Abstract
A new class of thermoplastic polyamide elastomers was successfully synthesized by polyaddition reactions between amino-terminated polyamide-6 and different diisocyanate-terminated polyether diols or polyester diols in a Haake torque rheometer. The chemical structure, phase separation, static and dynamic mechanical properties, thermal stability and hydrophilicity of TPAEs were characterized by Fourier transform infrared spectroscopy, differential scanning calorimeter (DSC), tensile tests and dynamic mechanical analysis (DMA), thermogravimetric analysis (TGA), and water contact angle test methods, in the order given. DSC results showed that these synthesized TPAEs exhibited two obvious endothermic peaks associated with melting transition of soft segments and hard segments. The mechanical results revealed that TPAEs possess outstanding mechanical properties with tensile strength of 22.2–35.9 MPa and the elongation-at-break of 360–467 %. TGA results showed TPAEs can be fabricated by traditional thermoplastic processing method without any decomposition once the processing temperature is below 300 °C. Furthermore, the TPAEs only dissolved in acetic acid and methanoic acid at room temperature, showing TPAEs can be applied to some harsh environment due to excellent organic solvent resistance. Especially, this friendly reactive processing can also be used to handle the environmental problems associated with volatile organic compound emission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermoplastic elastomers (TPEs) are segment copolymers prepared by copolymerization or blending [1]. TPEs are composed of rubber phase (soft segments) and plastic phase (hard segments), which are essential features of all TPEs. The hard phase with either crystalline or glassy state imparts good plasticity to the material, while the soft segments impart outstanding elasticity to the materials. TPEs show excellent elastomeric behavior within their service temperature. These segment copolymers can be used by thermoplastic processing at elevated temperatures, which arouse more interest due to significant commercial interest. In the past 30 years, many kinds of TPEs, such as thermoplastic polyurethane elastomers (TPU) [2, 3], poly(styrene-b-butadiene-b-styrene) (SBS) [4], thermoplastic polyolefin elastomers (TPO) [5] and thermoplastic polyester elastomers (TPE) [6] have been synthesized and used in production. Some of these materials were patented and commercialized in important industrial applications many years ago [7, 8].
Polyamide thermoplastic elastomers (TPAEs) based on polyamide are a new member of TPEs family and have been investigated for decades due to their favorable balance between processability and performance [9–11]. The TPAEs are segment copolymers consisting of hard segments based mainly on polyamide and soft segments based on polyether or polyester. Those remarkable advantages can be endowed with excellent processing and recycling [12]. Properties of TPAEs are affected by chemical nature and relative content of hard segments and soft segments. Therefore, it is possible to design a different segment structure for special application by altering the chemical compositions of hard and soft segments. In Castaldo’s [13] study, polyester and polyether with low glass transition temperatures (T g) were often used as soft blocks, which facilitated TPAEs’ low temperature performance; meanwhile, polyamide-6, polyamide-11 and polyamide-12 with high melting temperatures (T m) were often used as hard segments, which facilitated TPAEs’ high-temperature properties. Boulares et al. [14] and Biemond et al. [15] proved that the hydrogen bonds between amide groups of the hard segments acted as cross-linking points between TPAE chains, giving the material dimensional stability and solvent resistance. Fakirovet al. [16] and Yu et al. [17] designed a system for special application by altering the chemical compositions of hard and soft segments, combining the various hard segments with different soft segments to achieve great performance on mechanical properties, phase separation and crystallinity.
TPAEs are also well known as a class of high-performance materials with good elasticity and excellent thermal stability, widely applied in biomedicine, drug release [18], as antistatic [19], in spinning [20], and gas separation [21]. Hou et al. [22] and Mallakpour et al. [23] focused on introducing chains of polyimides with remarkable heat resistance and superior mechanical properties into PU backbones to prepare TPAEs. It was reported that TPAEs exhibited good persistent antistatic ability as permanent antistatic agent by polyaddition reaction at 260 °C, in which polyamide-6 acted as hard segments and polyether as soft segments [24, 25]. One of the most important applications of these segment copolymers is the fabrication of polymeric membranes for gas separation processes. Sridhar et al. [26] showed that TPAEs were found to be a promising choice to separate CO2 from CH4. So they prepared a composite film of poly(ether-b-amide) by solution casting and solvent evaporation on polyvinylidene fluoride (PVDF) ultra-porous substrate. Specially, it is reported that various methods are used to synthesize multi-block copolymers of TPAEs by polyaddition. Rached et al. [27] took a method to synthesize triblock copolymers through lauryl lactam and bifunctional macroinitiator at 200 °C by reactive extrusion processes. The characterization results reported that the thermal properties of triblock copolymers were strongly dependent upon their respective contents of soft and hard segments. Gaymans et al. [28, 29] concentrated on a class of poly(ether-esteramide) elastomers by transesterification of various aromatic diester–diamides and PTMG at 250 °C. The results illustrated phase separation during the polymerization and impeded the formation of high molecular weight polymers. To date, preparation of TPAEs based on polyamide-6, MDI and appropriate polyols, polyolefins, polyethers and polyesters by extrusion has been more acceptable to researchers [30–32]. However, all these mentioned synthetic routes must be performed under harsh condition (high temperatures, high vacuum and solution) with catalysts and initiators. The harsh reaction condition and the complicated process such as elimination of by-products (water and carbon dioxide) may limit the industrialization and application of TPAEs. Kong et al. [33] prepared a thermoplastic elastomer based on amino-terminated polyamide-6 and diisocyanate-terminated polytetramethylene glycol displaying excellent mechanical properties, in solvent at 140 °C.
In this paper, we successfully prepared TPAEs via polyaddition reactions between amino-terminated polyamide-6 and different diisocyanate-terminated polyether diols or polyester diols in a Haake torque rheometer. The friendly reactive processing without any solvent, catalyst and initiator has been rarely reported recently. The chemical structure, phase separation, static and dynamic mechanical properties, thermal stability and hydrophilicity of TPAEs were characterized by Fourier transform infrared spectroscopy (FTIR), differential scanning calorimeter (DSC), tensile tests and dynamic mechanical analysis (DMA), thermogravimetric analysis (TGA), and water contact angle test, accordingly. TPAEs can achieve high decomposition temperature, low glass transition temperature, and high elongation-at-break and tensile strength by polyaddition reactions using this method. In this respect, we report a detailed study of the synthesis of various TPAEs copolymer and the effects of soft segments on the properties of TPAEs.
Experimental
Materials
4,4′-Diphenylmethane diisocyanate (MDI), polyethylene glycol (PEG, M n 2000 g/mol), poly(butylene adipate) glycol (PBA, M n 2000 g/mol) and poly(tetramethylene ether) glycol (PTMG, M n 2000 g/mol) were supplied by Yantai Wanhua Polyurethane Co. (Shandong, China). Caprolactam and hexanediamine were purchased from Kelong Chemical Industry Incorporated Co. (Chengdou, China). De-ionized water was made in our lab. All reagents were analytically pure and used as received.
Preparation of amino-terminated polyamide-6 (OPA-6)
A stoichiometric ratio of caprolactam, hexanediamine and de-ionized water as catalyst was placed in a 500-mL three-neck flask equipped with a central mechanical stirrer, a thermometer and a nitrogen inlet. Deoxygenation was performed at room temperature by nitrogen bubbling for 30 min. Afterwards, the reaction was maintained for 30 min at 170 °C, and then conducted at 240 °C for 4 h. After the removal of the deionic water under vacuum at 240 °C, an OPA-6 was synthesized.
Preparation of diisocyanate-terminated polyether diol or polyester diol (OPUs)
A predetermined amount of MDI and diol, i.e., PTMG, PEG or PBA (molar ratio of MDI and diol = 2:1) was placed into a 500-mL three-neck flask equipped with a central mechanical stirrer, a thermometer and a reflux condenser. The reaction was carried out at 80 °C for 3 h, and then the OPU was obtained. The composition of OPUs is shown in Table 1. The synthesized OPUs by PTMG, PEG and PBA were named as OPU-1, OPU-2 and OPU-3, respectively.
Preparation of polyamide-6-based thermoplastic elastomers
A presetting amount of OPA-6 and OPU (molar ratio of MDI and OPU/1:1) was first mixed at 120 °C for 30 min to react efficiently. In the next step, the mixture was transferred and reacted in a Haake torque rheometer (Germany) at 180 °C for 10 min, and TPAE was obtained. Table 1 shows the composition of TPAEs, and the corresponding syntactic route is depicted in Scheme 1. These prepared TPAEs based on OPU-1, OPU-2 and OPU-3 were labeled as TPAE-1, TPAE-2 and TPAE-3, respectively.
Characterization
FTIR of the prepared TPAEs was carried out by a Nicolet FTIR (Nexus 670) spectrometer with a resolution of 4/cm. The scanning range was altered from 4000 to 400/cm.
Shore A hardness of the samples was obtained with a Shore hardness tester (LXAD, Shanghai, China) according to GB/T 2411-2008.
DSC was carried out on a DSC 204 Instruments apparatus (Netzsch, Germany) from −70 °C to 200 °C under nitrogen atmosphere at a heating rate of 10 °C/min. Samples of about 8.0 mg were needed for each testing.
DMA of the prepared TPAEs was performed on a TA Instrument Q800 (USA) equipped with liquid nitrogen cooling system from −100 to 50 °C at a frequency of 1 Hz and the heating rate of 3 °C/min. The typical dimensions of the specimens were 15 mm × 4 mm × 2 mm.
TGA of TPAEs was carried out in a TA Instrument SDT-Q600 thermal analyzer (USA) from 20 to 600 °C under a nitrogen atmosphere at a heating rate of 10 °C/min, and about 5.0 mg sample was taken in each testing.
The measurement of tensile strength and elongation-at-break of TPAEs was carried out with an Instron-4302 mechanical tester (USA) at a constant tensile rate of 200 mm/min. Each measurement was repeated at least five times.
Solvent resistance of TPAEs was carried out to estimate the resistance against dissolution and swelling in different solvents by the following procedures. Approximately, 1 g of the membrane specimen was soaked in different solvents for 48 h and each corresponding sample was observed.
Contact angle testing of TPAEs was measured to evaluate the hydrophilic nature of sample’s surface with a DAS25 contact angle analyzer (Kruss, Germany) using the sessile drop method. Briefly, a droplet of 1.5 μL of water was delivered onto a dry TPAE surface, and contact angle computation was performed using a DAS25 software assuming a circular profile of the droplet.
Results and discussion
FTIR of TPAEs
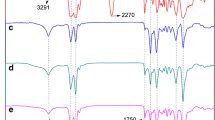
FTIR analysis was performed to verify the polyaddition reactions between OPA-6 and OPUs. In Fig. 1, it was noticed that the FTIR curve of TPAE-1 was similar to the characteristic absorption peaks of TPAE-2 and TPAE-3. As can be seen from Fig. 1, the characteristic absorption bands of TPAE-1 at 2971 and 2831/cm are attributed to the asymmetric and symmetric stretching vibrations of the –CH2– group and the corresponding bending vibrations appear at 1250 and 690/cm in TPAE-1. The characteristic absorption peaks at 1500–1750/cm are due to –CO–NH– stretching and bending vibrations of polyamide-6 segments, which display three infrared absorptions including the characteristic absorption peak of C=O (Amide I band), the bending vibration absorption peak of –NH– (Amide II band) and the stretching vibration of –C–N– (Amide III band) at the absorption bands. The C=O and –C–O– respective stretching vibrations of carboxyl group appear at 1726 and 1125/cm originating from carbamate of soft segments. The emerging two characteristic absorption peaks at 3500–3300/cm belong to –NH2 group. However, the characteristic peak of –N=C=O group at 2260/cm and the characteristic peaks of –NH2 at 3370 and 3292/cm are not detected in spectral curve of TPAE-1. It illustrates that the –NH2 group and –N=C=O group have totally reacted. Based on the above FTIR analysis, it can be included that TPAEs are successfully synthesized by reactions of OPA-6 and OPUs.
Phase separation of TPAEs
Figure 2 shows the DSC curves of TPAE-1, TPAE-2 and TPAE-3 and some data are shown in Table 2. DSC gives information on the influence of soft segments and hard segments on the crystallization of TPAEs. As can be seen from Fig. 2 and Table 2, the temperature of the first endothermic peak for TPAE-1, TPAE-2, and TPAE-3 is at around 10, 28, and 45 °C, in the order given. The temperature of the second endothermic peak for these TPAEs is around 165 °C. For TPAE-1, the curve has an apparently first endothermic peak connected to the melting transition of soft segments around 10 °C; while for TPAE-2 and TPAE-3, the endothermic peak has shifted toward high temperature. Especially, the melting transition of TPAE-3 appears approximately at 50 °C. The soft segments of PTMG, PEG and PBA with molecular weight of 2000 g/mol are known to crystallize at room temperature [15, 34]. This phenomenon is attributed to scarcely destroyed crystal structure of soft segments because of linking with polyamide-6 segments as hard segments. Owing to strong hydrogen bonding interaction between ester groups, it is easy to induce crystallization in PBA, which improves the melting transition of soft segments [35, 36]. Therefore, TPAE-3 has high melting transition of soft segments, while TPAE-1 and TPAE-2 have lower melting transition of soft segments. The curve of TPAE-1 also has an apparently endothermic peak at 165 °C, which is attributed to the melting of micro-crystalline hard segments. However, the TPAE-2 and TPAE-3 have no obvious endothermic peak assigned to the melting transition of hard segments. This result could be ascribed to the presence of small and imperfect crystallites of polyamide-6 segments in TPAE-2 and TPAE-3. On the other hand, there is a wide range of temperature between two endothermic peaks of TPAE-1, and both melting transitions of soft segments and hard segments approach the neat melting transition, which can clearly illustrate micro-phase separation between the two phases [37, 38].
DMA is strongly sensitive to the morphology and structure of TPAEs. The dependence of tanδon temperature for TPAEs is shown in Fig. 3. The tanδcurve of TPAEs exhibits the maximum peak at −58, −46 and −32 °C for TPAE-1, TPAE-2 and TPAE-3, respectively. The glass temperature of polyamide-6 appears around 50 °C. The T g of TPAE-3 is 12 °C higher than that of TPAE-2, and 26 °C higher than that of TPAE-1. The more the soft segment domains are contaminated with dissolved hard segments, the higher will be the T g of polyether domain [37]. For TPAE-3, these dramatic changes are relative to the glass transition of soft segments due to strong hydrogen bonding in PBA, which improves the T g of soft segments. As the temperature rises, there appears a small peak for tanδat 50 °C of TPAE-1 and 43 °C of TPAE-3, but no obvious peak of TPAE-2, which is associated with the melting of the micro-crystalline of soft segments. Compared with TPAE-2 and TPAE-3, TPAE-1 has an apparent T g peak around −58 °C. The closer the T g of soft segments of TPAE-1 to the bulk temperature of PTMG of around −72 °C, the more distinct TPAE-1 micro-phase separation would occur [39]. The micro-phase separation of the samples can be observed in Fig. 3.
Static and dynamic mechanical properties of TPAEs
The mechanical properties of TPAEs are essential in practical applications. It is, therefore, necessary to find the inter-relationship between the mechanical properties of TPAEs and its microstructure. To find out the effect of soft segments on mechanical properties of the polymer, the tensile strength, elongation-at-break and hardness tests were carried out at ambient temperature. As can be observed in Fig. 4, the TPAE-1 based on PTMG, as soft segments, has the best tensile strength of 35.9 MPa, and the TPAE-2 displays the lowest tensile strength of 22.2 MPa. The TPAE-3 has a higher value of tensile strength than that of TPAE-2 but still lower than TPAE-1. On the other hand, TPAE-1 and TPAE-3 show similar elongation-at-breaks of 431 and 467 %, and the TPAE-2 performs the worst elasticity whose elongation-at-break is 360 %. The value of hardness for three TPAEs has the analogous variation tendency with that of elongation-at-break. Above all, TPAE-1 displays all-inclusive mechanical properties. PTMG has the ability to form a micro-phase separation with polyamide-6, which produces better viscoelasticity and processability. Compared with TPEA-1 and TPAE-3, the all-inclusive mechanical properties of TPAE-2 are not as excellent as these two. Accounting for the flexible chain of PEG it is its poor compatibility with polyamide-6.
To further elucidate the mechanical behavior of TPAEs over a broad temperature range with respect to storage modulus (E′) of TPAE-1, TPAE-2 and TPAE-3, the dependency of E’ on temperature is shown for TPAEs in Fig. 5. TPAEs do not exhibit typical rubbery plateaus for thermoplastic elastomers as depicted in Fig. 5. This result shows that the three TPAEs have somewhat higher E′ values of about 2–4 GPa below T g. However, E′ value of PAE-1 decreases one order of magnitude upon approximately T g and then rises a little at −40 °C before modulus starts to drop again. Because of PTMG as soft segments of TPAE-1 with low crystallinity and crystallizing rate, TPAE-1 possesses a partially crystalline poly(tetrahydrofuran) soft segment at around T g which has been detected by DMA. E′ value of TPAE-2 and TPAE-3 decreases very slowly above T g, and its curve has no rubbery plateau before 50 °C, because TPAE-2 and TPAE-3 obtain integrated crystallization of polyethylene glycol and poly(butylene adipate) glycol as soft segments due to large crystallizing rate without obvious peaks at T g. Moreover, the crystallization of soft segments for TPAE-2 and TPAE-3 is not destroyed below the T g detected by DMA which is in good accordance with the result of DSC. E′ value of TPAE-1 is lower than that of TPAE-2 and TPAE-3 due to the crystallization of TPAE-1 based on PTMG segments because soft segments have low rate crystallization. The increase may be attributed to an increase in soft segments crystallinity and nature of modulus for soft segments based on polyether or polyester.
TGA analysis
Thermal properties are very important in TPAEs, because those polymers are usually processed by thermal-plastic molding methods such as extrusion and injection. Figure 6a and b presents TGA and DTG curves of TPAE-1, TPAE-2 and TPAE-3. In Fig. 6a, the result clearly shows that no obvious weight loss can be noticed up to 300 °C for TPAE-1, TPAE-2 and TPAE-3, suggesting that the three TPAEs have sufficient good thermal stability. Besides, the initial thermal decomposition temperature of TPAE-1, TPAE-2 and TPAE-3 is given as 361.3, 347.6 and 355.6 °C, respectively. Figure 6b shows that the decomposition rate of three TPAEs remains unchangeable before 300 °C based on DTG curve. This finding also suggests that the onset of thermal degradation of three TPAEs is primarily determined by the rupture of urethane bonds that link the hard and soft segments. As the urethane bonds that link the hard segments consist of polyamide and MDI with soft segments based on polyether or polyester and are in small proportions in TPAEs, the weight loss of the first main decomposition of TPAEs is only about 10 %.
As the temperature further rises, the remainder decomposes rapidly in 300–500 °C, with the maximum rate at 412.5 °C for TPAE-1; 387.5 °C for TPAE-2; 402.4 °C for TPAE-3, which is observed in each curve depicted in Fig. 6a and b. TPAE-2 displays slightly lower thermal stability compared to TPAE-1 and TPAE-3, owing to its relatively inferior intermolecular forces and non-polar molecular chain. Meanwhile, it can be observed that TPAE-1 shows the best thermal stability due to the low content of ether groups in PTMG. The thermal degradation of aliphatic polyester segments is initiated by a random scission of the ester linkage at the alkyl-oxygen bond, followed by pyrolysis at around 370–440 °C [40]. Therefore, the DTG curve of the three TPAEs at 380–410 °C is attributed to polyester degradations. As temperature further increases, the drop in weight percentage becomes very slow. After the high-temperature pyrolysis, TPAE-1 leaves increased char content compared to TPAE-2 and TPAE-3, in part due to its higher carbon/hydrogen ratio. As shown in Fig. 6, it can be concluded from the above discussion that the TPAEs have good thermal stability with the decomposition temperature higher than 300 °C.
Solvent resistance
As it is listed in Table 3, the TPAEs have only dissolved in acetic acid and methanoic acid at room temperature, and shown outstanding solvent resistance. This performance is related to polyamide-6 segments, because the neat and high-polarity polyamide-6 segments prevent the solvent from entering the bulk structure of TPAEs [41]. Hard segments of TPAEs experience a greater restriction on its swelling behavior due to its imposed polarity [42, 43]. The TPAEs have excellent organic solvent resistance, extending the application of TPAEs, especially in some harsh environment.
Contact angle testing
Hydrophilicity of the TPAEs is evaluated by contact angle measurement between the TPAEs surface and the air/water interface. The contact angle is measured with water to wet the solid surface. The contact angle measurements for TPAEs are presented in Fig. 7. Water contact angles of TPAE-1, TPAE-2 and TPAE-3 are 70.7°, 54.5° and 72.7°, respectively. Higher water contact angle means that TPAE-3 films have high surface hydrophobicity compared with TPAE-1 and TPAE-2 films.
Obviously, all three TPAEs have hydrophilic surfaces, due to the existence of polar groups of polyamide-6 as hard blocks and non-polar groups of polyether or polyester as soft blocks. The hydrophilicity of the membrane surface decreases in the order of TPAE-3 > TPAE-1 > TPAE-2, implying that TPAE-2 has the best hydrophilic surface with the contact angle of 54.5°. The reason is that PEG has more ether bonds as hydrophilic groups than PTMG and similarly polar groups compared with water. Therefore, TPAE-2 based on PEG is more hydrophilic than TPAE-1. However, PBA has ester bonds as hydrophobic group and good crystal structure of soft segments, which lead to best water resistance of TPAE-3 based on PBA. Above all, TPAE-2 has the greater ability to combine with the water compared to TPAE-1 and TPAE-3. The hydrophilicity of TPAEs can imply partly the expanding applications of TPAEs in various fields.
Conclusion
The TPAEs, i.e., TPAE-1, TPAE-2 and TPAE-3, have been successfully prepared in a Haake torque rheometer at 180 °C without solvent, catalyst and initiator. There are micro-phase separations in the TPAE samples and TPAEs have excellent thermal stability with high onset decomposition temperature at 300 °C. The mechanical properties of the prepared TPAEs possess promising tensile strength of 22.2–35.9 MPa and elongation-at-break of 360–467 %. Meanwhile, TPAE-3 films have higher surface hydrophobicity compared to TPAE-1 and TPAE-2 films. From the above analysis, TPAEs present good crystallization. These TPAEs have good mechanical properties between −40 and 50 °C and storage modulus particularly decreases slowly in this temperature range, which gives these TPAEs good application potential.
References
Krijgsman J, Husken D, Gaymans RJ (2003) Synthesis and properties of thermoplastic elastomers based on PTMO and tetra-amide. Polymer 44:7573–7588
Jia RP, Zong AX, He XY, XuJY Huang MS (2015) Synthesis of newly fluorinated thermoplastic polyurethane elastomers and their blood compatibility. Fiber Polym 16:231–238
Nanclares J, Petrović ZS, Javni I, Ionescu M, Jaramillo F (2015) Segmented polyurethane elastomers by non-isocyanate route. J Appl Polym Sci 132:42492–42502
Chernyy S, Ullah S, Jomaas G, Leistedb R, Mindykowskib PA, Ravnsbækc JB, Tordrupc SW, Almdala K (2015) Modification of poly(styrene-b-butadiene-b-styrene) [SBS] with phosphorus containing fire retardants. Eur Polym J 70:136–146
Li T, Ma LF, Bao RY, Qi GQ, Yang W, Xie BH, Yang MB (2015) A new approach to construct segregated structures in thermoplastic polyolefin elastomers towards improved conductive and mechanical properties. J Mater Chem A 3:5482–5490
Stempfle F, Schemmer B, Oechsle AL, Mecking S (2015) Thermoplastic polyester elastomers based on long-chain crystallizable aliphatic hard segments. Polym Chem UK 6:7133–7137
Chen Y, Puskas JE (2004) Neuethermoplastiscelastomere fur biomedizinische anwendungen (Teil 2). Gummi Fasern Kunstst 57:526–534
Deleens G (1980) Hydrolysis-resistant copolyether-ester amides. US Patent 4238528
Gaymans RJ, Schwering P, De Haan JL (1989) Nylon 4,6-polytetramethylene oxide segmented block copolymers. Polymer 30:974–977
Van Hutten PF, Walch E, Veeken AHM (1990) Segmented block copolymers based on polyamide-4,6 and poly(propylene oxide). Polymer 31:524–529
Chung LZ, Kou DL, Hu AT, Tsai HB (1992) Block copolyetheramides. II. Synthesis and morphology of polyamide-6 based block copolyetheramides. J Polym Sci Part A Polym Chem 30:951–953
Holden G (2000) In: Understanding thermoplastic elastomers. Hanser, München
Castaldo L, Maglio G, Palumbo R (1978) Synthesis of polyamide–polyether block copolymers. Polym Sci Polym Lett 16:643–645
Boulares A, Tessier M, Marechal E (2000) Synthesis and characterization of poly(copolyethers-b-polyamides). II. Characterization and properties of the multiblock copolymers. Polymer 41:3561–3580
Biemond GJE, Feijen J, Gaymans RJ (2007) Poly(ether amide) segmented block copolymers with adipic acid based tetraamide segments. J Appl Polym Sci 105:951–963
Fakirov S, Goranov K, Bosvelieva E, Du Chesne A (1992) Multiblock poly(ether-ester-amide)s based on polyamide-6 and poly(ethylene glycol). 1. Effect of polyether segment length on the properties of poly(ether-ester-amide)s with various polyamide/polyether ratios. Makromol Chem 193:2391–2404
Yu YC, Jo WH (1995) Segmented block copolyetheramides based on nylon 6 and polyoxypropylene. II. Structure and properties. J Appl Polym Sci 56:895–904
Geever LM, Higginbotham CL (2007) The effect of salts and pH buffered solutions on the phase transition temperature and swelling of thermo responsive pseudogels based on N-isopropylacrylamide. J Mater Sci 42:9845–9854
Wang GJ, Xue B (2010) Synthesis and characterization of poly(ether-block-amide) and application as permanent antistatic agent. J Appl Polym Sci 118:2448–2453
Niesten MCEJ, Krijgsman J, Harkema S, Gaymans RJ (2001) Melt spinnable spandex fibers from segmented copolyetheresteraramids. J Appl Polym Sci 82:2194–2203
Sijbesmaa H, Nymeijera K, Marwijkb RV, Heijboerb R, Potrecka J, Wesslinga M (2008) Flue gas dehydration using polymer membranes. J Membr Sci 313:263–276
Hou L, Liu H, Yang G (2006) Preparation and characterization of thermoplastic polyurethane elastomer and polyamide 6 blends by in situ anionic ring-opening polymerization of ϵ-caprolactam. Polym Eng Sci 46:1196–1203
Mallakpour S, Rafiemanzelat F (2005) Synthesis and characterization of new optically active poly(amide-imide-urethane) thermoplastic elastomers, derived from bis(p-amido benzoic acid)-N-trimellitylimido-l-leucine and polyoxyethylene-MDI. React Funct Polym 62:153–167
Yu YC, Jo WH (1994) Segmented block copolyetheramides based on nylon 6 and polyoxypropylene. I. Synthesis and characterization. J Appl Polym Sci 54:585–591
Liu L, Chakma A, Feng X (2004) Preparation of hollow fiber poly(ether-b amide)/polysulfone composite membranes for separation of carbon dioxide from nitrogen. J Membr Sci 105:43–51
Sridhar S, Suryamurali R, Smitha B, Aminabhavia TM (2007) Development of crosslinked poly(ether-b-amide) membrane for CO2/CH4 separation. Colloid Surf A 297:267–274
Rached R, Hoppe S, Jonquieres A, Lochon P, Pla F (2006) A new macroinitiator for the synthesis of triblock copolymers PA12-b-PDMS-b-PA12. J Appl Polym Sci 102:2818–2831
Niesten MCEJ, Ten Brinke JW, Gaymans RJ (2001) Structural changes of segmented copolyetheresteramides with uniform aramid units induced by melting and deformation. Polymer 42:1131–1142
Niesten MCEJ, ten Brinke JW, Gaymans RJ (2001) Segmented copolyetheresteraramids with extended poly(tetramethyleneoxide) segments. Polymer 42:1461–1469
Wang HH, Lin LE (2003) Modified polyurethane with improvement of acid dye dyeability. J Appl Polym Sci 89:1397–1404
Brauer M, Hupfer B, Nagel J, Lehmann D (2002) Chemical modification of polyurethane for two-component injection molding. Polym Eng Sci 42:859–869
Yi CW, Peng ZH, Wang HP, Li M, Wang CS (2011) Synthesis and characteristics of thermoplastic elastomer based on polyamide-6. Polym Int 60:1728–1736
Kong WB, Hu K, Fu XW, Guo DY, Lei JX (2016) Preparation and characterization of thermoplastic elastomer based on amino-terminated polyamide-6 and diisocyanate-terminated polytetramethylene glycol. Polym Plast Technol 55:1–8
Król P (2007) Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog Mater Sci 52:915–1015
Garrett JT, Xu R, Cho J, Runt J (2003) Phase separation of diamine chain-extended poly(urethane) copolymers: FTIR spectroscopy and phase transitions. Polymer 44:2711–2719
Chen KS, Yu TL, Chen YS, Lin TL, Liu WJ (2001) Soft- and hard-segment phase segregation of polyester-based polyurethane. J Polym Res 8:99–109
Hesketh TR, Van Bogart JWC, Cooper SL (1980) Differential scanning calorimetry analysis of morphological changes in segmented elastomers. Polym Eng Sci 20:190–197
Van Bogart JWC, Bluemke DA, Cooper SL (1981) Annealing-induced morphological changes in segmented elastomers. Polymer 22:1428–1438
Sheth JP, Xu J, Wilkes GL (2003) Solid state structure–property behavior of semicrystalline poly(ether-b-amide) PEBAX® thermoplastic elastomers. Polymer 44:743–756
Louiea JS, Pinnau I, Ciobanu I, Ishida PK, Ngb A, Reinhard M (2006) Effects of polyether–polyamide block copolymer coating on performance and fouling of reverse osmosis membranes. J Membr Sci 280:762–770
Chatterjee T, Basu D, Das A, Wiessner S, Naskar K, Heinrich G (2016) Super thermoplastic vulcanizates based on carboxylated acrylonitrile butadiene rubber (XNBR) and polyamide (PA12). Eur Polym J 78:235–252
Liaw DJ (1997) The relative physical and thermal properties of polyurethane elastomers: effect of chain extenders of bisphenols, diisocyanate, and polyol structures. J Appl Polym Sci 66:1251–1265
Zia KM, Ahmad A, Anjum S, Zuber M, Anjum MN (2015) Synthesis and characterization of siloxane-based polyurethane elastomers using hexamethylene diisocyanate. J Elastom Plast 47:625–663
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, L., Kong, W., Wu, B. et al. Reactive processing of thermoplastic elastomers based on polyamide-6: preparation and characterization. Iran Polym J 25, 765–773 (2016). https://doi.org/10.1007/s13726-016-0465-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-016-0465-1