Abstract
This paper presents hydrothermal decomposition of cellulosic polysaccharides in corncob using SnO2-Co3O4/C biochar catalyst to nine soluble monosaccharides including xylose, mannose, galactose, glucose, xylulose, arabinose, fructose, maltose and sucrose. H3O+ can convert hemicellulose and cellulose to soluble sugars by saccharification, following the Lewis acid sites from SnO2-Co3O4-2/C catalyst make the isomerization of xylose to xylulose or arabinose, as well as glucose to fructose. The xylulose and fructose would be furthermore dehydrated to small compounds due to the acid strength of catalyst. Maltose and sucrose could be produced by the condensation of monosaccharides with prolonged reaction time. The maximum yield of reducing sugars reached 83.3% under the optimized operation condition as 5 g dried corncob particles, 0.3 g SnO2-Co3O4/C biochar catalyst and 100 mL H2O charged in the reactor at 180 °C for duration of 170 min.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass is one of the most abundant natural sources of carbon resources for the production of a wide range of chemicals with various applications [1,2,3,4]. Biomass (lignocellulosic) has a complex structure consists of cellulose, hemicelluloses and lignin. Agricultural lignocellulose is the most abundant and less expensive type of biomass on earth, therefore, being a promising feedstock for the production of renewable energy, especially biofuels, and chemicals [5]. Corncob is a by-product of the corn process industry that is traditionally used as livestock feed, firewood substitute, or rotted in the farmland. The major compositions of the corncob are about 38 wt% hemicelluloses, 35 wt% cellulose and 20 wt% lignin [6]. Hemicellulose is heteropolysaccharide composed of different polysaccharides, such as mannans, xylans, arabinans, galactans and glucomannan. Cellulose is a homopolysaccharide composed of glucose units linked to each other through β-1-4-glucosidic bonds [7]. The biomass-derived starting materials have attracted much attention due to their convenient production from renewable sources [8,9,10,11]. Furfural, derived from hemicellulose, is a key platform compound which can be widely converted to a variety of chemicals and biofuels [12,13,14,15]. Hydrogenation is one of the potential routes for furfural conversion [16,17,18,19,20,21]. However, the furfural is produced not only by fructose conversion.
Lignocellulosic biomass materials are usually divided into monosaccharides, disaccharides, oligosaccharides and polysaccharides, wherein monosaccharides and disaccharides are commonly referred to sugars [22]. The past few decades have witnessed significant researches and development activities using different methods for hydrolysis and conversion hemicellulose and cellulose, such as mineral acids, bases and enzymes [23, 24]. Liquid acid-catalyzed hydrolysis of cellulose is efficient. However, corrosion, waste disposal and solvent recycle make this method unattractive.
Some attempts have been made on the hydrolysis of cellulose in ionic liquids since cellulose has good solubility in chloride and acetate anion ionic liquids. Zhou [25] reported that cellulose was degraded by WCl6 in 1-butyl-3-methyl imidazole chloride at 80 °C and lower, and 83% and 85.5% yield of total reducing sugar was obtained at 70 and 80 °C, respectively. Recently, sugarcane bagasse was pretreated with alkali and enzymatic delignification to be obtained de-lignified bagasse and then produced ethanol by saccharification and fermentation [26]. Daniel described the different methods of producing xylose, mannose, and arabinose sugars from hemicellulosic oligosaccharides of biomass by enzymatic and thermochemical pretreatments, and demonstrated that the oligosaccharides, such as xylooligosaccharides, arabinooligosaccharides and mannooligosaccharides have great potential from agricultural crop residues [27]. Ruppert had obtained the monosaccharide and disaccharides from carbohydrates by hydrogenolysis and hydrogenation [28]. In addition, some researcher works indicated that the contents of xylose and arabinose could be increased to 70% and 18%, respectively, by ionic liquid pretreatment to change the structure of hemicellulose from corncob [29]. Moreover, Liu demonstrated a novel and facile approach of conversion monosaccharides (glucose and xylose) to oligosaccharides [cello-oligosaccharides (COS) and xylo-oligosaccharides (XOS)], the yields of COS and XOS reached 4.62% (38 s) and 47.09% (30 s), respectively, at 500 °C reaction temperature coupled with sharp-quenching method [30]. Although these methods are improvements over the use of mineral acids alone, they are limited by the nature of the catalyst and its activity at the given conditions. Carbon materials derived from activated carbon, sugar or cellulose are solid acid catalysts that have the potential to eliminate many of the problem associated with solvent recycle and separation and can be made from renewable resources [31, 32].
In previous studies, we mainly investigated the process of furfural generation from cellulose. However, it was found that the furfural production process was not only through fructose. In this work, it is to produce soluble sugars by corncob hydrothermal degradation in the present of SnO2-Co3O4/C biochar catalyst, which was prepared by sugar solution and lignocelluloses residue from corncob degradation, as well as mixture precipitated Sn(OH)4 and Co(OH)2. There are few reports that nine kinds of sugars are obtained at the same time. It was included saccharification of hemicellulose and cellulose of corncob which produces a series of saccharides, and isomerization of xylose to xylulose or arabinose, as well as glucose to fructose. Meanwhile, it also found that there were condensation reactions of monosaccharide to disaccharide, and partly dehydration of monosaccharides to furfural (FF) or 5-hydroxymethylfurfural (HMF) along with the saccharification of corncob over SnO2-Co3O4/C biochar catalyst.
Experimental
Material
Corncob was supplied from a local farm located in Hebei Province, China. Corncob was firstly chopped into small pieces and dried at 60 °C under vacuum for 24 h. Then, the dried corncob particles were sieved through 20 and 80 meshes to collect particles sized between 0.9 and 0.2 mm for experiments. Xylose, xylulose, arabinose, mannose, galactose, glucose, fructose, maltose and sucrose were supplied from Bioreagent Company, Shanghai, China. The chemicals and organic solvents used in experiments were all of the analytical grades and purchased from Tianjin Kermel Chemical Reagents Co. Ltd, China. Distilled water was used in the preparation of all solutions.
Preparation of SnO2-Co3O4/C biochar catalyst
The amounts of 1.744 g SnCl4·5H2O and 0.6 g CoCl2·6H2O as the metal raw materials were used to prepare sol–gel Sn(OH)4 and Co(OH)2 mixture hydroxide. The sol–gel mixture hydroxide was directly added to 1.5 g lignocellulose residues and 150 mL degradation solution (containing 20.7 g soluble reducing sugars) and then concentrated to paste by rotary evaporation at 45 °C under 0.09 MPa absolute pressure. The paste carbonization was carried out in a tube-carbide furnace at 200 °C for 48 h with a nitrogen gas atmosphere. After cooling down to the ambient temperature, the carbonized solid mixture was ground into powder (60–80 meshes) to give the SnO2-Co3O4/C biochar catalyst. With a similar procedure, a group of biochar catalysts such as SnO2/C, Co3O4/C, SnO2-SiO2/C, SnO2-Al2O3/C and SnO2-TiO2/C were also prepared and employed to make comparison. In this work, there was no need to consider the recovery of biochar catalysts because they were utilized from lignocellulose residues to make new biochar catalyst in next cycle.
Determination of acid density of SnO2-Co3O4/C biochar catalyst
The acid density of SnO2-Co3O4/C biochar catalyst was estimated by neutralized titration method [33] as the following steps: 0.2 g of SnO2-Co3O4/C biochar catalyst was added to 40 mL of 2 mol/L NaCl solution and stirred at room temperature for 24 h so that Na+ and H+ equilibrium on the catalyst surface was changed. After separation of the catalyst by filtration, the filtrate was titrated with 10 mmol/L NaOH solution. The acid density of SnO2-Co3O4/C biochar catalyst was calculated using the following formulas, and the equivalent concentration of sulphuric acid employed in corncob hydrolysis was also determined by stoichiometric proportion according to the acid density of SnO2-Co3O4/C biochar catalyst.
where C is the concentration of NaOH solution, V is the volume of using NaOH solution, M is the relative molecular mass of sulphuric acid and m is the quality of SnO2-Co3O4/C biochar catalyst.
Catalytic hydrolysis of corncob
The catalytic hydrolysis of corncob was performed in a stainless steel batch autoclave equipped with a liner of polytetrafluoroethylene. As a typical run, 5 g dried corncob particles, 0.1–0.4 g SnO2-Co3O4/C biochar catalyst and 80–140 mL water were charged in the reactor, fasten the cover of autoclave and the mixture was then heated at 160–200 °C with a duration of 160–200 min in the oven. After the hydrolysis reactions, the mixture of corncob degradation solution and unreacted solid residue were separated by filtration. In this study, the effect of different operation conditions (time, temperature, catalyst dosage, the ratio of corncob to water) on the corncob hydrolysis to soluble sugars was investigated intensively.
Product analysis and corncob conversion ratio
Based on the calibration curve, FF and 5-HMF concentration in the products of corncob degradation was determined quantitatively using a high-performance liquid chromatography (HPLC) instrument (LC-20AD, Shimadzu, Japan) equipped with a SPD-M20AV UV detector and an Inertsil ODS-EP C18 reversed-phase column (4.6 × 250 mm) at 40 °C column temperature. In the measurement, a mixture of water and methanol (77:23, v/v) was used as the mobile phase with a flow rate of 1.0 mL/min and injecting sample solution of 20 μL in volume.
The total reducing sugar was measured using 3,5-dinitrosal-icylic acid (DNS) method following procedures previously reported in the literature [34]. The analysis of compositions in the reducing sugar was performed using an HPLC instrument (LC-20AD, Shimadzu, Japan) equipped with an evaporative light scattering detector (ELSD) (Alltech LC-2000ES) and an XBridge BEH Amide Column (4.6 mm × 250 mm). The chromatograms of standard sugars mixture were also shown as reference. The pre-set chromatographic conditions are listed in Table 2.
The yields of products and the corncob conversion ratio were calculated based on the following equations:
Results and discussion
Characterization of biochar catalyst
The determination of type (Brønsted or Lewis) of acid sites of the catalysts was performed by Fourier transform infrared (FTIR) spectroscopy combined with in situ adsorption of pyridine. The absorption bands appearing at 1545 and 1455 cm−1 in the IR difference spectra were acceptedly assigned to adsorbed pyridinium ions and pyridine coordinated to Lewis acid sites, respectively [35]. The peak at 1457 cm−1 was considered to relate to the characteristic vibrations of Lewis acid sites, as shown in Fig. 1. After SnO2 and Co3O4 loading on biochar carrier, the acid site of SnO2-Co3O4/C biochar catalyst was changed from Brønsted acid to Lewis.
To identify the elemental compositions and chemical states of the product, X-ray photoelectron spectroscopy (XPS) analysis was carried out for SnO2-Co3O4-2/C catalyst. Figure 2 shows the survey spectrum of Co, Sn and O elements in the region of 0–1000 eV. It was certain that the content of C and O was about 94%, and the remaining content was made up with N, Sn, Co and few Si. The SnO2 species were the main Sn species on the surfaces of the bimetallic SnO2-Co3O4-2/C catalyst, and the Co3O4 was also existed.
Figure 3 shows the X-ray diffraction (XRD) patterns of SnO2-Co3O4-2/C catalyst. The diffraction peaks 2θ at 31°–36° correspond to crystal planes of Co3O4 and SnO2. In addition, the diffraction peaks 2θ at 21.5°, 23.5°, 35.2°, 45.1°, 53.3°, 61.4°, 68.7° in the SnO2-Co3O4-2/C catalyst can be indexed as carbon carrier. It indicates that there are more SnO2 and Co3O4 particles attached to the biomass carrier.
Corncob saccharification
Lignocellulose could be hydrolyzed under acid conditions and produce arabinose, xylose, glucose and other compounds [36]. In the degradation solution of corncob hydrolysis over SnO2-Co3O4/C biochar catalyst with Lewis acid circumstance in water medium, nine sugar components were identified, as shown in Fig. 4. The chromatograms of nine standard sugars mixture were also shown as reference. There were also other unknown sugars from corncob hydrolysis.
In this work, the degradation solution contained about 83.3% reducing sugars was formed from the saccharification of hemicellulose and cellulose in corncob. The experimental data in Fig. 4 indicate that the glucose and xylose were the main products among monosaccharides, their contents were up to 34.1%. Because xylose could further be converted to xylulose or FF under the Lewis acid function [37], the content of glucose remained in degradation solution higher than that of xylose. It was demonstrated that the SnO2-Co3O4/C biochar catalyst was more suitable for cellulose and hemicellulose hydrolysis. In addition, the disaccharide was also synthesized from the corresponding monosaccharide by condensation reaction.
Optimized conditions for corncob saccharification with SnO2-Co3O4/C biochar catalyst
It has reported that acid strength has a significantly influence on corncob saccharification [38]. The aldose could be isomerized to ketose by Lewis acidity, and some metal oxides containing Sn (IV) were also regarded as a strong Lewis acid due to the availability of Lewis acid sites [39]. The effect of different biochar catalysts on the sugars yields is illustrated in Fig. 5I. The content of reducing sugars was the highest with 83.3% yield. The yields of monosaccharides, such as xylose, glucose, mannose and galactose were 11.9%, 22.2%, 3.29% and 3.01%, respectively, when 0.3 g SnO2-Co3O4/C biochar catalyst was added in the reactor, compared with single SnO2/C and Co3O4/C catalyst. The BET surface area of SnO2-Co3O4-2/C catalyst was 6.338 m2/g and its acid density was 0.538 mmol/g. The SnO2 played an important role in supplying Lewis acid sites on the surface of SnO2-Co3O4-2/C catalyst. There was a synergetic action between SnO2 and Co3O4 for corncob hydrolysis. The elemental contents of Sn and Co in the SnO2-Co3O4-2/C catalyst were 391.43 and 90.24 μg/g by AAS analysis, respectively. However, the catalytic performance of SnO2-SiO2/C, SnO2-Al2O3/C and SnO2-TiO2/C catalysts was not better than that of SnO2/C. Perhaps they were more suited to generate terminal products than saccharification process to give monosaccharides, such as FF, HMF, lactic acid, levulinic, ethanol, etc. [40, 41].
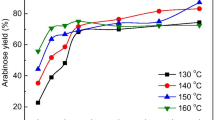
Figure 5II shows the yields of sugars which are dramatically increased with the reaction temperature from 160 to 180 °C. However, the enhancement of xylulose, arabinose and fructose yields were not high as compared with the reducing sugars. This was just according to a report by Vinit Chuhdhary who claimed that aldose isomerization to ketosis does not require much energy. It was proved that the corncob saccharification needed higher reaction temperature than isomerization [42].
The amount of catalyst has mainly affected the acidity of catalyst in the reaction of corncob saccharification. As shown in Fig. 5III, when the dosage of catalyst was 0.3 g, the catalytic effect was better and the yield of reducing sugar was up to 83.3%. The yield of monosaccharide increased with the amount of catalyst in the range of 0–0.3 g. The increased yield of xylose, arabinose and fructose is due to the isomerization of xylose and glucose under the SnO2-Co3O4/C catalyst. In addition, with the increase in the amount of catalyst, the yield of soluble sugar decreased, mainly because the increase of acidity converted the cellulose and semi-fiber in the corncob to other small molecular compounds.
The principle of cellulose hydrolysis could be described as the chemical formulation: (C6H10H5)n + n H2O → n (C6H12O6). It can be clearly seen in Fig. 5IV that the water volume and suitable ratio of corncob to water have a great effect on the corncob saccharification. When the amount of water was less than 16 mL per 1 g of corncob, the corncob saccharification was insufficient and the sugars yields were lower, even no mannose and galactose produced. Based on the hydronium ion H3O+ from overstock water and hot temperature [43], the production of xylose and glucose decreased due to the isomerization to xylulose and fructose, respectively, and then FF and HMF were generated by dehydration.
Accompanied by the saccharification of corncob over SnO2-Co3O4/C biochar catalyst at temperature 180 °C for the duration of 160–200 min, it was also found that there were isomerization, condensation and dehydration simultaneously. It can be seen that in Table 1 the yields of xylulose, arabinose and fructose were lower when 4.5 wt% H2SO4 was employed as Brønsted acid catalyst, which had almost equivalent acidity to the SnO2-Co3O4/C biochar catalyst (0.542 mmol/g). Beyond the corrosive action to instrument, the H2SO4 could make the dehydration of xylose to FF and glucose to HMF directly. There was no isomerization reaction observed during corncob saccharification with H2SO4 catalyst.
When corncob saccharification was performed under the optimum operation condition as 5 g corncob, 0.2 g SnO2-Co3O4/C biochar catalyst and 120 mL H2O at 180 °C for 170 min duration, the highest yields of xylose, mannose, galatose, glucose were 13.9%, 3.65%, 3.78%, 25.2%, respectively. The optimized condition for monosaccharides isomerization could give to xylulose (5.89%), arabinose (3.42%) and fructose (4.25%). The condensation reaction proceeded with the reaction time prolonged to 190 min with 0.3 g SnO2-Co3O4/C biochar catalyst and 100 mL H2O at 180 °C, and the yield of maltose and sucrose reached 3.23% and 2.89%, respectively.
Saccharification and isomerization of corncob with SnO2-Co3O4/C biochar catalyst
The hemicellulose in corncob is a typical heterogeneous polymer constructed with pentose and hexose sugars [44], such as xylose, arabinose, glucose, galactose and mannose, etc. Cellulose is considered to be a major source of glucose, so it is readily available and renewable. Therefore, the saccharification was taken as a depolymerization of hemicellulose and cellulose to sugars by acidic hydrolysis. It is an interesting process especially for the production of rare sugars which are high value-added compounds in the future biorefinery. Daizo had employed carbon-based solid acid catalyst to catalytic conversion of starch to glucose and obtained the glucose yield of 77.54% [45]. Although there were only 13.9% xylose and 25.2% glucose by corncob saccharification with SnO2-Co3O4/C biochar catalyst in our experiment, the final nine monosaccharides, as the special bio-energy resources, were made from cellulose and hemicellulose in non-food crop.
Typical simplified saccharification and isomerization reaction schemes of hemicellulose and cellulose are shown in Fig. 6a, b, respectively. The hemicellulose in corncob was saccharified to xylose by hydronium ion H3O+ [40] under hydrothermal condition at temperature 180 °C. Then, Lewis acid sites on the surface of SnO2-Co3O4-2/C catalyst facilitated the isomerization of xylose to xylulose that is an interconversion reaction with aldose to ketose of pentose sugars [46]. Because the aldose-ketose isomerization is a reversible reaction, the distribution of products was kept up with chemical equilibrium. For increasing the selectivity and yield of a monosaccharide, suitable reaction condition for saccharification and isomerization of corncob with SnO2-Co3O4/C biochar catalyst is indispensable. As Table 2 shows, when 5 g corncob, 0.2 g SnO2-Co3O4/C biochar catalyst and 120 mL H2O were charged in the reactor and the corncob was hydrolyzed at 180 °C for 170 min duration, the total yield of monosaccharides was up to 49.77% and disaccharides was only 2.5%.
Condensation and dehydration of monosaccharide with SnO2-Co3O4/C biochar catalyst
Xylose and glucose, as the raw material of biomass-based chemicals, were correspondingly converted to FF and HMF under acidity circumstance by dehydration [47]. The suggested pathway of condensation and dehydration of xylose and glucose, which were the products of corncob degradation by hydrothermal process with SnO2-Co3O4/C biochar catalyst, is illustrated in Fig. 7. The hydronium ion H3O+ from hot water provides weak acidity, so the yields of FF and HMF are improved with the increasing volume of water. As shown in Table 2, the highest yields of FF and HMF have reached 30.1% and 16.0% with 5 g corncob, 0.3 g SnO2-Co3O4/C biochar catalyst and 120 mL water at 180 °C for 200 min duration. Meanwhile, two molecules of glucose could couple to maltose by condensation reaction under acidic catalysis, like glucose and fructose coupling to sucrose. That was the reason why the content of sucrose and maltose increased with the longer reaction time. When 5 g corncob, 0.3 g SnO2-Co3O4/C biochar catalyst and 100 mL H2O were charged in the reactor and the corncob was hydrolyzed at 180 °C for 190 min duration, the total yield of maltose reached 3.23% and sucrose was 2.89%.
The validation experiment for preparation of furfural, as a comparison with H2SO4 catalyst, was performed, and the yield of furfural was only 6.8% with the same content of SnO2-Co3O4/C biochar catalyst. The experimental results indicated that the SnO2-Co3O4/C biochar catalyst was more suitable for saccharification of hemicellulose and cellulose, as well as for isomerization of xylose to xylulose, rather than dehydration of xylulose.
Conclusion
This study investigated a hydrothermal process for efficient conversion of corncob into reducing sugars (as much as 3.04 g/5 g corncob) using SnO2-Co3O4/C biochar catalyst. After optimizing the hydrolysis parameters at 180 °C for 170 min, the total yield of monosaccharides was up to 49.77% and a maximum level of 13.9% xylose, 3.65% mannose, 3.78% galatose and 25.2% glucose were produced. There were also xylulose, arabinose and fructose in products as isomerization reaction of aldose to ketose during corncob saccharification with SnO2-Co3O4/C biochar catalyst. The advantage of this process was hydrolysis of cellulose and hemicellulose in corncob to soluble sugars without conflicting food crop (cornstarch), and the degradation solution containing monosaccharides had no acidic properties and did not corrode the instrument.
References
Wang G, Yao R, Xin H, Guan Y, Wu P, Li X (2018) At room temperature in water: efficient hydrogenation of furfural to furfuryl alcohol with a Pt/SiC-C catalyst. RSC Adv 8:37243–37253
Singh SK (2018) Heterogeneous bimetallic catalysts for upgrading biomass-derived furans. Asian J Org Chem 7:1901–1923
Guo H, Zhang H, Zhang L, Wang C, Peng F, Huang Q, Xiong L, Huang C, Ouyang X, Chen X, Qiu X (2018) Selective hydrogenation of furfural to furfuryl alcohol over acid-activated attapulgite-supported NiCoB amorphous alloy catalyst. Ind Eng Chem Res 57:498–511
Sadjadi S, Farzaneh V, Shirvani S, Ghashghaee M (2017) Preparation of Cu-MgO catalysts with different copper precursors and precipitating agents for the vapor-phase hydrogenation of furfural. Korean J Chem Eng 34:692–700
Ribeiro LS, de Melo Órfão JJ, Pereira MFR (2017) Direct catalytic production of sorbitol from waste cellulosic materials. Bioresour Technol 232:152–158
Liu Q, Yang F, Sun X, Liu Z, Li G (2017) Preparation of biochar catalyst with saccharide and lignocellulose residues of corncob degradation for corncob hydrolysis into furfural. J Mater Cycles Waste 19:134–143
Kannam SK, Oehme DP, Doblin MS, Gidley MJ, Bacic A, Downton MT (2017) Hydrogen bonds and twist in cellulose microfibrils. Carbohydr Polym 175:433–439
Liu CG, Xiao Y, Xia XX, Zhao XQ, Peng L, Srinophakun P, Bai FW (2019) Cellulosic ethanol production: progress, challenges and strategies for solutions. Biotechnol Adv 37:491–504
Shirvani S, Ghashghaee M, Farzaneh V, Sadjadi S (2018) Influence of catalyst additives on vapor-phase hydrogenation of furfural to furfuryl alcohol on impregnated copper/magnesia. Biomass Conv Biorefin 8:79–86
Ghashghaee M, Shirvani S, Ghambarian M (2017) Kinetic models for hydroconversion of furfural over the ecofriendly Cu-MgO catalyst: an experimental and theoretical study. Appl Catal A-Gen 545:134–147
Ghashghaee M, Sadjadi S, Shirvani S, Farzaneh V (2017) A Novel consecutive approach for the preparation of Cu-MgO catalysts with high activity for hydrogenation of furfural to furfuryl alcohol. Catal Lett 147:318–327
Guo H, Zhang H, Tang W, Wang C, Chen P, Chen X, Ouyan X (2017) Furfural hydrogenation over amorphous alloy catalysts prepared by different reducing agents. BioResources 12:8755–8774
Gilkey MJ, Xu B (2016) Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal 6:1420–1436
Gong W, Chen C, Zhang H, Wang G, Zhao H (2018) Highly dispersed Co and Ni nanoparticles encapsulated in N-doped carbon nanotubes as efficient catalysts for the reduction of unsaturated oxygen compounds in aqueous phase. Catal Sci Technol 8:5506–5514
Ghashghaee M, Shirvani S, Farzaneh V (2017) Effect of promoter on selective hydrogenation of furfural over Cu-Cr/TiO2 catalyst. Russ J Appl Chem 90:304–309
Yang Z, Huang YB, Guo QX, Fu Y (2013) RANEY® Ni catalyzed transfer hydrogenation of levulinate esters to γ-valerolactone at room temperature. Chem Commun 49:5328–5330
Shumeiko B, Schlackl K, Kubička D (2019) Hydrogenation of bio-oil model compounds over Raney-Ni at ambient pressure. Catalysts 9:268
Gundekari S, Srinivasan K (2019) Screening of solvents, hydrogen source, and investigation of reaction mechanism for the hydrocyclisation of levulinic acid to γ-valerolactone using Ni/SiO2–Al2O3 catalyst. Catal Lett 149:215–227
Ghashghaee M, Shirvani S, Farzaneh V, Sadjadi S (2018) Hydrotalcite-impregnated copper and chromium-doped copper as novel and efficient catalysts for vapor-phase hydrogenation of furfural: effect of clay pretreatment. Braz J Chem Eng 35:669–678
Banik BK, Barakat KJ, Wagle DR, Manhas MS, Bose AK (1999) Microwave-assisted rapid and simplified hydrogenation. J Org Chem 64:5746–5753
Villaverde MM, Garetto TF, Marchi AJ (2015) Liquid-phase transfer hydrogenation of furfural to furfuryl alcohol on Cu-Mg-Al catalysts. Catal Commun 58:6–10
Dunning JW, Lathrop EC (1945) The saccharification of agricultural residues. Ind Eng Chem Res 37:24–29
Chen H, Liu J, Chang X, Chen D, Xue Y, Liu P, Li H, Han S (2017) A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process Technol 160:196–206
Varma AJ, Kulkarni MP (2002) Oxidation of cellulose under controlled conditions. Polym Degrad Stabil 77:25–27
Zhou L, Zhang S, Li Z, Zhang Z, Liu R, Yun J (2019) WCl6 catalyzed cellulose degradation at 80 °C and lower in [BMIM]Cl. Carbohydr Polym 212:289–296
Asgher M, Ahmad Z, Ipbal HMN (2013) Alkali and enzymatic delignification of sugarcane bagasse to expose cellulose polymers for saccharification and bio-enthanol production. Ind Crop Prod 44:488–495
Daniel O, Ahring BK (2012) The potential for oligosaccharide production from the hemicellulose fraction of biomasses through pretreatment processes: xylooligosaccharides (XOS), arabino-oligosaccharides (AOS), and mannooligosaccharides (MOS). Carbohydr Res 360:84–92
Ruppert AM, Weinber K, Palkoits R (2012) Hydrogenolysis goes bio: from carbohydrates and sugar alcohols to platform chemicals. Angew Chem Int Edit 51:2564–2601
Sun SN, Li MF, Yuan TQ, Xu F, Sun RC (2012) Effect of ionic liquid pretreatment on the structure of hemicelluloses from corncob. J Agric Food Chem 60:11120–11127
Liu X, Wei W, Wu S, Lei M, Liu Y (2018) A promptly approach from monosaccharides of biomass to oligosaccharides via sharp-quenching thermo conversion (SQTC). Carbohydr Polym 189:204–209
Liu Q, Yang F, Liu Z, Li G (2015) Preparation of SnO2-Co3O4/C biochar catalyst as a Lewis acid for corncob hydrolysis into furfural in water medium. J Ind Eng Chem 26:46–54
Cao X, Sun S, Sun R (2017) Application of biochar-based catalysts in biomass upgrading: a review. RSC Adv 7:48793–48805
Ormsby R, Kastner JR, Miller J (2012) Hemicellulose hydrolysis using solid acid catalysts generated from biochar. Catal Today 190:89–97
Liu X, Ai N, Zhang H, Lu M, Ji D, Yu F (2012) Quantification of glucose, xylose, arabinose, furfural, and HMF in corncob hydrolysate by HPLC-PDA-ELSD. Carbohydr Res 353:111–114
Marianou AA, Michailof CM, Pineda A, Iliopouloua EF, Triantafyllidis KS, Lappas AA (2018) Effect of Lewis and Brønsted acidity on glucose conversion to 5-HMF and lactic acid in aqueous and organic media. Appl Catal A Gen 555:75–87
Yamaguchi D, Hara M (2010) Starch sacchsrification by carbon-based solid acid catalyst. Solid State Sci 12:1018–1023
Román-Leshkov Y, Davis ME (2011) Activation of carbonyl-containing molecules with solid Lewis acid in aqueous media. ACS Catal 1:1566–1580
Jiang N, Qi W, Huang R, Wang M, Su R, He Z (2013) Production enhancement of 5-hydroxymethyl furfural from fructose via mechanical stirring control and high-fructose solution addition. J Chem Technol Biotechnol 89:56–64
Cao X, Peng X, Sun S, Zhong L, Chen W, Wang S (2015) Hydrothermal conversion of xylose, glucose, and cellulose under the catalysis of transition metal sulfates. Carbohydr Polym 118:44–51
Li S, Deng W, Li Y, Zhang Q, Wang Y (2019) Catalytic conversion of cellulose-based biomass and glycerol to lactic acid. J Energy Chem 32:138–151
Choudhary V, Caratzoulas S, Vlachos DG (2013) Insights into the isomerization of xylose to xylulose and lyxose by a Lewis acid catalyst. Carbohydr Res 368:89–95
Maki-Arvela P, Salmi T, Holmbom B, Willfor S, Murzin DY (2011) Synthesis of sugars by hydrolysis of hemicelluloses—a review. Chem Rev 111:5638–5666
Li B, Relue P, Varanasi S (2012) Simultaneous isomerization and reactive extraction of biomass sugars for high yield production of ketose sugars. Green Chem 14:2436–2444
Yamaguchi D, Hara M (2010) Starch saccharification by carbon-based solid acid catalyst. Solid State Sci 12:1018–1023
Dong H, Nimlos MR, Himmel ME, Johnson DK (2009) The effect of water on β-d-xylose condensation reaction. J Phys Chem A 113:8577–8585
Lamminpää K, Ahola J, Tanskanen J (2012) Kinetic of xylose dehydration into furfural in formic acid. Ind Eng Chem Res 51:6297–6303
Rinaldi R, Palkovits R, Schuth F (2008) Depolymerization of cellulose using solid catalysts in ionic liquids. Angew Chem Int Ed 47:8047–8050
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21576067).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Q., Fan, H., Qi, J. et al. Catalytic hydrolysis of corncob cellulosic polysaccharide into saccharides using SnO2-Co3O4/C biochar catalyst. Iran Polym J 29, 383–392 (2020). https://doi.org/10.1007/s13726-020-00805-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-020-00805-9