Abstract
Effect of incorporation of carbon nanotubes (CNTs) into diglycidyl ether of bisphenol A (DGEBA)-based epoxy resin (HY5052/LY5052 system) on cure reaction was investigated via thermal analysis. Keeping their length constant, CNTs of different diameters were chosen. Samples based on epoxy resin and different type and content of CNTs were prepared and characterized. The cure behavior of epoxy matrix in the presence of CNTs was studied in both glassy and rubbery states. The results indicated that the final cure characteristics of epoxy nanocomposites were controlled by the competition of viscosity-increasing effect and heat-sink effect of CNTs. Isothermal analysis showed that accelerating or decelerating effect of CNTs on the cure process depends on CNT content, its aspect ratio and temperature of isothermal cure. The presence of the CNTs physically hindered the mobility of the epoxy and hardener monomers preventing the cure reaction (viscosity-increasing effect). In contrast, inherent high thermal conductivity of CNTs can act as a heat sink to accelerate the heat absorption of the epoxy (heat-sink effect). Below glass–rubber transition temperature (T g), heat-sink effect of CNTs was dominant due to the restricted mobility of polymer chains in glassy state, whereas at temperatures higher than T g, viscosity-increasing effect was dominant. CNTs physically hindered the mobility of reactive species despite the inherent tendency of polymer chains for long-range molecular motions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, the proper dispersion of CNT in polymer matrix has been the most critical issue for achieving appropriate properties of CNT/polymer composites [1–3]. Furthermore, it has been already reported in the literature that the choice of optimal dimensions and content of CNTs are a promising approach to improve the efficiency of CNTs as reinforcing/conductive nano-filler [4–6].
Wu et al. [7] studied the effect of CNTs with two aspect ratios (length-to-diameter, A = l/d) including high aspect ratio (HAR) and low aspect ratio (LAR) on network structure of the CNTs in biodegradable polylactide (PLA) matrix. They concluded that the composites with HAR CNTs present a higher modulus than that of the LAR CNT composites owing to the mesoscopic dispersion structure of CNTs which directly depends on the aspect ratio [7]. Zhang et al. [8] observed that the fatigue crack growth rates of epoxy matrix can be significantly reduced by increasing the aspect ratio of multi-walled carbon nanotubes (MWNT). Zhang et al. [9] also showed that longer nanotubes produce a higher toughening efficiency in polypropylene/CNT nanocomposites than the shorter ones. In contrast, Dubnikova et al. [10] reported that increase in the MWNTs aspect ratio reduces their efficiency due to the low flexibility and less entanglement of thick (LAR) nanotubes. Ayatollahi et al. [4] proposed a correction factor based of MWNT’s dimensions to modify the predictions of Halpin–Tsai theory for modulus of epoxy nanocomposite. Hernandez-Perez et al. [11] pointed out that epoxy/HAR mixture presented higher viscosity which does not favor CNT’s proper dispersion.
From a macro-kinetic point of view, the study of cure kinetics of polymers as a function of the processing and material parameters is of a great importance in the analysis and design of processing operations [12]. Many studies have verified that introduction of carbon nanotubes can affect the cure behavior of epoxy resins [13–15].
Lau et al. [16] dispersed CNTs into epoxy matrix incorporating various solvents and found that even small traces of residual solvent had a significant impact on the cure reaction of epoxy resin. Puglia et al. [17, 18] demonstrated that incorporation of carbon nanotubes increased the rate of cure reaction and the thermal degradation of diglycidyl ether segment of the DGEBA epoxy matrix. The findings on cure kinetics and thermal degradation of the epoxy nanocomposites can be correlated to the ultrahigh thermal conductivity of CNTs and the ability of the epoxy resin to disperse the CNTs, providing a larger surface area for heat propagation. The effects of different grades of single-walled carbon nanotubes (SWNTs) on the curing behavior of epoxy were studied via a differential scanning calorimetry (DSC) method. It was observed that CNTs initiate the curing process of epoxy at lower temperatures, whereas the overall cure reaction was slowed down [19].
DSC was used to elucidate differences in the cure behavior of DGEBA epoxy originated from the surface chemistry of CNT. The comparison of the results hinted that the cure mechanisms of nanocomposites containing fluorinated CNT are similar to those of the neat resin, but different from that of the nanocomposites with carboxylated CNT [20]. Higher extent of reaction was reported for the composite obtained using the plasma-fluorinated SWNTs due to the covalently attached amine groups on CNTs [21]. In another study, the accelerating effect of CNTs functionalized by liquid crystal epoxy resin (ef-CNTs) on cure of epoxy matrix was demonstrated [22].
As mentioned, a number of studies have been conducted on the cure reaction of epoxy system versus CNT content, but scarcely any published papers have been available to date on the influence of CNT aspect ratio on the cure kinetics of the epoxy system. The novel target of this study is to seek the influence of CNT aspect ratio on the cure kinetics of epoxy resin. Also, the purpose of this work is to provide a comprehensive understanding about the key role of temperature in the cure kinetics of epoxy. Particular emphasis is given to the glass–rubber transition temperature (T g) and the experimental work has been elaborated to explain the influence of T g on the cure kinetics of epoxy system for the first time.
Experimental
Materials
The polymer matrix (Araldite LY 5052/Aradur HY 5052, Huntsman, Switzerland) was used as a standard low viscosity epoxy [23] along with a hardener based on modified cycloaliphatic amines (HY5052). The chemical structures of the epoxy resin system are shown in Scheme 1 [24]. Different types of nanotubes were employed and their corresponding dimensions are listed in Table 1.
Preparation of nanocomposites and neat epoxy specimens
In the preparation of nanocomposites, CNTs were sonicated in the hardener for 30 min prior to curing. Afterward, the epoxy was mixed with a hardener and stirred in a water–ice bath at 100 rpm to avoid bubbles and curing. The mixture was poured into a rectangular cavity silicone mold and subject to 24 h of cure at room temperature followed by 4 h of post-cure at 100 °C.
Characterization
The overall quality of the CNTs’ dispersion in epoxy matrix was determined via observing their cryo-fractured surfaces under a Vega/Tescan (Czech Republic) scanning electron microscope (SEM). A priori, the SEM specimens were prepared by fracturing the samples in liquid nitrogen. The dynamic thermo-mechanical tests were carried out on a cured sample using single cantilever bending fixture on a dynamic mechanical analyzer (DMA-TRITON, Tritec 2000 DMA, Lincolnshire, UK) according to ASTM-E 1640. The curing cycle was 24 h at ambient temperature followed by 4 h at 100 °C. The heating rate and frequency were set at 10 °C/min and 1 Hz, in the temperature range from 25 to 170 °C. Isothermal calorimetric tests were performed on a differential scanning calorimeter (DSC) Netzsch 200F3 (Germany) coupled with an intercooler. The procedure performed in isothermal scans was as follows. Samples of about 22 mg were heated from ambient temperature to isothermal temperatures (110, 120, 130 °C) at a scan rate of 70 °C/min. Dynamic scan was then carried out on the same specimen to make sure that the heat of reaction required to complete the cure process (post-cure heat or residual heat) was zero (not shown here).
Theoretical concept
The characteristics obtained from DSC thermograms are given in the following equation:
where H tot, H iso and H res are the total heat of the reaction, the heat evolved during an isothermal scanning at the desired temperature and the residual heat obtained via dynamic scanning, respectively [18]. The degree of cure ( \(\alpha\)) is defined as follows:
where H t is the partial area under the DSC isotherm trace up to time t [17]. The general curing kinetic mechanism is described as follows:
where k(T), f(α) and \(\frac{d\alpha }{dt}\) are the Arrhenius rate constant at temperature T (1/s), the reaction model and reaction rate (1/s), respectively [25]. The curing kinetics of epoxy resins can be classified in two main groups: nth order and autocatalyzed. The former obeys the general form given by Eq. (4) [26]:
and the latter has the following form:
where n and m are reaction orders [27]. The activation energy (E a, J/mol) and exponential factor (Z, 1/s) are calculated from Eq. (6) (R is the gas constant) as follows:
Results and discussion
Surface fracture morphology
The two main critical issues in the preparation of epoxy/CNT nanocomposites are adequate dispersion of the CNTs within the matrix and strong interfacial adhesion between the CNT and epoxy matrix [20, 28]. Several research works have recently been devoted to investigate the effect of CNT’s aspect ratio on dispersion of CNTs in the epoxy matrix [4, 11, 16]. They observed that the higher aspect ratio of CNTs promoted tube agglomeration.
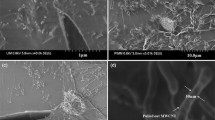
Due to the mentioned fact, the epoxy sample filled with 0.5 wt% of SWNTs was chosen for morphology studies. Figure 1a shows the cryo-fractured surface morphology of the 0.5 wt% epoxy/SW sample. It is observed that SWNTs were effectively dispersed in the epoxy resin matrix. It can be concluded that the employed mixing process is beneficial to obtain a good dispersion of SWNTs in the epoxy resin. Interaction between SWNTs and epoxy resin was clearly witnessed by comparison of SEM micrographs in Fig. 2b, c. The nanocomposites (Fig. 1b) showed a rough fracture surface characterized by the presence of thick river patterns [29], whereas, the fracture surface of a neat epoxy resin (Fig. 1c) appears typically cleavage like [30].
As seen, the fracture surface of the nanocomposite is very rough, suggesting that the failure phenomenon occurred through a plastic deformation. Increasing surface roughness causes the path of the crack front to deviate when it interacts with SWNTs, which makes crack propagation more difficult. On the contrary, the smooth fracture surface of neat epoxy indicates that brittle failure is dominant. These observations are in agreement with the surface morphology reported by others [28].
Isothermal curing kinetics
CNTs’ content effect
Isothermal curing studies were accomplished with the aim of determining the kinetic parameters of the curing reactions in epoxy nanocomposites. A priori, DMA tests were performed to determine the glass–rubber transition temperatures (T g) of neat epoxy resin and its nanocomposites to obtain an appropriate temperature range for the isothermal cure test.
The storage modulus (E′) and loss factor (tan δ) of the nanocomposites were measured by DMA as a function of temperature for neat epoxy and the sample with 0.5 wt% of mixed CNTs (m-CNTs), which are given in Fig. 2. Mixed CNTs were generally prepared by physical mixing of equal weights of the four CNT types given in Table 1. Over the whole range of temperature, the epoxy nanocomposite exhibits a higher storage modulus than the neat epoxy. Although the addition of m-CNTs to the epoxy matrix slightly affected (8 % increase) the storage modulus in the glassy region, there was a considerable enhancement (25 % increase) in rubbery region storage modulus. This behavior could be associated with an improved interfacial interaction due to the extensive special surface area (SSA) of the CNTs [31]. Increasing trend of modulus at higher CNT contents has been reported previously [32]. This interfacial interaction hinders the mobility of the epoxy matrix segments in the vicinity of the CNTs and leads to an observable increase in E′ value [33, 34].
Table 2 shows a summary of values of storage modulus, both in glassy and rubbery states for the neat epoxy and its nanocomposite. Also, the peak of tan δ corresponding to α-relaxation was reported as glass to rubber transition temperature (T g). Besides the influence on E′, introduction of m-CNTs into the epoxy matrix results in a shift of T g toward lower temperatures. This effect has also been reported by other authors, although several causes are suggested for the occurrence of this phenomenon. It might be initiated due to lack of stoichiometry generated by the preferential adsorption of any of the reactive constituents onto the hollow CNTs which may lead to inhibition of cross-linking reaction between the epoxy and hardener [35, 36].
Another possibility is that CNTs can act as high-quality plasticizers and interact strongly with the epoxy matrix. They can create an interphase region, restraining the epoxy system from exchanging energy with the surroundings [35]. For both the examined materials, the loss factor (tan δ) shows a sharp peak around 120 °C corresponding to T g of the epoxy system. According to the datasheet of LY5052/HY5052 by Huntsman Inc. [23], the maximum attainable T g for this epoxy system is about 130 °C. Thus, based on the datasheet and DMA result presented in the manuscript, the authors are almost sure that at 110 °C the system is in its glassy state. Therefore, taking into account DMA data, isothermal curing tests were carried out at different temperatures in the range of 110–130 °C to investigate cure reaction at both glassy and rubbery states. To avoid any effect of CNTs’ dimensions, m-CNTs (a mixture of all four types of CNTs mentioned in Table 1) were used in the study of the effect of CNTs’ content on cure kinetics of the epoxy resin.
To exclude the endothermic heat flow required to raise the temperature of a substance to a given isothermal temperature, the following procedure was employed. The heat flow versus time thermogram of non-reacted epoxy resin and amine hardener (exactly at the same weight ratio) was used as baseline to calculate the cure thermogram of epoxy and its nanocomposites. In other words, the heat flow curve of the non-reacted epoxy/hardener system was subtracted from the heat flow curve of the samples obtained from DSC to extract the accurate exotherm of the cure process. For a clearer conception, a schematic of the approach is shown in Fig. 3.
The effect of CNT content on the cure of epoxy resin extracted from the DSC isotherm is shown in Figs. 4, 5, 6. All degree of cure curves present a sigmoidal form, indicative of an autocatalytic kinetics, due to the structural and molecular variations that occur during the cure process via amine–epoxide reaction [33, 37]. It is obvious from the cure curves that maximum reaction rates are attained at time t > 0. Thus, the nth order kinetics becomes invalid pointing to the autocatalytic model. The nth order kinetic model is suitable only if the maximum reaction rate occurs at t = 0. But in the autocatalytic model, the rate shows a maximum at an intermediate conversion [36]. Frequently, ‘‘autocatalytic’’ behavior, explicitly with a maximum reaction rate at non-zero times, was reported for epoxy systems [14, 38]. The cure process includes initially the “gelation” process, increasing the viscosity of resin due to a progressive free volume reduction [39], since “gelation” followed by diffusion-controlled effects take place by the “vitrification” process [14].
The total area under the thermogram, based on the non-reacted LY5052/HY5052 composite, was calculated as the total heat of cure reaction. The maximum of the exotherm peak (t p) and the total heat of reaction (H tot) as a function of the m-CNTs’ content are reported in Table 3. Namely, the introduction of m-CNTs does not affect the overall cure mechanism of epoxy resin. However, exothermic peaks and total heat of reaction of epoxy/m-CNT nanocomposites were found to be smaller than that of the neat epoxy which has also been reported by others [13, 14, 20].
The values of isothermal enthalpy (H tot) increased by increasing the isothermal cure temperature. This is related to the fact that the increase in isothermal reaction temperature for exothermic reactions generates heat in a higher proportion than the heat generated by the reaction itself [20]. For a given reaction temperature, the enthalpy of epoxy/CNT nanocomposites decreased relative to the neat resin. The decrease of H tot with increasing m-CNT content can be directly attributed to the proportional decrease of epoxy concentration in the nanocomposite [14]. Furthermore, this suggests that the presence of the nanotubes causes an increase in viscosity which lowers the mobility of the reactive species and results in a lower enthalpy [20].
As known, the activation energy is the extent of energy barrier for a reaction [22]. Therefore, increase in viscosity which hinders the curing process is expected to raise activation energy. At elevated temperatures, thermal energy becomes comparable to or higher than the energy barrier, which make it easier to overcome the heights of potential barriers [40]. This is consistent with the observed fact that the increase in isothermal temperatures decreases the total enthalpy difference between neat epoxy and epoxy/CNT samples. It can be concluded that the potential barrier of cure behavior (activation energy) has a range of validity at low temperatures, but are gradually be washed out as the temperature is raised. A similar effect was observed by Abdalla et al. [20]: by increasing temperature for a DGEBA/carboxyl-modified MWNT nanocomposite, the difference between the total heat of neat epoxy and epoxy/CNT decreased.
The maximum of the exothermic peaks of the neat epoxy resin is located around 2.29, 2.12 and 1.84 min at 110, 120 and 130 °C, respectively. A similar trend with increasing isotherm temperature was also observed for m-CNT-filled epoxy nanocomposite. This small decrease of the maximum of the exotherm peak is expected to be due to an increase in the isotherm cure temperature that can accelerate the cure reaction. Another notable observation is the slight shift of t p for epoxy nanocomposites which depends on the temperature of isotherm cure. On detecting the early stage of cure reaction, one can observe that at 110 °C the cure reaction of m-CNT-filled epoxy resin accelerates at the initial stage, whereas the opposite behavior is observed at higher temperatures at (130 °C).
Earlier reports revealed that there were several factors such as the thermal properties of CNTs [29], reduced molecular chain mobility [38], increased viscosity of epoxy in the presence of CNTs [41] and chemical species on CNTs’ surfaces [34] that can influence the cure kinetics of epoxy resin. In the case of unmodified CNTs, two competitive effects arose from the presence of CNTs: (a) accelerating effect due to high thermal conductivity of CNTs, and (b) decelerating effect due to the restriction in molecular mobility imposed by CNTs (or increased viscosity). Accelerating effect of CNTs due to their high thermal conductivity might be dominant at 110 °C compared to 130 °C, owing to restricted motion of polymer chains leading to lower t p values. Thus, the high thermal conductivity of the CNTs enhances the cure kinetics at high temperatures (130 °C) even at low m-CNT content. Although the change in t p value of isotherm curing process is evident at the lowest CNT content, there is a negligible change with further increase of m-CNT content.
Tables 4, 5 summarize the activation energy and reaction order for the neat resin and nanocomposites using the autocatalytic model (Eq. 5). When the autocatalytic equation is employed for epoxy/amine system, the overall order of the reaction is 2, with m + n ≈2. The higher activation energy of the nanocomposites compared to that of the neat resin is reported in the literature [33, 42].
As known, during the cure reaction, the system undergoes gelation and vitrification transitions. Intensive cross-linking occurring in the region between the above transitions restricts molecular mobility, causing the cure reaction to change from a kinetic-controlled mechanism to a diffusion-controlled regime [25]. The accelerating effect of CNTs due to their high thermal conductivity and decelerating effect of CNTs due to the restriction in molecular mobility imposed by CNTs (or increased viscosity) are predominant in diffusion-controlled and kinetic-controlled regimes, respectively. The K parameter governs the autocatalytic reaction after the initial autocatalytic stage [43], i.e., between gelation and vitrification transition. It is found that the cure temperature shows a positive effect on the K value, similar to that observed for epoxy/carboxylated CNT system [20]. With increasing temperature, vitrification occurs faster and the diffusion-controlled regime is extended. Thus, the accelerating effect of CNTs owing to high thermal conductivity can overcome the decreasing effect of CNTs due to increase of viscosity. The decreasing effect of CNTs is gradually washed out by increasing the temperature of curing. Diminishing decelerating effect of CNTs is more obvious at high content of CNTs (the sample with 0.5 wt% of CNTs), which show a higher value of K than neat epoxy.
It is interesting to observe that by increasing the curing time, in the last stage of cure reaction there is a significant influence of the presence of CNTs. At the last stage of cure reaction after vitrification transition, T g plays a key role in cure behavior of epoxy resin. Considering the vitrification phenomenon, the cure is mainly diffusion controlled at times above 6 min. Below T g (110 °C), insignificant modification in the last stage of cure reaction is expected by addition of CNTs due to the scarce molecular motion after vitrification. Above T g (130 °C), the effect of CNTs on cure behavior becomes clear due to the possible molecular motion of the epoxy matrix.
CNTs’ aspect ratio effect
In spite of changing the cure kinetic parameters of epoxy, by increasing the CNT content, the overall cure mechanism of epoxy remains unchanged. To minimize the effect of CNT content, a very low content of every type of CNTs (0.01 wt%) was chosen for further investigation on the effect of CNTs’ aspect ratio on cure kinetics. Increasing the length over diameter ratio (l/d) of CNTs initiates numerous phenomena in the epoxy matrix, which can cause opposite effects on the curing behavior as well as other mechanical [43], electrical [4] and thermo-mechanical [11] properties. Among these different issues, increase in thermal conductivity and viscosity of epoxy resin upon increasing l/d were found to affect epoxy nanocomposite cure behavior significantly.
Increasing thermal conductivity (λ, W/mK) of nano-fillers with increasing aspect ratio is the result of two facts: (a) the inherently higher individual thermal conductivity of CNTs which possesses higher l/d ratio (λ SW ≥ λ DW ≥ λ MW-A ≥ λ MW-B) [44] and (b) easier formation of heat-conductive network in epoxy matrix, e.g., thermal percolation threshold (φ C and wt%) for CNTs with higher aspect ratio [45].
Percolation threshold [46] characterized by a sharp drop of several orders of magnitude in thermal resistivity decreased with increasing aspect ratio of CNTs. Depending on the aspect ratio of CNTs and processing procedure, percolation thresholds between 0.0025 and 10 wt% have been experimentally observed for epoxy/CNT nanocomposites. Diffusion processes and particle–particle interactions in a matrix of low viscosity filled by nano-particles play a key role in the agglomeration and network formation [45]. Decreasing thermal percolation threshold of nano-fillers with increasing aspect ratio can be interpreted by some analytical models such as excluded volume theory [47]. The concept of excluded volume is a reliable approach to estimate the percolation threshold of nanocomposites containing randomly dispersed non-spherical nano-particles including CNTs [48]. A conducting network may be formed if the excluded volumes of two conductive nano-particles overlap. As the excluded volume reduces, the percolation threshold decreases which means a conductive network is formed at lower concentrations of conductive nano-particles [4]. The increase of aspect ratio of conductive nano-particles has proven to be effective in reducing the excluded volume [49]. Therefore, with higher aspect ratio, more heat-conductive CNTs are definitely expected. Furthermore, the presence of CNTs causes an increase in viscosity of the epoxy matrix at the expense of lowering the mobility of reactive species of the curing reaction [20]. The introduction of high aspect ratio CNTs causes a viscosity increase of epoxy monomers markedly compared to that of low aspect ratio CNTs, due to their higher specific area [35].
Heat flow and degree of cure versus time (t) curves obtained at different isothermal temperatures for HY5052/LY5052/CNT nanocomposites for different CNT dimensions are reproduced in Figs. 7, 8, 9. The results of the neat epoxy and the analogous nanocomposites t p and H tot with the same CNT content for different types of CNT are summarized in Table 3.
Below T g in glassy region (at 110 °C), in general, one can say that the addition of small amount of SWNTs and double-walled carbon nanotubes (DWNTs) significantly increased the cure rate in the initial stage of cure reaction, whereas MWNTs did not significantly influence the first 3 min of curing. The comparison of the results in Fig. 7b leads to an overall picture which opens new perspectives in modifying the thermal conductivity of polymer matrix systems via introduction of CNTs. The reported thermal conductivity for SWNT and MWNTs at room temperature is 6,600 and 200–3,000 W/mK, respectively [50].
According to the literature [51], a certain enhancement of the thermal conductivity of epoxy nanocomposites, by incorporation of CNTs with high l/d may be expected. It is also expected that a low percolation threshold could be obtained by dispersing SWNTs in an epoxy matrix. Therefore, the accelerated cure of epoxy in the presence of the high aspect ratio CNTs (SWNTs and DWNTs) can be associated with high thermal conductivity of the epoxy nanocomposites. It has been proven that introduction of SWNT can improve the thermal stability and accelerate the heat absorption of the epoxy (heat-sink effect) [16]. But the stronger catalytic effect of MWNTs compared to that of SWNTs in the early stage of the cure process (the first 3 min of curing process) still needs to be investigated in depth
This inconsistency may be solved when looking at the relative surface area of CNTs. Compared to SWNTs and DWNTs, MWNTs showed the highest potential for the efficient enhancement of initial cure reaction. This is due to the relatively lower surface area of MWNTs which decreases their ability to adsorb curing species on their surfaces [50]. In addition, lower H tot can be accounted for by the fact that the presence of the CNTs subordinates the mobility of the reactive species of cure reaction.
Above T g, it can be seen that epoxy/CNTs nanocomposite exhibits a longer t p and larger H tot values. From Fig. 9b, it is evident that the acceleration effect of CNTs on the early stage of curing is not noticeable at high temperatures. This may be due to the fact that above T g, an increase in viscosity reduces the mobility of reactive species. Here, large segments of the molecules can start to wiggle around, whereas below T g the polymer molecules are frozen. In addition, rapid molecular motions above T g negatively affect the initial cure rate of epoxy by suppressing the formation of high thermal-conductive network of CNTs, as a prerequisite for accelerating epoxy cure.
Application of least squares method to autocatalytic equation gives values of empirical parameters m, n and k listed in Table 4. Activation energies and pre-exponential parameter obtained by nonlinear regression are given in Table 5. Since CNTs can hinder curing reactions by influencing the molecular diffusivity, it is expected that pure CNTs show a negative effect on cure reaction and increase its activation energy. But in this case, due to a very low CNT content, some high thermal-conductive CNTs such as SWNTs can partially compensate this effect and reduce the activation energy of cure reaction. A slight uneven change in activation energy is correlated to competition between heat-sink and viscosity-increasing effects of CNTs of various aspect ratios. As shown above, the key points here are effect of both content and dimensions of CNT on the cure reaction of epoxy which are determined by isothermal DSC measurements below or above the T g value.
Conclusion
Investigation of isothermal curing using DSC indicates that the effective parameters of CNTs in curing reaction of epoxy are (1) specific surface area, (2) aspect ratio (l/d) and (3) thermal conductivity. These parameters are correlated to heat-sink and viscosity-increasing effects of CNTs on cure process. It was observed that H tot decreased on introducing a small quantity of CNTs. This is followed by a further significant decrease with increasing CNT loadings which can be directly attributed to the proportional decrease in reactive species concentration. Furthermore, below T g, t p is hastened with introduction of CNTs into the epoxy matrix, but above T g the peak is somehow delayed compared to that of the neat epoxy. The shift of t p is well apparent at the lowest CNT content, but there is no significant change in t p with further increase in CNT content. DSC results focusing on the effect of CNT dimensions indicated that the composite cure is influenced not only by the viscosity-increasing effect of CNTs, but also by the heat-sink effect. The competition between these two factors determines the final cure characteristics of epoxy nanocomposites. The increased thermal conductivity (heat-sink effect) induced by high aspect ratio CNTs and its effect on initial cure is more obvious at the low isothermal temperatures. Increasing l/d of CNTs at high isothermal temperatures does not produce any relative accelerating effect, suggesting a predominant viscosity-increasing effect of CNTs against the heat-sink effect. It was observed that CNTs were able to change the cure process of the epoxy system depending on the temperature of isothermal cure. In the glassy state, the accelerating effect of CNTs due to their high thermal conductivity is dominant owing to restricted motion of polymer chains. In the rubbery state, the decelerating effect of CNTs is predominant due to their viscosity-increasing effect.
References
Ghorabi S, Rajabi L, Madaeni S, Zinadini S, Derakhshan A (2012) Effects of three surfactant types of anionic, cationic and non-ionic on tensile properties and fracture surface morphology of epoxy/MWCNT nanocomposites. Iran Polym J 21:121–130
Hajibaba A, Naderi G, Ghoreishy M, Bakhshandeh G, Nouri M (2012) Effect of single-walled carbon nanotubes on morphology and mechanical properties of NBR/PVC blends. Iran Polym J 21:505–511
Alimardani M, Abbassi-Sourki F, Bakhshandeh G (2012) Preparation and characterization of carboxylated styrene butadiene rubber (XSBR)/multiwall carbon nanotubes (MWCNTs) nanocomposites. Iran Polym J 21:809–820
Ayatollahi M, Shadlou S, Shokrieh M, Chitsazzadeh M (2011) Effect of multi-walled carbon nanotube aspect ratio on mechanical and electrical properties of epoxy-based nanocomposites. Polym Test 30:548–556
Zamani M, Fereidoon A, Sabet A (2012) Multi-walled carbon nanotube-filled polypropylene nanocomposites: high velocity impact response and mechanical properties. Iran Polym J 21:887–894
Lavorgna M, Romeo V, Martone A, Zarrelli M, Giordano M, Buonocore GG, Qu MZ, Fei GX, Xia HS (2013) Silanization and silica enrichment of multiwalled carbon nanotubes: synergistic effects on the thermal-mechanical properties of epoxy nanocomposites. Eur Polym J 49:428–438
Wu D, Wu L, Zhou W, Sun Y, Zhang M (2010) Relations between the aspect ratio of carbon nanotubes and the formation of percolation networks in biodegradable polylactide/carbon nanotube composites. J Polym Sci Pol Phys 48:479–489
Zhang W, Picu R, Koratkar N (2008) The effect of carbon nanotube dimensions and dispersion on the fatigue behavior of epoxy nanocomposites. Nanotechnology 19:285709
Zhang H, Zhang Z (2007) Impact behaviour of polypropylene filled with multi-walled carbon nanotubes. Eur Polym J 43:3197–3207
Dubnikova I, Kuvardina E, Krasheninnikov V, Lomakin S, Tchmutin I, Kuznetsov S (2010) The effect of multiwalled carbon nanotube dimensions on the morphology, mechanical, and electrical properties of melt mixed polypropylene-based composites. J Appl Polym Sci 117:259–272
Hernandez-Perez A, Aviles F, May-Pat A, Valadez-Gonzalez A, Herrera-Franco PJ, Bartolo-Perez P (2008) Effective properties of multiwalled carbon nanotube/epoxy composites using two different tubes. Compos Sci Technol 68:1422–1431
Hayaty M, Honarkar H, Beheshty M (2013) Curing behavior of dicyandiamide/epoxy resin system using different accelerators. Iran Polym J 22:591–598
Wang S, Liang Z, Liu T, Wang B, Zhang C (2006) Effective amino-functionalization of carbon nanotubes for reinforcing epoxy polymer composites. Nanotechnology 17:1551–1557
Valentini L, Armentano I, Puglia D, Kenny J (2004) Dynamics of amine functionalized nanotubes/epoxy composites by dielectric relaxation spectroscopy. Carbon 42:323–329
Allaoui A, El Bounia N-E (2009) How carbon nanotubes affect the cure kinetics and glass transition temperature of their epoxy composites? A review. Express Polym Lett 3:588–594
Lau K-t, Lu M, C-k Lam, H-y Cheung, Sheng F-L, Li H-L (2005) Thermal and mechanical properties of single-walled carbon nanotube bundle-reinforced epoxy nanocomposites: the role of solvent for nanotube dispersion. Compos Sci Technol 65:719–725
Puglia D, Valentini L, Kenny J (2003) Analysis of the cure reaction of carbon nanotubes/epoxy resin composites through thermal analysis and Raman spectroscopy. J Appl Polym Sci 88:452–458
Puglia D, Valentini L, Armentano I, Kenny J (2003) Effects of single-walled carbon nanotube incorporation on the cure reaction of epoxy resin and its detection by Raman spectroscopy. Diam Relat Mater 12:827–832
Tao K, Yang S, Grunlan JC, Kim YS, Dang B, Deng Y, Thomas RL, Wilson BL, Wei X (2006) Effects of carbon nanotube fillers on the curing processes of epoxy resin-based composites. J Appl Polym Sci 102:5248–5254
Abdalla M, Dean D, Robinson P, Nyairo E (2008) Cure behavior of epoxy/MWCNT nanocomposites: the effect of nanotube surface modification. Polymer 49:3310–3317
Valentini L, Puglia D, Carniato F, Boccaleri E, Marchese L, Kenny JM (2008) Use of plasma fluorinated single-walled carbon nanotubes for the preparation of nanocomposites with epoxy matrix. Compos Sci Technol 68:1008–1014
Chen S, Hsu SH, Wu MC, Su WF (2011) Kinetics studies on the accelerated curing of liquid crystalline epoxy resin/multiwalled carbon nanotube nanocomposites. J Polym Sci Pol Phys 49:301–309
Araldite LY5052/Aradur HY5052 (2010) Datasheet, Huntsman advanced materials. http://www.swiss-composite.ch/pdf/t-Araldite-LY5052-Aradur5052-e.pdf
Suckley DR (2000) Microwave processing of the araldite LY5052: HY5052 epoxy resin system. University of Manchester, Manchester
Vyazovkin S, Sbirrazzuoli N (1996) Mechanism and kinetics of epoxy-amine cure studied by differential scanning calorimetry. Macromolecules 29:1867–1873
Borchardt HJ, Daniels F (1957) The application of differential thermal analysis to the study of reaction kinetics. J Am Chem Soc 79:41–46
Keenan MR (1987) Autocatalytic cure kinetics from DSC measurements: zero initial cure rate. J Appl Polym Sci 33:1725–1734
Cheng Q, Wang J, Jiang K, Li Q, Fan S (2008) Fabrication and properties of aligned multiwalled carbon nanotube-reinforced epoxy composites. J Mater Res 23:2975–2984
Visco A, Calabrese L, Milone C (2009) Cure rate and mechanical properties of a DGEBF epoxy resin modified with carbon nanotubes. J Reinf Plast Comp 28:937–949
Visco AM, Calabrese L, Cianciafara P, Bonaccorsi L, Proverbio E (2007) Fiber reinforced polyester resins polymerized by microwave source. J Mater Eng Perform 16:792–799
Dai H (2002) Carbon nanotubes: opportunities and challaenges. Surf Sci 500:218–241
Susin SB, Pistor V, Amico SC, Coelho LAF, Pezzin SH, Zattera AJ (2014) Investigation of cure kinetics in epoxy/multiwalled carbon nanotube nanocomposites. J Appl Polym Sci 131(1–6):39857. doi:10.1002/app.39857
Rahaman A, Mohanty A (2014) Effect of carbon nanotubes on the curing and thermomechanical behavior of epoxy/carbon nanotubes composites. Polym Compos 35:441–449
Gojny FH, Schulte K (2004) Functionalisation effect on the thermo-mechanical behavior of multiwall carbon nanotube/epoxy composite. Compos Sci Technol 64:2303–2308
Prolongo SG, Gude MR, Ureña A (2009) Synthesis and characterisation of epoxy resins reinforced with carbon nanotubes and nanofibers. J Nanosci Nanotechnol 9:6181–6187
Shen J, Huang W, Wu L, Hu Y, Ye M (2007) The reinforcement role of different amino-functionalized multi-walled carbon nanotubes in epoxy nanocomposites. Compos Sci Technol 67:3041–3050
Yang K, Gu M, Jin Y (2008) Cure behavior and thermal stability analysis of multiwalled carbon nanotube/epoxy resin nanocomposites. J Appl Polym Sci 110:2980–2988
Qiu J, Wang S (2010) Reaction kinetics of functionalized carbon nanotubes reinforced polymer composites. Mater Chem Phys 121:295–301
Macan J, Brnardi I, Ivankovi M, Mencer HJ (2005) Dsc study of cure kinetics of DGEBA-based epoxy resin with Poly(oxypropylene) diamine. J Therm Anal Calorim 81:369–373
Goldstein M (1969) Viscous liquids and the glass transition: a potential energy barrier picture. J Chem Phys 51:3728–3739
Siddiqui NA, Khan SU, Ma PC, Li CY, Kim JK (2011) Manufacturing and characterization of carbon fibre/epoxy composite prepregs containing carbon nanotubes. Compos Part A-Appl Sci 42:1412–1420
Xie H, Liu B, Yuan Z, Shen J, Cheng R (2004) Cure kinetics of carbon nanotube/tetrafunctional epoxy nanocomposites by isothermal differential scanning calorimetry. J Polym Sci Pol Phys 42:3701–3712
Martone A, Faiella G, Antonucci V, Giordano M, Zarrelli M (2011) The effect of the aspect ratio of carbon nanotubes on their effective reinforcement modulus in an epoxy matrix. Compos Sci Technol 71:1117–1123
Baughman RH, Zakhidov AA, de Heer WA (2002) Carbon nanotubes—the route toward applications. Science 297:787–794
Sandler JKW, Kirk JE, Kinloch IA, Shaffer MSP, Windle AH (2003) Ultra-low electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer 44:5893–5899
Guadagno L, De Vivo B, Di Bartolomeo A, Lamberti P, Sorrentino A, Tucci V, Vertuccio L, Vittoria V (2011) Effect of functionalization on the thermo-mechanical and electrical behavior of multi-wall carbon nanotube/epoxy composites. Carbon 49:1919–1930
Deng H, Zhang R, Bilotti E, Loos J, Peijs T (2009) Conductive polymer tape containing highly oriented carbon nanofillers. J Appl Polym Sci 113:742–751
Bauhofer W, Kovacs JZ (2009) A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos Sci Technol 69:1486–1498
Balberg I, Anderson CH, Alexander S, Wagner N (1984) Excluded volume and its relation to the onset of percolation. Phys Rev B 30:3933–3940
Gojny FH, Wichmann MHG, Fiedler B, Kinloch IA, Bauhofer W, Windle AH (2006) Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer 47:2036–2045
Kapadia RS, Louie BM, Bandaru PR (2014) The influence of carbon nanotube aspect ratio on thermal conductivity enhancement in nanotube–polymer composites. J Heat Transfer 136(1–6):011303. doi:10.1115/1.4025047
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmizadeh, E., Yousefi, A.A. & Naderi, G. Effect of type and aspect ratio of different carbon nanotubes on cure behavior of epoxy-based nanocomposites. Iran Polym J 24, 1–12 (2015). https://doi.org/10.1007/s13726-014-0281-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0281-4