthesis and regulation of Aureobasidium melanogenum P16. Curr Genet 63:471–485. https://doi.org/10.1007/s00294-016-0650-y
Rødkær SV, Færgeman NJ (2014) Glucose-and nitrogen sensing and regulatory mechanisms in Saccharomyces cerevisiae. FEMS Yeast Res 14:683–696. https://doi.org/10.1111/1567-1364.12157
Badhwar P, Kumar A, Yadav A, Kumar P, Siwach R, Chhabra D, Dubey KK (2020) Improved pullulan production and process optimization using novel GA-ANN and GA-ANFIS hybrid statistical tools. Biomolecules 10:124. https://doi.org/10.3390/biom10010124
Singh RS, Kaur N, Kennedy JF (2015) Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydr Polym 123:190–207. https://doi.org/10.1016/j.carbpol.2015.01.032
Singh RS, Kaur N, Singh D, Bajaj BK, Kennedy JF (2022) Downstream processing and structural confirmation of pullulan-A comprehensive review. Int J Biol Macromol 208:553–564. https://doi.org/10.1016/j.ijbiomac.2022.03.163
Sutherland IW (1990) Biotechnology of Microbial Exopolysaccharides (No. 9). Cambridge University Press
Sreekanth MS, Vijayendra SVN, Joshi GJ, Shamala TR (2013) Effect of carbon and nitrogen sources on simultaneous production of α-amylase and green food packaging polymer by Bacillus sp. CFR 67. J Food Sci Technol 50:404–408. https://doi.org/10.1007/s13197-012-0639-6
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18:2328–2375. https://doi.org/10.3390/molecules18022328
Raychaudhuri R, Naik S, Shreya AB, Kandpal N, Pandey A, Kalthur G, Mutalik S (2020) Pullulan based stimuli responsive and sub cellular targeted nanoplatforms for biomedical application: synthesis, nanoformulations and toxicological perspective. Int J Biol Macromol 161:1189–1205. https://doi.org/10.1016/j.ijbiomac.2020.05.262
Vimala K, Sundarraj S, Paulpandi M, Vengatesan S, Kannan S (2014) Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Proc Biochem 49:160–172. https://doi.org/10.1016/j.procbio.2013.10.007
Ganeshkumar M, Ponrasu T, Raja MD, Subamekala MK, Suguna L (2014) Green synthesis of pullulan stabilized gold nanoparticles for cancer targeted drug delivery. Spectrochim Acta Part A 130:64–71. https://doi.org/10.1016/j.saa.2014.03.097
Ganduri VRK, Mangamuri U, Muvva V, Poda S (2016) Pullulan-stabilized silver nanoparticles-their synthesis, characterization and application as bactericidal agents. J Appl Pharm Sci 6:27–37
Wu S, Chen J (2013) Using pullulan-based edible coatings to extend shelf-life of fresh-cut ‘Fuji’apples. Int J Biol Macromol 55:254–257. https://doi.org/10.1016/j.ijbiomac.2013.01.012
Nešić A, Cabrera-Barjas G, Dimitrijević-Branković S, Davidović S, Radovanović N, Delattre C (2019) Prospect of polysaccharide-based materials as advanced food packaging. Molecules 25:135. https://doi.org/10.3390/molecules25010135
Ghaffarlou M, İlk S, Hammamchi H, Kıraç F, Okan M, Güven O, Barsbay M (2022) Green and facile synthesis of pullulan-stabilized silver and gold nanoparticles for the inhibition of quorum sensing. ACS Appl Bio Mater 5:517-527 https://doi.org/10.1021/acsabm.1c00964
Salleh MSN, Ali RR, Shameli K, Hamzah MY, Kasmani RM, Nasef MM (2021) Interaction insight of pullulan-mediated gamma-irradiated silver nanoparticle synthesis and its antibacterial activity. Polymers 13:3578. https://doi.org/10.3390/polym13203578
Hong L, Kim WS, Lee SM, Kang SK, Choi YJ, Cho CS (2019) Pullulan nanoparticles as prebiotics enhance the antibacterial properties of Lactobacillus plantarum through the induction of mild stress in probiotics. Front Microbiol 10:142. https://doi.org/10.3389/fmicb.2019.00142
Jayeoye TJ, Supachettapun C, Muangsin N (2023) Toxic Ag+ detection based on Au@ Ag core shell nanostructure formation using Tannic acid assisted synthesis of Pullulan stabilized gold nanoparticles. Sci Rep 13:1844 https://doi.org/10.1038/s41598-023-27406-9
Pavaloiu RD, Sha’at F, Hlevca C, Sha’at M, Sevcenco C, Petrescu M, Moscovici M (2020) Preliminary Evaluation of Pullulan Nanoparticles Loaded with Valsartan. Chem Proc 3:139 https://doi.org/10.3390/ecsoc-24-08428
Kanmani P, Lim ST (2013) Synthesis and characterization of pullulan-mediated silver nanoparticles and its antimicrobial activities. Carbohydr Polym 97:421–428. https://doi.org/10.1016/j.carbpol.2013.04.048
Al-Owasi Y, Elshafie A, Sivakumar N, Al-Bahry S (2019) Synthesis of pullulan-mediated silver nanoparticles (AgNPs) and their antimicrobial activities. Sultan Qaboos University J Sci (SQUJS) 24:88–94 https://doi.org/10.24200/squjs.vol24iss2pp88-94
Slavin YN, Asnis J, Hńfeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol 15:1–20. https://doi.org/10.1186/s12951-017-0308-z
Priyadarshini S, Gopinath V, Priyadharsshini NM, MubarakAli D, Velusamy P (2013) Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surf B Biointerfaces 102:232–237. https://doi.org/10.1016/j.colsurfb.2012.08.018
Raza MA, Kanwal Z, Rauf A, Sabri AN, Riaz S, Naseem S (2016) Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 6:74. https://doi.org/10.3390/nano6040074
Alshareef A, Laird K, Cross RBM (2017) Shape-dependent antibacterial activity of silver nanoparticles on Escherichia coli and Enterococcus faecium bacterium. Appl Surf Sci 424:310–315. https://doi.org/10.1016/j.apsusc.2017.03.176
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720. https://doi.org/10.1128/AEM.02218-06
Cheon JY, Kim SJ, Rhee YH, Kwon OH, Park WH (2019) Shape-dependent antimicrobial activities of silver nanoparticles. Int J Nanomedic 2019:2773–2780. https://doi.org/10.2147/IJN.S196472
Nešić MD, Dučić T, Liang X, Algarra MMi L, Korićanac L, Petković M, (2020) SR-FTIR spectro-microscopic interaction study of biochemical changes in HeLa cells induced by Levan-C60, Pullulan-C60, and their cholesterol-derivatives. Int J Biol Macromol 165:2541–2549. https://doi.org/10.1016/j.ijbiomac.2020.10.141
Guilger-Casagrande M, Germano-Costa T, Bilesky-José N, Pasquoto-Stigliani T, Carvalho L, Fraceto LF, de Lima R (2021) Influence of the capping of biogenic silver nanoparticles on their toxicity and mechanism of action towards Sclerotinia sclerotiorum. J Nanobiotechnol 19:1–18. https://doi.org/10.1186/s12951-021-00797-5
Pinto RJ, Almeida A, Fernandes SC, Freire CS, Silvestre AJ, Neto CP, Trindade T (2013) Antifungal activity of transparent nanocomposite thin films of pullulan and silver against Aspergillus niger. Colloids Surf B Biointerfaces 103:143–148. https://doi.org/10.1016/j.colsurfb.2012.09.045
Shahabi-Ghahfarrokhi I, Khodaiyan F, Mousavi M, Yousefi H (2015) Preparation of UV-protective kefiran/nano-ZnO nanocomposites: physical and mechanical properties. Int J Biol Macromol 72:41–46. https://doi.org/10.1016/j.ijbiomac.2014.07.047
Hassannia-Kolaee M, Khodaiyan F, Shahabi-Ghahfarrokhi I (2016) Modification of functional properties of pullulan–whey protein bionanocomposite films with nanoclay. J Food Sci Technol 53:1294–1302. https://doi.org/10.1007/s13197-015-1778-3
Uyama H, Kuwabara M, Tsujimoto T, Nakano M, Usuki A, Kobayashi S (2003) Green nanocomposites from renewable resources: plant oil-clay hybrid materials. Chem Mater 15:2492–2494. https://doi.org/10.1021/cm0340227
Mulla MZ, Rostamabadi H, Habibi N, Falsafi SR (2023) Pullulan nanocomposites: effect of nanoparticles and essential oil reinforcement on its performance and food packaging applications. Food Humanity 1:887–894. https://doi.org/10.1016/j.foohum.2023.08.006
Xie L, Shen M, Wang Z, Xie J (2021) Structure, function and food applications of carboxymethylated polysaccharides: a comprehensive review. Trends Food Sci Technol 118:539–557. https://doi.org/10.1016/j.tifs.2021.09.016
Mapelli C, Musatti A, Barbiroli A, Saini S, Bras J, Cavicchioli D, Rollini M (2019) Cellulose nanofiber (CNF)-sakacin-A active material: production, characterization and application in storage trials of smoked salmon. J Sci Food Agric 99:4731–4738. https://doi.org/10.1002/jsfa.9715
Hassan AH, Cutter CN (2020) Development and evaluation of pullulan-based composite antimicrobial films (CAF) incorporated with nisin, thymol and lauric arginate to reduce foodborne pathogens associated with muscle foods. Int J Food Microbiol 320:108519. https://doi.org/10.1016/j.ijfoodmicro.2020.108519
Liang Q, Gao Q (2023) Effect of amylose content on the preparation for carboxymethyl starch/pullulan electrospun nanofibers and their properties as encapsulants of thymol. Food Hydrocoll 136:108250. https://doi.org/10.1016/j.foodhyd.2022.108250
Wypij M, Rai M, Zemljič LF, Bračič M, Hribernik S, Golińska P (2023) Pullulan-based films impregnated with silver nanoparticles from the Fusarium culmorum strain JTW1 for potential applications in the food industry and medicine. Front Bioeng Biotechnol 11:1241739. https://doi.org/10.3389/fbioe.2023.1241739
Khan MJ, Ramiah SK, Selamat J, Shameli K, Sazili AQ, Mookiah S (2022) Utilisation of pullulan active packaging incorporated with curcumin and pullulan mediated silver nanoparticles to maintain the quality and shelf life of broiler meat. Ital J Anim Sci 21:244–262. https://doi.org/10.1080/1828051X.2021.2012285
Sheng L, Su P, Han K, Chen J, Cao A, Zhang Z, Ma M (2017) Synthesis and structural characterization of lysozyme–pullulan conjugates obtained by the Maillard reaction. Food Hydrocoll 71:1–7. https://doi.org/10.1016/j.foodhyd.2017.04.026
Tomé LC, Silva NH, Soares HR, Coroadinha AS, Sadocco P, Marrucho IM, Freire CS (2015) Bioactive transparent films based on polysaccharides and cholinium carboxylate ionic liquids. Green Chem 17:4291–4299. https://doi.org/10.1039/C5GC00416K
Unalan IU, Wan C, Figiel Ł, Olsson RT, Trabattoni S, Farris S (2015) Exceptional oxygen barrier performance of pullulan nanocomposites with ultra-low loading of graphene oxide. Nanotechnology 26:275703. https://doi.org/10.1088/0957-4484/26/27/275703
Atila D, Karataş A, Keskin D, Tezcaner A (2022) Pullulan hydrogel-immobilized bacterial cellulose membranes with dual-release of vitamin C and E for wound dressing applications. Int J Biol Macromol 218:760–774. https://doi.org/10.1016/j.ijbiomac.2022.07.160
Hu H, Catchmark JM, Demirci A (2022) Effects of pullulan additive and co-culture of Aureobasidium pullulans on bacterial cellulose produced by Komagataeibacter hansenii. Bioprocess Biosyst Eng 45:573–587. https://doi.org/10.1007/s00449-021-02680-x
Yeasmin S, Yeum JH, Yang SB (2020) Fabrication and characterization of pullulan-based nanocomposites reinforced with montmorillonite and tempo cellulose nanofibril. Carbohydr Polym 240:116307. https://doi.org/10.1016/j.carbpol.2020.116307
Yeasmin S, Yeum JH, Ji BC, Choi JH, Yang SB (2021) Electrically conducting pullulan-based nanobiocomposites using carbon nanotubes and TEMPO cellulose nanofibril. Nanomaterials 11:602. https://doi.org/10.3390/nano11030602
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2:MR17-MR71 https://doi.org/10.1116/1.2815690
Moris H, Ghaee A, Karimi M, Nouri-Felekori M, Mashak A (2022) Preparation and characterization of Pullulan-based nanocomposite scaffold incorporating Ag-Silica Janus particles for bone tissue engineering. Biomater Adv 135:212733. https://doi.org/10.1016/j.bioadv.2022.212733
Dai M, Xiong X, Cheng A, Zhao Z, Xiao Q (2023) Development of pullulan-based nanocomposite films reinforced with starch nanocrystals for the preservation of fresh beef. J Sci Food Agric 103:1981–1993. https://doi.org/10.1002/jsfa.12280
Shah A, Ashames AA, Buabeid MA, Murtaza G (2020) Synthesis, in vitro characterization and antibacterial efficacy of moxifloxacin-loaded chitosan-pullulan-silver-nanocomposite films. J Drug Deliv Sci Technol 55:101366. https://doi.org/10.1016/j.jddst.2019.101366
Huang L, Chaurasiya B, Wu D, Wang H, Du Y, Tu J, Sun C (2018) Versatile redox-sensitive pullulan nanoparticles for enhanced liver targeting and efficient cancer therapy. Nanomedicine: NBM 14:1005–1017 https://doi.org/10.1016/j.nano.2018.01.015
Gong R, Chen G (2016) Preparation and application of functionalized nano drug carriers. Saudi Pharm J 24:254–257. https://doi.org/10.1016/j.jsps.2016.04.010
Bruneel D, Schacht E (1994) Chemical modification of pullulan: 3) succinoylation. Polymer 35:2656–2658. https://doi.org/10.1016/0032-3861(94)90395-6
Niu B, Shao P, Chen H, Sun P (2019) Structural and physiochemical characterization of novel hydrophobic packaging films based on pullulan derivatives for fruits preservation. Carbohydr Polym 208:276–284. https://doi.org/10.1016/j.carbpol.2018.12.070
Yuan H, Zhong W, Wang R, Zhou P, Nie Y, Hu W, Yang P (2020) Preparation of cholesteryl-modified aminated pullulan nanoparticles to evaluate nanoparticle of hydrophobic degree on drug release and cytotoxicity. J Nanomater 2020:1–10. https://doi.org/10.1155/2020/7171209
Yuan L, Guo B, Zhong W, Nie Y, Yao X, Peng X, Zhang Q (2019) Interaction of mitoxantrone-loaded cholesterol modified pullulan nanoparticles with human serum albumin and effect on drug release. J Nanomater 2019:8036863. https://doi.org/10.1155/2019/8036863
Yuan L, Cao Y, Luo Q, Yang W, Wu X, Yang X, Zhan Q (2018) Pullulan-based nanoparticle-HSA complex formation and drug release influenced by surface charge. Nanoscale Res Lett 13:1–14. https://doi.org/10.1186/s11671-018-2729-5
Shibata M, Asahina M, Teramoto N, Yosomiya R (2001) Chemical modification of pullulan by isocyanate compounds. Polymer 42:59–64. https://doi.org/10.1016/S0032-3861(00)00321-9
Zia F, Zia KM, Zuber M, Kamal S, Aslam N (2015) Starch based polyurethanes: a critical review updating recent literature. Carbohydr Polym 134:784–798. https://doi.org/10.1016/j.carbpol.2015.08.034
Rebocho AT, Pereira JR, Neves LA, Alves VD, Sevrin C, Grandfils C, Reis MA (2020) Preparation and characterization of films based on a natural p (3hb)/mcl-pha blend obtained through the co-culture of Cupriavidus necator and Pseudomonas citronellolis in apple pulp waste. Bioengineering 7:34. https://doi.org/10.3390/bioengineering7020034
Parisi C, Vigani M, Rodríguez-Cerezo E (2015) Agricultural nanotechnologies: what are the current possibilities? Nano Today 10:124–127. https://doi.org/10.1016/j.nantod.2014.09.009
Nile SH, Baskar V, Selvaraj D, Nile A, Xiao J, Kai G (2020) Nanotechnologies in food science: applications, recent trends, and future perspectives. Nanomicro Lett 12:1–34. https://doi.org/10.1007/s40820-020-0383-9
Aboul anean HED, Mallasi O (2022) The use of nano natural edible coating and films to Prolong Shelf Life of fruit vegetable. J Nutr Health Food Eng 12:8–12 https://https://doi.org/10.15406/jnhfe.2022.12.00348
Kraśniewska K, Pobiega K, Gniewosz M (2019) Pullulan-biopolymer with potential for use as food packaging. Int J Food Eng 15:30. https://doi.org/10.1515/ijfe-2019-0030
Shukla P, Chaurasia P, Younis K, Qadri OS, Faridi SA, Srivastava G (2019) Nanotechnology in sustainable agriculture: studies from seed priming to post-harvest management. Nanotechnol Environ Eng 4:1–15. https://doi.org/10.1007/s41204-019-0058-2
Muthukumaran P, Babu PS, Shyamalagowr S, Aravind J, Kamaraj M, Govarthanan M (2022) Polymeric biomolecules based nanomaterials: Production strategies and pollutant mitigation as an emerging tool for environmental application. Chemosphere 2022:136008. https://doi.org/10.1016/j.chemosphere.2022.136008
Mythili R, Selvankumar T, Kamala-Kannan S, Sudhakar C, Ameen F, Al-Sabri A, Kim H (2018) Utilization of market vegetable waste for silver nanoparticle synthesis and its antibacterial activity. Mater Lett 225:101–104. https://doi.org/10.1016/j.matlet.2018.04.111
Ameen F, AlYahya S, Govarthanan MAL, jahdali N, Al-Enazi N, Alsamhary K, Alharbi SA (2020) Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. J Mol Struct 1202:127233 https://doi.org/10.1016/j.molstruc.2019.127233
Balachandar R, Navaneethan R, Biruntha M, Kumar KKA, Govarthanan M Karmegam N (2022) Antibacterial activity of silver nanoparticles phytosynthesized from Glochidion candolleanum leaves. Mater Lett 311:131572 https://doi.org/10.1016/j.matlet.2021.131572
Khalaf HH, Sharoba AM, El-Tanahi HH, Morsy MK (2013) Stability of antimicrobial activity of pullulan edible films incorporated with nanoparticles and essential oils and their impact on turkey deli meat quality. J Food Dairy Sci 4:557–573 https://doi.org/10.21608/jfds.2013.72104
Wu S, Jiang Q, Han D, Yuan S, Zhao X, Duan J, Hou B (2023) An ecofriendly coaxial antibacterial and anticorrosion nanofiber pullulan-ethyl cellulose embedded with carvacrol coating for protection against marine corrosion. Int J Biol Macromol 246:125653. https://doi.org/10.1016/j.ijbiomac.2023.125653
Morsy MK, Sharoba AM, Khalaf HH, El-Tanahy HH, Cutter CN (2015) Efficacy of antimicrobial pullulan-based coating to improve internal quality and shelf-life of chicken eggs during storage. J Food Sci 80:M1066–M1074. https://doi.org/10.1016/S1570-0232(03)00258-7
Priyadarshi R, Kim SM, Rhim JW (2021) Pectin/pullulan blend films for food packaging: effect of blending ratio. Food Chem 347:129022. https://doi.org/10.1016/j.foodchem.2021.129022
Li Y, Yokoyama W, Wu J, Ma J, Zhong F (2015) Properties of edible films based on pullulan–chitosan blended film-forming solutions at different pH. RSC Adv 5:105844–105850. https://doi.org/10.1039/C5RA21876D
Khan MJ, Kumari S, Selamat J, Shameli K, Sazili AQ (2020) Reducing meat perishability through pullulan active packaging. J Food Qual 2020:1–10. https://doi.org/10.1155/2020/8880977
Morsy MK, Khalaf HH, Sharoba AM, El-Tanahi HH, Cutter CN (2014) Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J Food Sci 79:M675–M684. https://doi.org/10.1111/1750-3841.12400
Gniewosz M, Kraśniewska K, Woreta M, Kosakowska O (2013) Antimicrobial activity of a pullulan–caraway essential oil coating on reduction of food microorganisms and quality in fresh baby carrot. J Food Sci 78:M1242–M1248. https://doi.org/10.1016/S1570-0232(03)00258-7
Mohamed AL, Hassabo AG (2018) Composite material based on pullulan/silane/ZnO-NPs as pH, thermo-sensitive and antibacterial agent for cellulosic fabrics. Adv Nat Sci Nanosci Nanotechnol 9:045005. https://doi.org/10.1088/2043-6254/aaeee0
Silva NH, Vilela C, Almeida A, Marrucho IM, Freire CS (2018) Pullulan-based nanocomposite films for functional food packaging: exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocoll 77:921–930. https://doi.org/10.1016/j.foodhyd.2017.11.039
Shanmugam R, Mayakrishnan V, Kesavan R, Shanmugam K, Veeramani S, Ilangovan R (2022) Mechanical, barrier, adhesion and antibacterial properties of pullulan/graphene bio nanocomposite coating on spray coated nanocellulose film for food packaging applications. J Polym Environ 2022:1–9. https://doi.org/10.1007/s10924-021-02311-2
Roy S, Priyadarshi R, Rhim JW (2021) Development of multifunctional pullulan/chitosan-based composite films reinforced with ZnO nanoparticles and propolis for meat packaging applications. Foods 10:2789. https://doi.org/10.3390/foods10112789
Food US (2000) Drug Administration Center for Food Safety and Applied Nutrition, Office of Nutritional Products, Labeling and Dietary Supplements. Letter regarding dietary supplement health claim for omega-3 fatty acids and coronary heart disease (Docket No. 91N-0103)
Haas TA, Plow EF (1994) Integrin-ligarid interactions: a year in review. Curr Opin Cell Biol 6:656–662. https://doi.org/10.1016/0955-0674(94)90091-4
Fundueanu G, Constantin M, Mihai D, Bortolotti F, Cortesi R, Ascenzi P, Menegatti E (2003) Pullulan-cyclodextrin microspheres: a chromatographic approach for the evaluation of the drug-cyclodextrin interactions and the determination of the drug release profiles. J Chromatogr B 791:407–419. https://doi.org/10.1016/S1570-0232(03)00258-7
Vinogradov SV, Batrakova EV, Kabanov AV (2004) Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem 15:50–60. https://doi.org/10.1021/bc034164r
Akiyoshi K, Kobayashi S, Shichibe S, Mix D, Baudys M, Kim SW, Sunamoto J (1998) Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J Control Release 54:313–320. https://doi.org/10.1016/S0168-3659(98)00017-0
Suginoshita Y, Tabata Y, Matsumura T, Toda Y, Nabeshima M, Moriyasu F, Chiba T (2002) Liver targeting of human interferon-β with pullulan based on metal coordination. J Control Release 83:75–88. https://doi.org/10.1016/S0168-3659(02)00197-9
Masuda K, Sakagami M, Horie K, Nogusa H, Hamana H, Hirano K (2001) Evaluation of carboxymethylpullulan as a novel carrier for targeting immune tissues. Pharm Res 18:217–223. https://doi.org/10.1023/A:1011040703915
Bevilacqua MP (1993) Endothelial-leukocyte adhesion molecules. Annu Rev Immunol 11:767–804. https://doi.org/10.1146/annurev.iy.11.040193.004003
Satoh K, Chen F, Aoyama A, Date H, Akiyoshi K (2008) Nanoparticle of cholesterol-bearing pullulan as a carrier of anticancer drugs. EJC Suppl 9:139. https://doi.org/10.1016/S1359-6349(08)71707-5
Constantin M, Fundueanu G, Bortolotti F, Cortesi R, Ascenzi P, Menegatti E (2007) A novel multicompartimental system based on aminated poly (vinyl alcohol) microspheres/succinoylated pullulan microspheres for oral delivery of anionic drugs. Int J Pharm 330:129-137 https://doi.org/10.1016/j.ijpharm.2006.09.005
Laha B, Maiti S (2019) Design of core-shell stearyl pullulan nanostructures for drug delivery. Mater Today Proc 11:620–627. https://doi.org/10.1016/j.matpr.2019.03.019
Horie K, Sakagami M, Kuramochi K, Hanasaki K, Hamana H, Ito T (1999) Enhanced accumulation of sialyl Lewis X-carboxymethylpullulan conjugate in acute inflammatory lesion. Pharm Res 16:314–320. https://doi.org/10.1023/A:1018849029727
Jung SW, Jeong YI, Kim SH (2003) Characterization of hydrophobized pullulan with various hydrophobicities. Int J Pharm 254:109–121. https://doi.org/10.1016/S0378-5173(03)00006-1
Lin K, Yi J, Mao X, Wu H, Zhang LM, Yang L (2019) Glucose-sensitive hydrogels from covalently modified carboxylated pullulan and concanavalin A for smart controlled release of insulin. React Funct Polym 139:112–119. https://doi.org/10.1016/j.reactfunctpolym.2019.01.016
Park KH, Song HC, Na K, Bom HS, Lee KH, Kim S, Lee DH (2007) Ionic strength-sensitive pullulan acetate nanoparticles (PAN) for intratumoral administration of radioisotope: Ionic strength-dependent aggregation behaviour and 99mTechnetium retention property. Colloids Surf B Biointerfaces 59:16–23. https://doi.org/10.1016/j.colsurfb.2007.04.010
Zhang HZ, Li XM, Gao FP, Liu LR, Zhou ZM, Zhang QQ (2010) Preparation of folate-modified pullulan acetate nanoparticles for tumor-targeted drug delivery. Drug Deliv 17:48–57. https://doi.org/10.3109/10717540903508979
Barker SA, Tun HC, Doss SH, Gray CJ, Kennedy JF (1971) Preparation of cellulose carbonate. Carbohydr Res 17:471–474. https://doi.org/10.1016/S0008-6215(00)82559-8
Na K, Park KM, Jo EA, Lee KS (2006) Self-organized pullulan/deoxycholic acid nanogels: physicochemical characterization and anti-cancer drug-releasing behaviour. Biotechnol Bioprocess Eng 11:262–267. https://doi.org/10.1007/BF02932041
Priya SS, Rekha MR (2017) Redox sensitive cationic pullulan for efficient gene transfection and drug retention in C6 glioma cells. Int J Pharm 530:401–414. https://doi.org/10.1016/j.ijpharm.2017.08.004
Patil SB, Inamdar SZ, Das KK, Akamanchi KG, Patil AV, Inamadar AC, Kulkarni RV (2020) Tailor-made electrically-responsive poly (acrylamide)-graft-pullulan copolymer based transdermal drug delivery systems: synthesis, characterization, in-vitro and ex-vivo evaluation. J Drug Deliv Sci Technol 56:101525. https://doi.org/10.1016/j.jddst.2020.101525
Li H, Bian S, Huang Y, Liang J, Fan Y, Zhang X (2014) High drug loading pH-sensitive pullulan-DOX conjugate nanoparticles for hepatic targeting. J Biomed Mater Res Part A 102:150–159. https://doi.org/10.1002/jbm.a.34680
Shen S, Li H, Yang W (2014) The preliminary evaluation on cholesterol-modified pullulan as a drug nanocarrier. Drug Deliv 21:501–508. https://doi.org/10.3109/10717544.2014.895068
Yang XC, Niu YL, Zhao NN, Mao C, Xu FJ (2014) A biocleavable pullulan-based vector via ATRP for liver cell-targeting gene delivery. Biomaterials 35:3873–3884. https://doi.org/10.1016/j.biomaterials.2014.01.036
Tao X, Tao T, Wen Y, Yi J, He L, Huang Z, Yang X (2018) Novel delivery of mitoxantrone with hydrophobically modified pullulan nanoparticles to inhibit bladder cancer cell and the effect of nano-drug size on inhibition efficiency. Nanoscale Res Lett 13:1–12. https://doi.org/10.1186/s11671-018-2769-x
Kyogoku N, Ikeda H, Tsuchikawa T, Abiko T, Fujiwara A, Maki T, Hirano S (2016) Time-dependent transition of the immunoglobulin G subclass and immunoglobulin E response in cancer patients vaccinated with cholesteryl pullulan-melanoma antigen gene-A4 nanogel. Oncol Lett 12:4493–4504. https://doi.org/10.3892/ol.2016.5253
Vora L, Tyagi M, Patel K, Gupta S, Vavia P (2014) Self-assembled nanocomplexes of anionic pullulan and polyallylamine for DNA and pH-sensitive intracellular drug delivery. J Nanopart Res 16:1–13. https://doi.org/10.1007/s11051-014-2781-8
Tao X, Xie Y, Zhang Q, Qiu X, Yuan L, Wen Y, Feng X (2016) Cholesterol-modified amino-pullulan nanoparticles as a drug carrier: comparative study of cholesterol-modified carboxyethyl pullulan and pullulan nanoparticles. Nanomaterials 6:165. https://doi.org/10.3390/nano6090165
Wu D, Chen Y, Wen S, Wen Y, Wang R, Zhang Q, Deng X (2019) Synergistically enhanced inhibitory effects of pullulan nanoparticle-mediated co-delivery of lovastatin and doxorubicin to triple-negative breast cancer cells. Nanoscale Res Lett 14:1–12. https://doi.org/10.1186/s11671-019-3146-0
Zhang C, An T, Wang D, Wan G, Zhang M, Wang H, Wang Y (2016) Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly (β-amino ester)/poly (lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. J Control Release 226:193–204. https://doi.org/10.1016/j.jconrel.2016.02.030
Yu KS, Oh JY, Kim MC, Kang SH, Lee NS, Han SY, Kim DK (2018) Controlled release of ursodeoxycholic acid from pullulan acetate nanoparticles to modulate glutamate-induced excitotoxicity in PC-12 cells. J Nanomater 2018:7130450. https://doi.org/10.1155/2018/7130450
Song L, Zhou X, Dai X, Wang R, Cheng G, Zhao N, Xu FJ (2018) Self-destructible polysaccharide nanocomposites with unlockable Au nanorods for high-performance photothermal therapy. NPG Asia Mater 10:509–521. https://doi.org/10.1038/s41427-018-0053-2
Sui J, Cui Y, Cai H, Bian S, Xu Z, Zhou L, Zhang X (2017) Synergistic chemotherapeutic effect of sorafenib-loaded pullulan-Dox conjugate nanoparticles against murine breast carcinoma. Nanoscale 9:2755–2767. https://doi.org/10.1039/C6NR09639E
Laksee S, Sansanaphongpricha K, Puthong S, Sangphech N, Palaga T, Muangsin N (2020) New organic/inorganic nanohybrids of targeted pullulan derivative/gold nanoparticles for effective drug delivery systems. Int J Biol Macromol 162:561–577. https://doi.org/10.1016/j.ijbiomac.2020.06.089
Laksee S, Puthong S, Kongkavitoon P, Palaga T, Muangsin N (2018) Facile and green synthesis of pullulan derivative-stabilized Au nanoparticles as drug carriers for enhancing anticancer activity. Carbohydr Polym 198:495–508. https://doi.org/10.1016/j.carbpol.2018.06.119
Huang L, Wang Y, Ling X, Chaurasiya B, Yang C, Du Y, Sun C (2017) Efficient delivery of paclitaxel into ASGPR over-expressed cancer cells using reversibly stabilized multifunctional pullulan nanoparticles. Carbohydr Polym 159:178–187. https://doi.org/10.1016/j.carbpol.2016.11.094
Huang L, Tu J, Sun C (2017) Reversibly disulfide-crosslinked pullulan nanoparticles for dual-targeted and bio-triggered anti-tumor drug delivery. J Control Release 259:e87. https://doi.org/10.1016/j.jconrel.2017.03.189
Chen L, Qian M, Zhang L, Xia J, Bao Y, Wang J, Li Y (2018) Co-delivery of doxorubicin and shRNA of Beclin1 by folate receptor targeted pullulan-based multifunctional nanomicelles for combinational cancer therapy. RSC Adv 8:17710–17722. https://doi.org/10.1039/C8RA01679H
Zheng Y, Lv X, Xu Y, Cheng X, Wang X, Tang R (2019) pH-sensitive and pluronic-modified pullulan nanogels for greatly improved antitumor in vivo. Int J Biol Macromol 139:277–289. https://doi.org/10.1016/j.ijbiomac.2019.07.220
Li H, Yu C, Zhang J, Li Q, Qiao H, Wang Z, Zeng D (2019) pH-sensitive pullulan-doxorubicin nanoparticles loaded with 1, 1, 2-trichlorotrifluoroethane as a novel synergist for high intensity focused ultrasound mediated tumor ablation. Int J Pharm 556:226–235. https://doi.org/10.1016/j.ijpharm.2018.12.006
Sarika PR, James NR, Nishna N, Kumar PA, Raj DK (2015) Galactosylated pullulan–curcumin conjugate micelles for site specific anticancer activity to hepatocarcinoma cells. Colloids Surf B Biointerfaces 133:347–355. https://doi.org/10.1016/j.colsurfb.2015.06.020
Wang D, Zhang S, Zhang T, Wan G, Chen B, Xiong Q, Wang Y (2017) Pullulan-coated phospholipid and Pluronic F68 complex nanoparticles for carrying IR780 and paclitaxel to treat hepatocellular carcinoma by combining photothermal therapy/photodynamic therapy and chemotherapy. Int J Nanomedicine 12:8649. https://doi.org/10.2147/IJN.S147591
Xia J, Zhang L, Qian M, Bao Y, Wang J, Li Y (2017) Specific light-up pullulan-based nanoparticles with reduction-triggered emission and activatable photoactivity for the imaging and photodynamic killing of cancer cells. J Colloid Interface Sci 498:170–181. https://doi.org/10.1016/j.jcis.2017.03.059
Lee I, Akiyoshi K (2004) Single molecular mechanics of a cholesterol-bearing pullulan nanogel at the hydrophobic interfaces. Biomaterials 25:2911–2918. https://doi.org/10.1016/j.biomaterials.2003.09.065
Ikuta Y, Katayama N, Wang L, Okugawa T, Takahashi Y, Schmitt M, Shiku H (2002) Presentation of a major histocompatibility complex class 1-binding peptide by monocyte-derived dendritic cells incorporating hydrophobized polysaccharide-truncated HER2 protein complex: implications for a polyvalent immuno-cell therapy. Blood 99:3717–3724. https://doi.org/10.1182/blood.V99.10.3717
Gupta M, Gupta AK (2004) Hydrogel pullulan nanoparticles encapsulating pBUDLacZ plasmid as an efficient gene delivery carrier. J Control Release 99:157–166. https://doi.org/10.1016/j.jconrel.2004.06.016
Kang JH, Tachibana Y, Kamata W, Mahara A, Harada-Shiba M, Yamaoka T (2010) Liver-targeted siRNA delivery by polyethylenimine (PEI)-pullulan carrier. Bioorg Med Chem 18:3946–3950. https://doi.org/10.1016/j.bmc.2010.04.031
Ambattu LA, Rekha MR (2015) Collagen synthesis promoting pullulan–PEI–ascorbic acid conjugate as an efficient anti-cancer gene delivery vector. Carbohydr Polym 126:52–61. https://doi.org/10.1016/j.carbpol.2015.03.023
Ambattu LA, Rekha MR (2015) Betaine conjugated cationic pullulan as effective gene carrier. Int J Biol Macromol 72:819–826. https://doi.org/10.1016/j.ijbiomac.2014.09.043
San Juan A, Ducrocq G, Hlawaty H, Bataille I, Guénin E, Letourneur D, Feldman LJ (2007) Tubular cationized pullulan hydrogels as local reservoirs for plasmid DNA. J Biomed Mater Res Part A 83:819–827. https://doi.org/10.1002/jbm.a.31413
Nagane K, Kitada M, Wakao S, Dezawa M, Tabata Y (2009) Practical induction system for dopamine-producing cells from bone marrow stromal cells using spermine-pullulan-mediated reverse transfection method. Tissue Eng Part A 15:1655–1665. https://doi.org/10.1089/ten.tea.2008.0453
Thakor DK, Teng YD, Tabata Y (2009) Neuronal gene delivery by negatively charged pullulan–spermine/DNA anioplexes. Biomaterials 30:1815–1826. https://doi.org/10.1016/j.biomaterials.2008.12.032
Kanatani I, Ikai T, Okazaki A, Jo JI, Yamamoto M, Imamura M, Tabata Y (2006) Efficient gene transfer by pullulan–spermine occurs through both clathrin-and raft/caveolae-dependent mechanisms. J Control Release 116:75–82. https://doi.org/10.1016/j.jconrel.2006.09.001
Park JS, Park JK, Nam JP, Kim WS, Choi C, Kim MY, Nah JW (2012) Preparation of pullulan-g-poly (L-lysine) and its evaluation as a gene carrier. Macromol Res 20:667–672. https://doi.org/10.1007/s13233-012-0098-y
Liu Y, Wang Y, Zhang C, Zhou P, Liu Y, An T, Wang Y (2014) Core–shell nanoparticles based on pullulan and poly (β-amino) ester for hepatoma-targeted codelivery of gene and chemotherapy agent. ACS Appl Mater Interfaces 6:18712–18720. https://doi.org/10.1021/am504203x
Kim H, Na K (2010) Evaluation of succinylated pullulan for long-term protein delivery in poly (lactide-co-glycolide) microspheres. Macromol Res 18:812–819. https://doi.org/10.1007/s13233-010-0814-4
Singh RS, Kaur N, Sharma R, Rana V (2018) Carbamoylethyl pullulan: QbD based synthesis, characterization and corneal wound healing potential. Int J Biol Macromol 118:2245–2255. https://doi.org/10.1016/j.ijbiomac.2018.07.107
Singh RS, Kaur N, Sharma R, Rana V (2019) Investigating the potential of carboxymethyl pullulan for protecting the rabbit eye from systematically induced precorneal tear film damage. Exp Eye Res 184:91–100. https://doi.org/10.1016/j.exer.2019.04.017
Hashimoto Y, Mukai SA, Sawada SI, Sasaki Y, Akiyoshi K (2015) Nanogel tectonic porous gel loading biologics, nanocarriers, and cells for advanced scaffold. Biomaterials 37:107–115. https://doi.org/10.1016/j.biomaterials.2014.10.045
Anseth KS, Bowman CN, Brannon-Peppas L (1996) Mechanical properties of hydrogels and their experimental determination. Biomaterials 17:1647–1657. https://doi.org/10.1016/0142-9612(96)87644-7
Bang S, Lee E, Ko YG, Kim WI, Kwon OH (2016) Injectable pullulan hydrogel for the prevention of postoperative tissue adhesion. Int J Biol Macromol 87:155–162. https://doi.org/10.1016/j.ijbiomac.2016.02.026
Loke WK, Lau SK, Yong LL, Khor E, Sum CK (2000) Wound dressing with sustained anti-microbial capability. J Biomed Mater Res 53:8–17. https://doi.org/10.1002/(SICI)1097-4636(2000)53:1%3C8::AID-JBM2%3E3.0.CO;2-3
Lay-Flurrie K (2004) The properties of hydrogel dressings and their impact on wound healing. Professional Nurse (London, England) 19:269–273
Fujioka-Kobayashi M, Ota MS, Shimoda A, Nakahama KI, Akiyoshi K, Miyamoto Y, Iseki S (2012) Cholesteryl group-and acryloyl group-bearing pullulan nanogel to deliver BMP2 and FGF18 for bone tissue engineering. Biomaterials 33:7613–7620. https://doi.org/10.1016/j.biomaterials.2012.06.075
Schlaubitz S, Derkaoui SM, Marosa L, Miraux S, Renard M, Catros S, Fricain JC (2014) Pullulan/dextran/nHA macroporous composite beads for bone repair in a femoral condyle defect in rats. PLoS ONE 9:e110251. https://doi.org/10.1371/journal.pone.0110251
Chen F, Yu S, Liu B, Ni Y, Yu C, Su Y, Yan D (2016) An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci Rep 6:1–12. https://doi.org/10.1038/srep20014
Galvez MG, Wong VW, Chang EI, Major M, Carre L, Kandimalla R, Gurtner GC (2009) Pullulan-collagen hydrogel scaffold as a dermal substitute. J Am Coll Surg 209:S78. https://doi.org/10.1016/j.jamcollsurg.2009.06.191
Kobayashi H, Katakura O, Morimoto N, Akiyoshi K, Kasugai S (2009) Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. J Biomed Mater Res Part B 91:55–60. https://doi.org/10.1002/jbm.b.31373
Li X, Xue W, Zhu C, Fan D, Liu Y (2015) Novel hydrogels based on carboxyl pullulan and collagen crosslinking with 1, 4-butanediol diglycidylether for use as a dermal filler: initial in vitro and in vivo investigations. Mater Sci Eng C 57:189–196. https://doi.org/10.1016/j.msec.2015.07.059
Atila D, Keskin D, Tezcaner A (2015) Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr Polym 133:251–261. https://doi.org/10.1016/j.carbpol.2015.06.109
Wong VW, Rustad KC, Galvez MG, Neofytou E, Glotzbach JP, Januszyk M, Gurtner GC (2011) Engineered pullulan–collagen composite dermal hydrogels improve early cutaneous wound healing. Tissue Eng Part A 17:631–644. https://doi.org/10.1089/ten.tea.2010.0298
Jeong HG, Kim YE, Kim YJ (2013) Fabrication of poly (vinyl acetate)/polysaccharide biocomposite nanofibrous membranes for tissue engineering. Macromol Res 21:1233–1240. https://doi.org/10.1007/s13233-013-1155-x
Xu F, Weng B, Gilkerson R, Materon LA, Lozano K (2015) Development of tannic acid/chitosan/pullulan composite nanofibers from aqueous solution for potential applications as wound dressing. Carbohydr Polym 115:16–24. https://doi.org/10.1016/j.carbpol.2014.08.081
Alban S, Schauerte A, Franz G (2002) Anticoagulant sulfated polysaccharides: Part I. Synthesis and structure–activity relationships of new pullulan sulfates. Carbohydr Polym 47:267–276. https://doi.org/10.1016/S0144-8617(01)00178-3
Shiku H, Kageyama SI (2008) U.S. Patent Application No. 11/722,019
Kageyama S, Wada H, Muro K, Niwa Y, Ueda S, Miyata H, Shiku H (2013) Dose-dependent effects of NY-ESO-1 protein vaccine complexed with cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and survival benefits of esophageal cancer patients. J Transl Med 11:1–10. https://doi.org/10.1186/1479-5876-11-246
Yuki Y, Kong I, Sato A, Nochi T, Mejima M, Kurokawa S, Kiyono H (2012) Adjuvant-free nanogel-based PspA nasal vaccine for the induction of protective immunity against Pneumococcus (166.7). J Immunol 2012:166–167. https://doi.org/10.4049/jimmunol.188.Supp.166.7
Kong IG, Sato A, Yuki Y, Nochi T, Takahashi H, Sawada S, Kiyono H (2013) Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae. Infect Immun 81:1625–1634. https://doi.org/10.1128/iai.00240-13
Nochi T, Yuki Y, Takahashi H, Sawada SI, Mejima M, Kohda T, Kiyono H (2010) Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater 9:572–578. https://doi.org/10.1038/nmat2784
Hasegawa U, Shin-ichiro MN, Kaul SC, Hirano T, Akiyoshi K (2005) Nanogel-quantum dot hybrid nanoparticles for live cell imaging. Biochem Biophys Res Commun 331:917–921. https://doi.org/10.1016/j.bbrc.2005.03.228
Kong SH, Noh YW, Suh YS, Park HS, Lee HJ, Kang KW, Yang HK (2015) Evaluation of the novel near-infrared fluorescence tracers pullulan polymer nanogel and indocyanine green/γ-glutamic acid complex for sentinel lymph node navigation surgery in large animal models. Gastric Cancer 18:55–64. https://doi.org/10.1007/s10120-014-0345-3
Yim H, Yang SG, Jeon YS, Park IS, Kim M, Lee DH, Na K (2011) The performance of gadolinium diethylene triamine pentaacetate-pullulan hepatocyte-specific T1 contrast agent for MRI. Biomaterials 32:5187–5194. https://doi.org/10.1016/j.biomaterials.2011.03.069
Kim S, Chae SY, Na K, Kim SW, Bae YH (2003) Insulinotropic activity of sulfonylurea/pullulan conjugate in rat islet microcapsule. Biomaterials 24:4843–4851. https://doi.org/10.1016/S0142-9612(03)00382-X
Shingel KI, Petrov PT (2002) Behavior of γ-ray-irradiated pullulan in aqueous solutions of cationic (cetyltrimethylammonium hydroxide) and anionic (sodium dodecyl sulfate) surfactants. Colloid Polym Sci 280:176–182. https://doi.org/10.1007/s00396-001-0599-2
Akiyoshi K, Sasaki Y, Sunamoto J (1999) Molecular chaperone-like activity of hydrogel nanoparticles of hydrophobized pullulan: thermal stabilization with refolding of carbonic anhydrase B. Bioconjug Chem 10:321–324. https://doi.org/10.1021/bc9801272
Shingel KI, Petrov PT (2001) Hydrodynamic and molecular characteristics of γ-irradiated pullulan. Bыcoкoмoлeкyляpныe coeдинeния Cepия Б 43:562–565
Acknowledgements
Figures are created by Biorender.com.
Author information
Authors and Affiliations
Contributions
RPM—conceptualisation, data curation, formal analysis, writing-original draft and review; SK—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahato, R.P., Kumar, S. A review of pullulan nanopolymer derived from agro-food waste and its applications. Iran Polym J 33, 1493–1526 (2024). https://doi.org/10.1007/s13726-024-01338-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-024-01338-1