Abstract
A flame-retardant epoxy resin (EP) composite maintaining good mechanical properties was prepared by the combination of aluminum diethyl phosphonate (ADP) with piperazine pyrophosphate (PAPP). The flame-retardant synergistic effect between ADP and PAPP was explored. When 3% ADP and 1% PAPP were added into EP simultaneously, the obtained EP composite (EPAP-3) showed good flame retardancy with the 39.3% limit oxygen index value and the V-0 classification in the vertical combustion test. Compared with the pure EP, the peak heat release rate value of EPAP-3 obtained in the microscale combustion calorimeter test decreased by 18.9%. Thermogravimetric analysis results indicated that the simultaneous introduction of ADP and PAPP increased the char residual of the prepared flame-retardant EP composites. Scanning electron microscopy observation on the morphology of the char layer after the vertical combustion test revealed the continuous and dense char layer structure with the shielding effect existing in the EPAP-3. Meanwhile, the low addition of ADP and PAPP hardly decreased the mechanical properties of the prepared flame-retardant EP composites. ADP and PAPP not only showed a good flame-retardant synergistic effect in EP but also hardly decreased the mechanical properties of EP, which made them the potential high-performance synergistic flame retardant in the preparation of EP composite.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer materials have become an increasingly important part of industrial production and everyday life [1,2,3]. As an important polymer material, epoxy resin (EP) is used more and more widely due to its excellent mechanical properties, dielectric properties, bonding properties, chemical corrosion resistance, and thermal properties. It can be used as paint and adhesive in aerospace, electrical, automotive, construction, and other fields [4, 5]. However, the relatively low limiting oxygen index value of around 23.5% for EP indicates the flammability of EP [6]. When EP burns, the generated burning droplet and the released toxic smoke can not only spread the fire but also endanger the life safety of people. Thus, improving the fire resistance of EP-based materials is the premise of its safe application, which has aroused extensive research by researchers around the world [7,8,9]. Halogen-containing flame retardants (HCFRs) were widely used in polymer materials due to their superior flame-retardant properties and relatively low cost [10]. However, toxic fumes and persistent organic pollutants can be released during the combustion of HCFRs. Thus, with the gradual improvement of environmental requirements, HCFRs are limited in some countries and substituted by halogen-free flame retardants (HFFRs) containing phosphorus, nitrogen, silicon, and other flame-retardant elements [11, 12]. Nevertheless, single-component HFFRs are often added to EP in large amounts to meet the fire safety requirement, leading to the deterioration of the mechanical properties of polymer material [13]. To reduce the impact of the excessive addition of single-component HFFRs on the mechanical properties of polymer material, reducing the total amount of HFFRs added through the synergistic effect of various flame retardants has been proved an efficient way [14,15,16].
Aluminum diethylphosphinate (ADP) is a phosphorous HFFR with good water resistance and heat stability [17]. Zhu et al. studied the mechanism of ADP in improving the flame retardancy of polymer materials and pointed out that ADP achieved flame retarding performance mainly through the gas phase flame retarding [18]. Wang et al. studied the effect of the addition of ADP on the flame retardancy of EP and found, when the addition amount of ADP in EP increased to 15%, the obtained composite passed the V-0 classification in the UL-94 test [19]. Piperazine pyrophosphate (PAPP) is an HFFR containing P and N elements simultaneously [20]. The excellent charring ability made PAPP a good flame retardant in polypropylene, polylactic acid, EP, etc. [21, 22]. The flame-retardant EP composite with the addition of 20% PAPP could pass the UL-94V-0 classification [23]. Meanwhile, it was found that, unlike ADP, PAPP achieved its flame retarding performance mainly through condense phase flame retarding [24].
When ADP or PAPP are used alone, the addition amount of them is always higher than 10% to achieve the high flame retardancy, which can significantly reduce the mechanical properties of the polymer materials as other HFFRs [25, 26]. To solve this problem, the synergistic effect between different flame retardants was used to reduce the amount of flame retardants [27]. For example, Li et al. found the synergistic effect of PAPP with epoxy vinyl siloxanes (EVOS) in EP. When the weight ratio of PAPP to EVOS was 9:1 and the amount of flame retardant in EP was 10%, in the UL-94 test, the prepared EP composite exhibited high flame retardancy and obtained the V-0 classification [28]. When PAPP and ADP mixture with a mass ratio of 4:1 was used as the flame retardant for acrylonitrile–butadiene–styrene (ABS) copolymer, the obtained flame-retardant ABS reached V-0 classification with the 25% flame-retardant loading [29].
By now, ADP and PAPP have not been simultaneously used in the preparation of flame-retardant EP. Thus, ADP and PAPP were used simultaneously to prepare flame-retardant EP. The synergistic effect of ADP with PAPP in flame-retardant EP was analyzed through the thermogravimetric analysis (TGA), limiting oxygen index (LOI) test, and microscale combustion calorimeter test (MCC). In addition, the mechanical properties of the prepared flame-retardant EP containing PAPP and ADP were studied.
Experimental
Materials
A commercial diglycidyl ether of bisphenol-A (EP: E-44) was bought from Guangzhou Suixin Chemical Company, China. Piperazine pyrophosphate (PAPP, GP300) was purchased from Zhongshan Sutebao New Materials Company, China. Aluminum diethylphosphinate (ADP, phosphorus content of 23.4–23.7%) and 4,4ʹ-diaminodiphenylmethane (DDM, 97.0%) were obtained from Shanghai Macklin Biochemical Company, China. Figure 1 shows the chemical structure of ADP and PAPP.
Preparation procedure of the EP sample
Firstly, a certain amount of E-44, ADP, and PAPP was weighed in a glass beaker according to the formulation in Table 1. The E-44 mixture was heated in an oil bath at 80 °C and blended by a magnetic stirrer for 20 min. The obtained E-44 mixture after heating was put on an ultrasound cell crusher and treated for 30 min until the flame retardants were evenly dispersed. Then, melted curing agent DDM was poured into the E-44 mixture and magnetically blended for 10 min at 80 °C. The uniform mixture was transferred in a vacuum oven and degassed at 80 °C for 10 min, after which the degassed mixture was slowly poured into a Teflon mold. The flame-retardant EP composites were obtained after curing at 120 °C and 150 °C for 2 h, respectively. The formulation of the prepared samples is listed in Table 1.
Characterization
Vertical burning (UL-94) test was performed on a vertical combustion apparatus (BKSSOD, China) according to the ASTM D3801 standard with the sample dimension of 127 mm × 12.7 mm × 3.2 mm. The limiting oxygen index (LOI) test was carried out on an oxygen index tester (YZS-75A, China) according to the ASTM D2863-97 standard with the sample thickness of 3.2 mm. The thermal decomposition behavior of the samples was investigated by a thermogravimeter (TA Q50, USA) under the N2 atmosphere with the heating rate of 10 °C min−1 in the temperature range of room temperature to 700 °C. The flammability property of the EP composites was determined by a microscale combustion calorimetry (FAA-PCFC, US Pat.No.5, 981,290). The surface morphology of the sample char layer after the UL-94 test was observed by a scanning electron microscope (SEM, SU8010N, Japan), which was also applied to detect the sample element composition due to the energy-dispersive spectrometer (EDS) equipped in it. A tensile test instrument (TCS-2000, China) was used to test the tensile properties of the prepared samples according to the GB/T 1040.2-2006 standard. An impact test apparatus (JJ-20, China) was applied to test the unnotched impact resistance of the flame-retardant EP composites according to the GB/T 1843-2008 standard. A contact angle test apparatus (JC2000D8, China) was used to analyze the polarity of the samples.
Results and discussion
LOI and UL-94 tests
Firstly, the prepared EP and EP composites were evaluated by limiting oxygen index (LOI) and vertical combustion (UL-94) tests, which are commonly used to determine the flame retardancy of materials. The video screenshots of the prepared samples during the UL-94 test are presented in Fig. 2. The detailed test results are shown in Table 2. The LOI value of pure EP reached 25.3% and the UL-94 test result was no rating because the pure EP was completely burned with the serious dripping phenomena after the first 10 s of flame ignition (Fig. 2a). When 4% ADP was added, the LOI value of EPAP-1 increased to 35.9%, but the UL-94 test of EPAP-1 only obtained the V-1 classification for the combustion time longer than 10 s. With the addition of a small amount of PAPP with ADP, the EP flame retardancy was enhanced obviously. The LOI value of EPAP-3 with the weight ratio of ADP to PAPP at 3:1 increased to 39.3%. Meanwhile, EPAP-3 achieved the V-0 classification in the UL-94 test with the total combustion time of fewer than 5 s. With the decrease of ADP content, the UL-94 test result of EPAP-4 was no grade. When only PAPP was applied in the preparation of flame-retardant EP composite, the deterioration of EP flame retardancy further happened. The LOI value of EPAP-5 was only 25.5% and, in the UL-94 test, the EPAP-5 sample combusted heavily. Therefore, the LOI and UL-94 investigation results indicated that, when ADP and PAPP are used together, the relatively good flame retardancy of the flame-retardant EP can be obtained, demonstrating the synergistic effect of ADP with PAPP in preparing the flame-retardant EP.
Table 3 shows the comparison of the flame retardancy of ADP/PAPP with the reported works. It can be observed that the synergistic flame-retardant system containing commercial flame retardants such as ammonium polyphosphate (APP), ADP, and PAPP has drawn much attention in these years. PAPP and ADP can be used together with epoxy-octavinyl silsesquioxane (EVOS) and ammonium phosphomolybdate (AMP) to achieve high flame retardancy. However, compared with the reported synergistic flame-retardant system, the synergistic flame-retardant system composed by ADP and PAPP in this work showed the advantages on the aspect of low addition amount and high LOI value, indicating the relatively good synergistic effect of ADP with PAPP in EP.
Thermal decomposition behavior
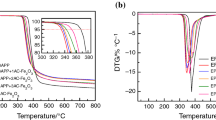
TGA was used to study the thermal decomposition behavior of ADP, PAPP, and the prepared EP samples. The TGA and corresponding DTG curves are presented in Fig. 3. The detailed TGA analysis data such as the 5% weight loss temperature (T5%WL), the maximum weight loss rate temperature (TMWLR), the maximum weight loss rate (MWLR), and the weight residual at 700 °C (WR700) are shown in Table 4. The T5%WL values of ADP and PAPP were 427.3 and 362.1 °C, respectively (Fig. 3a). The decomposition of ADP mainly happened between 300 and 500 °C with a weight loss of about 75.8%. In this temperature range, ADP firstly decomposed into diethyl phosphoric acid and ethylene. Eventually, aluminum phosphate was left in the residual char layer [31,32,33]. The thermal decomposition of PAPP happened in a relatively broad temperature range from 270 to 700 °C. In the temperature range from 270 to 320 °C, the decomposition is mainly caused by the release of water, which comes from the dehydration reaction between P–OH and –NH in the PAPP molecule [34]. In the temperature range from 320 to 440 °C, the char residual begins to come into being under the catalytic action of phosphoric acid derivatives. The final decomposition in the temperature range from 440 to 700 °C corresponds to the further decomposition of the formed char residual [35]. The addition of ADP and PAPP affected the EP decomposition behavior (Fig. 3b, c). The addition of 4% ADP in EPAP-1 reduced the T5%WL from 392.6 °C of EPAP-0 to 383.7 °C of EPAP-1. Compared with EPAP-0, the MWLR of EPAP-1 decreased from 1.76 to 1.30%°C−1, and the WR700 of EPAP-1 increased to 18.6%, indicating that the addition of ADP can decrease the EP decomposition rate and increase the EP char residue. The EP decomposition rate cannot be reduced by the addition of PAPP due to the 1.89%°C−1 MWLR of EPAP-5, which is higher than that of EPAP-0. However, compared with EPAP-0, PAPP exhibited relatively better char residue forming ability because the WR700 of EPAP-5 increased to 24.3% with the addition of 4% PAPP in EPAP-5. When ADP and PAPP were used simultaneously, EPAP-2, EPAP-3, and EPAP-4 showed similar abilities on the aspect of decrease the EP decomposition rate and increase the EP char residue. The TMWLR decreased and WR700 increased with the increase of the PAPP content. EPAP-3 has the lowest MWLR of 1.13%°C−1 among all the prepared flame-retardant EP samples. The change of the thermal decomposition behavior of the prepared flame-retardant EP is due to the relatively low thermal stability of PAPP and ADP and high char residue generated after the decomposition of PAPP and ADP [31]. The TGA investigation data proved that the thermal decomposition behavior of EP can be greatly changed by the addition of ADP and PAPP, leading to the different flame retardancy of the flame-retardant EP samples.
SEM analysis of char residual after UL-94 test
The char residual generated during combustion can isolate combustible gas and protect the unburned polymer materials [36]. Since the dense char layer can isolate the polymer from the contact with the combustible gases and prevent the rapid combustion of the polymer, the denser the char layer is, the better the protection effect is [37]. Therefore, the performance of flame retardant can be judged by studying the morphology of char residual produced after combustion. The char residue morphology of the EPAP-0, EPAP-1, EPAP-3, and EPAP-5 after the UL-94 test was observed by SEM. The char residual of pure EP exhibited a fractured morphology with obvious cracks and holes (Fig. 4a). With the addition of 4% ADP, a relatively smooth morphology with a lot of loose and small pores could be seen on the char residual surface of EPAP-1. In contrast, with the addition of 4% PAPP, the char residual of EPAP-5 presented a morphology with a lot of big holes (Fig. 4d). When ADP and PAPP were used with the weight ratio of 3:1 in EPAP-3, a dense and smooth char residual morphology could be observed. No cracks or holes were observed on the char residual surface of EPAP-3. Thus, among the four char residue samples observed, the char residue of EPAP-3 is the densest and smooth, which makes it have a relatively better isolation effect to protect the unburned EP. The dense and smooth char residue morphology can only be formed when ADP and PAPP flame retardants are used simultaneously in EPAP-3, which indicates that ADP and PAPP have synergistic flame retardancy.
MCC test
Microscale combustion calorimetry (MCC) is a new and rapid-developing testing method, which can quickly and conveniently measure the main fire parameters of materials [38]. The heat release rate (HRR) curves of the EP samples from the MCC test are presented in Fig. 5. The temperature of the peak heat release rate (TPHRR) of EPAP-5 is around 350 °C, which is lower than the TPHRR of other samples at about 380 °C. This is due to the lower decomposition temperature of PAPP, which lowers the decomposition temperature of EPAP-5 [38].
The detailed combustion parameters obtained from MCC, the peak heat release rate (PHRR), including heat release capacity (HRC), and the total heat release (THR), are shown in Table 5 in detail. EPAP-0 has the highest PHRR of 435.1 W g−1, THR of 26.8 kJ·g−1, and HRC of 468.6 Jg−1 K−1 among the tested samples, indicating the poor flame retardancy of EP. When ADP and PAPP were added to EP, HRC, PHRR, and THR of EPAP-1, EPAP-3, and EPAP-5, all decreased to varying degrees. In the prepared flame-retardant EP composites, EPAP-3 has the lowest HRC, PHRR, and THR. The PHRR of EPAP-3 decreased by 18.9% to 352.8 W g−1, the HRC of EPAP-3 decreased by 19.1% to 379.3 Jg−1 K−1, and the THR of EPAP-3 decreased by 6.3% to 25.1 kJ·g−1 compared with EPAP-0. In general, the lower HRC, PHRR, and THR mean that fewer materials burn completely, leading to the high flame retardancy of materials [39,40,41]. Therefore, according to MCC test results, ADP and PAPP play a synergistic role in improving the flame retardancy of EP samples.
Possible synergistic flame-retardant mechanism
In the prepared flame-retardant EP samples, since EPAP-3 has the best flame retardancy, the synergistic flame-retardant effect of ADP with PAPP was explored by analyzing the elemental composition of the EPAP-3 char residue first. In Fig. 6a, the EDS results of the EPAP-3 char residue showed that the EPAP-3 char residue was composed of aluminum, carbon, nitrogen, oxygen, and phosphorus elements with the contents of 0.56%, 83.97%, 7.13%, 6.25%, and 2.10%, respectively. This result indicates that the N, P, and Al elements in ADP and PAPP participated in and promoted the char forming of EP during the combustion process [42,43,44]. Based on the above analysis, the possible synergistic mechanism of ADP with PAPP in EP is shown in Fig. 6. During the EP combustion process, the phosphorous-free radical produced by the decomposition of ADP in the gas phase can prevent the free radical chain reaction of EP combustion [19, 32, 45]. The NH3 produced during the PAPP decomposition process can decrease the concentration of combustible gas in the gas phase [18]. In the condensed phase, due to the dehydration and decomposition of PAPP to produce polyphosphoric acid, the catalytic carbonization effect of PAPP enhanced the carbonization effect of the condensed phase [35]. Meanwhile, aluminum phosphate can be formed in the char residual with the decomposition of ADP, leading to the formation of the dense char residual [30]. The continuous and dense char residual caused by the decomposition of PAPP and ADP can effectively block the heat transfer and the flow of combustible gas to protect the EP material under the char layer. Thus, ADP and PAPP effectively increased EP flame retardancy through the above synergistic effect.
Mechanical properties
The presence of flame retardant in polymer materials usually can reduce the mechanical properties of materials, and in some cases, the reduction can even exceed 50% [46]. Therefore, it is necessary to clarify whether the addition of ADP and PAPP can greatly decrease the EP mechanical properties. The relevant tensile and impact test results are shown in Fig. 7. The obtained tensile strength and impact strength values of EPAP-0 are 76.4 MPa and 35.8 kJ·m−2, respectively. Regardless of the weight ratio of ADP to PAPP, the tensile strength and impact strength values of the prepared EP samples are about 68.6 MPa and 24.2 kJ·m−2, respectively. With the addition of the ADP and PAPP flame retardants, the tensile strength and impact strength values of the EP samples decreased by about 10.2% and 32.4%, respectively. The relatively small addition of less than 4% flame retardant and the good compatibility between ADP and EP led to the slight decrease in EP mechanical properties [30, 45, 47]. Therefore, when the ratio of ADP to PAPP is 3:1, the prepared EP composite, EPAP-3, has excellent flame retardancy and good mechanical properties. It has been reported that the polarity of additives has a significant impact on the mechanical properties of composite materials. When the polarity of the additive is similar to that of the polymer, the compatibility between the additive and the polymer is better, and the prepared composite has relatively good mechanical properties. When the polarity of additives and the polarity of polymer are greatly different, the compatibility between additives and polymer is poor, and the mechanical properties of the prepared composite are reduced. Contact angle test is an important method to study the surface polarity of materials. Figure 8 shows the contact angle test results of ADP, PAPP, and EP. The contact angle of ADP is 100.13°, the contact angle of PAPP is 17.45°, and the contact angle of EP is 85.05°. The difference in contact angle between ADP and EP is relatively small, indicating the relatively good compatibility between ADP and EP. Therefore, when ADP was added to EP, the mechanical properties of flame-retardant EP decreased slightly. The significant difference in contact angle between PAPP and EP indicates the poor compatibility between PAPP and EP. However, the addition amount of PAPP in EPAP-3 is only 1%, which can only slightly decrease the mechanical properties of EPAP-3. In summary, the amount of ADP and PAPP in EPAP-3 is not high, which cannot significantly decrease the mechanical properties of the prepared flame-retardant EP.
Conclusion
In this paper, the synergistic effect of ADP with PAPP in EP was studied. When the content of ADP and PAPP flame retardants was 4% and the weight ratio of ADP to PAPP was 3:1, the prepared flame-retardant EP composites, EPAP-3, showed the best flame retardancy with the UL-94V-0 classification and the 39.3% LOI value. The introduction of ADP and PAPP in EPAP-3 increased the char residue at 700 °C and promoted the formation of the continuous and smooth char residual during the combustion process. The PHRR, HRC, and THR of EPAP-3 decreased by 18.9%, 19.1%, and 6.3%, respectively, compared with pure EP. The synergistic mechanism of ADP and PAPP in EP was explored, and it was found that, during the EP burning process, ADP and PAPP have a synergistic effect in the gas phase and condensed phase simultaneously to improve the flame retardancy of EP. Because high flame retardancy can be achieved with the low addition of ADP and PAPP, good EP mechanical properties can be maintained. In conclusion, there is a synergistic effect of ADP with PAPP in flame-retardant EP. The synergistic flame retardant composed of ADP and PAPP has potential application in the preparation of high-performance flame-retardant EP composites.
Data availability
The data that support the findings of this study are available from the corresponding author.
References
Li C, Wang B, Zhou L, Hou X, Su S (2022) Effects of lignin-based flame retardants on flame-retardancy and insulation performances of epoxy resin composites. Iran Polym J 31:949–962
Wang S, Wu WD, Chen Q, Ding Z, Li SX, Zhang AL, Tang T, Liu J, Okoye PU (2022) Preparation of DOPO-derived magnesium phosphate whisker and its synergistic effect with ammonium polyphosphate on the flame retardancy and mechanical property of epoxy resin. J Appl Polym Sci 140:e53430
Wang S, Zhang T, Li J, Hua Y, Dou J, Chen X, Li S (2023) Potassium citrate-derived porous carbon with high CO2 capture and Congo red adsorption performance. Environ Sci Eur 35:9–22
Li Y, Qian Y, Jiang Q, Haruna AY, Luo Y, Yang J (2022) Thermally conductive polymer-based composites: fundamentals, progress and flame retardancy/anti-electromagnetic interference design. J Mater Chem C 10:14399–14430
Tian ZS, Wang YQ, Hou XL (2022) Review of chemical recycling and reuse of carbon fiber reinforced epoxy resin composites. New Carbon Mater 37:1021–1041
Qian LJ, Qiu Y, Wang JY, Xi W (2015) High-performance flame retardancy by char-cage hindering and free radical quenching effects in epoxy thermosets. Polymer 68:262–269
Zhi M, Yang X, Fan R, Yue S, Zheng L, Liu Q, He Y (2022) A comprehensive review of reactive flame-retardant epoxy resin: fundamentals, recent developments, and perspectives. Polym Degrad Stab 201:109976
Kandola BK, Magnoni F, Ebdon JR (2022) Flame retardants for epoxy resins: application-related challenges and solutions. J Vinyl Addit Technol 28:17–49
Chen Q, Wang S, Li S, Zhang A (2023) Highly efficient phosphorous-containing flame retardant for transparent epoxy resin with good mechanical properties. J Polym Res 30:32
Chen L, Wang YZ (2009) A review on flame retardant technology in China. Part I: development of flame retardants. Polym Adv Technol 21:1–26
Yuan Y, Yu B, Shi Y, Mao L, Xie J, Pan H, Liu Y, Wang W (2020) Insight into hyper-branched aluminum phosphonate in combination with multiple phosphorus synergies for fire-safe epoxy resin composites. Polymers 12:64
Zhao B, Kolibaba TJ, Lazar S, Grunlan JC (2021) Environmentally-benign, water-based covalent polymer network for flame retardant cotton. Cellulose 28:5855–5866
Rajaei M, Wang DY, Bhattacharyya D (2017) Combined effects of ammonium polyphosphate and talc on the fire and mechanical properties of epoxy/glass fabric composites. Compos B 113:381–390
Xu Y, Li J, Shen R, Wang Z, Hu P, Wang Q (2021) Experimental study on the synergistic flame retardant effect of bio-based magnesium phytate and rice husk ash on epoxy resins. J Therm Anal Calorim 146:153–164
Xia L, Wang X, Ren T, Luo L, Li D, Dai J, Dai L (2022) Green construction of multi-functional fire resistant epoxy resins based on boron nitride with core-shell structure. Polym Degrad Stab 203:110059
Zhou R, Wu X, Bao X, Wu F, Han X, Wang J (2023) The effect of polyepoxyphenylsilsesquioxane and diethyl bis-(2-hydroxyethyl) aminomethylphosphonate on the thermal stability of epoxy resin. ACS Omega 8:2077–2084
Langfeld K, Wilke A, Sut A, Greiser S, Ulmer B, Andrievici V, Schartel B (2015) Halogen-free fire retardant styrene-ethylene-butylene-styrene-based thermoplastic elastomers using synergistic aluminum diethylphosphinate-based combinations. J Fire Sci 33:157–177
Zhu P, Xu M, Li S, Zhan Z, Li B (2019) Preparation and investigation of efficient flame retardant TPE composites with piperazine pyrophosphate/aluminum diethylphosphinate system. J Appl Polym Sci 137:47711
Wang A, Zhang F, Xing L, Zhu Y, Xie W, Chen X, Cheng Y (2021) Effect of aluminum diethylphosphinate and its synergist on flame-retardant effects of epoxy resin. J Therm Anal Calorim 147:7277–7287
Li L, Huang Y, Tang W, Zhang Y, Qian L (2022) Synergistic effect between piperazine pyrophosphate and melamine polyphosphate in flame retardant coatings for structural steel. Polymers 14:3722
Chen HX, Xia W, Wang N, Liu Y, Fan P, Wang S, Chen Q (2022) Flame retardancy of biodegradable polylactic acid with piperazine pyrophosphate and melamine cyanurate as flame retardant. J Fire Sci 40:254–273
Liu W, Wang Z, Su S, Wu H, Sun M, Tang L (2022) Synergistic flame retardancy of ZnO and piperazine pyrophosphate/melamine cyanurate in polypropylene. J Vinyl Addit Technol 29:202–219
Sun Z, Hou Y, Hu Y, Hu W (2018) Effect of additive phosphorus-nitrogen containing flame retardant on char formation and flame retardancy of epoxy resin. Mater Chem Phys 214:154–164
Liu L, Xu Y, He Y, Xu M, Wang W, Li B (2020) A facile strategy for enhancing the fire safety of unsaturated polyester resins through introducing an efficient mono-component intumescent flame retardant. Polym Adv Technol 31:1218–1230
Kaynak C, Polat O (2014) Influences of nanoclays on the flame retardancy of fiber-filled and unfilled polyamide-6 with and without aluminum diethylphosphinate. J Fire Sci 33:87–112
Xiao X, Zhai J, Chen T, Mai Y, Hu S, Ye W, Yin L (2017) Flame retardant properties of polyamide 6 with piperazine pyrophosphate. Plast Rubber Compos 46:193–199
Zhang C, Guo H, Zhou X, Yu L, Li H, Yang ZB (2022) Effects of different boron-based flame retardants on the combustibility of bamboo filaments. Wood Res 67:221–230
Li S, Liu Y, Liu Y, Wang Q (2021) Synergistic effect of piperazine pyrophosphate and epoxy-octavinyl silsesquioxane on flame retardancy and mechanical properties of epoxy resin. Compos B 223:109115
Yuan Z, Wen H, Liu Y, Wang Q (2021) Synergy between piperazine pyrophosphate and aluminum diethylphosphinate in flame retarded acrylonitrile-butadiene-styrene copolymer. Polym Degrad Stab 190:109639
Lou S, Yu R, Wang S, Fan P, Liu J, Tang T (2023) Synergies between phosphomolybdate and aluminum diethylphosphinate acting as temperature-response microparticles for promoting fire safety of epoxy resin. Polymer 268:125715
Zhong L, Zhang KX, Wang X, Chen MJ, Xin F, Liu ZG (2018) Synergistic effects and flame-retardant mechanism of aluminum diethyl phosphinate in combination with melamine polyphosphate and aluminum oxide in epoxy resin. J Therm Anal Calorim 134:1637–1646
Feng H, Li D, Cheng B, Song T, Yang R (2022) A cross-linked charring strategy for mitigating the hazards of smoke and heat of aluminum diethylphosphonate/polyamide 6 by caged octaphenyl polyhedral oligomeric silsesquioxanes. J Hazard Mater 424:127420
Chen W, Liu P, Cheng Y, Liu Y, Wang Q, Duan W (2018) Flame retardancy mechanisms of melamine cyanurate in combination with aluminum diethylphosphinate in epoxy resin. J Appl Polym Sci 136:47223
Yuan Z, Wen H, Liu Y, Wang Q (2021) Synergistic effect between piperazine pyrophosphate and melamine polyphosphate in flame retarded glass fiber reinforced polypropylene. Polym Degrad Stab 184:109477
Liu W, Ding L, Wang L, Zhang C, Yang W, Liu D, Gui Z, Hu W (2023) A rational design of functionalized black phosphorus cooperates with piperazine pyrophosphate to significantly suppress the fire hazards of polypropylene. Chemosphere 314:137686
Wang L, Zhu J, Deng H, Lyu R, Wei Y, Lan X (2022) Improved pyrolysis performance of a cyclic phosphate-melamine intumescent fire-retardant coating system using ceria as an additive. ChemistrySelect 7:e202200062
Meng ZH, Liu Y, Wang S, Zhang AL, Li SX (2023) Application of the PAPP/MCA/APP intumescent flame retardant system in polypropylene. Fire Mater 2023:3152
Xu Q, Mensah RA, Jin C, Jiang L (2021) A critical review of the methods and applications of microscale combustion calorimetry for material flammability assessment. J Therm Anal Calorim 147:6001–6013
Lu X, Gu X (2022) Fabrication of a bi-hydroxyl-bi-DOPO compound with excellent quenching and charring capacities for lignin-based epoxy resin. Int J Biol Macromol 205:539–552
Rodriguez-Melendez D, Langhansl M, Helmbrecht A, Palen B, Zollfrank C, Grunlan JC (2022) Biorenewable polyelectrolyte nanocoating for flame-retardant cotton-based paper. ACS Omega 7:32599–32603
Li C, Yu X, Tan Y, Xie G, Liu H, Tang G (2021) Investigation on thermal properties and flame retardancy of glass-fiber reinforced poly(butylene succinate) composites filled with aluminum hypophosphite and melamine cyanurate. J Appl Polym Sci 139:51739
Mu XW, Jin ZY, Chu FK, Cai W, Zhu YL, Yu B, Song L, Hu Y (2022) High-performance flame-retardant polycarbonate composites: mechanisms investigation and fire-safety evaluation systems establishment. Compos B 238:109873
Cao CF, Yu B, Huang J, Feng XL, Lv LY, Sun FN, Tang LC, Feng JB, Song PA, Wang H (2022) Biomimetic, mechanically strong supramolecular nanosystem enabling solvent resistance, reliable fire protection and ultra-long fire warning. ACS Nano 16:20865–20876
Li WS, Yin ZT, Qi LY, Yu B, Xing WY (2023) Scalable production of bioinspired MXene/black phosphorene nanocoatings for hydrophobic and fire-safe textiles with tunable electromagnetic interference and exceeding thermal management. Chem Eng J 460:141870
Liu C, Li P, Xu YJ, Liu Y, Zhu P (2022) Nickel alginate-enhanced fire safety of aluminum diethylphosphinate on epoxy resin. J Appl Polym Sci 140:e53552
Yotkuna K, Chollakup R, Imboon T, Kannan V, Thongmee S (2021) Effect of flame retardant on the physical and mechanical properties of natural rubber and sugarcane bagasse composites. J Polym Res 28:1–13
Zhao P, Zeng W, Yang Z, Yang Y, Li J, Shi J, Chen D (2021) Preparation of a novel functionalized magnesium-based curing agent as an intrinsic flame retardant for epoxy resin. Chemosphere 273:129658
Acknowledgements
This work received financial support from the General Project Fund of the Liaoning Education Department (No. LJGD2019014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, Z., Wang, S., Ge, J. et al. Flame-retardant epoxy resin: synergistic effect between aluminum diethylphosphinate and piperazine pyrophosphate. Iran Polym J 33, 119–129 (2024). https://doi.org/10.1007/s13726-023-01238-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-023-01238-w