Abstract
A 76-year-old man was diagnosed with resectable pancreatic ductal adenocarcinoma (PDAC) of the pancreatic head. Concurrently, the patient had an approximately 2-cm cystic mass originating from the pancreatic tail. After preoperative chemotherapy for the resectable PDAC, the patient is presented with dyspnea and lower left thoracic pain. Chest X-ray revealed massive left pleural effusion, and laboratory analysis of the pleural fluid showed a very high amylase level. Computed tomography confirmed a fistula directly connecting the pancreatic tail pseudocyst to the left diaphragm. These findings suggested pancreatic-pleural fistula (PPF) from the pancreatic tail to the left pleura. Medical treatments of thoracic drainage, endoscopic pancreatic ductal drainage, and antibiotics were unsuccessful; therefore, a distal pancreatectomy, fistula closure, and thoracoscopic pleural decortication were performed before the pancreaticoduodenectomy for the PDAC. After surgery, the pleural effusion resolved and the symptoms were improved immediately. PPF is an uncommon complication in which pancreatic enzymes drain directly into the pleural cavity. Herein, we present a rare case of PPF after preoperative chemotherapy for PDAC with a review of the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic-pleural fistula (PPF) is a rare complication in which pancreatic enzymes drain directly into the pleural cavity [5]. Rupture of a pancreatic pseudocyst is the main considered cause of PPF. Its diagnosis is radiographically and biochemically confirmed. Computed tomography (CT) can confirm the fistula tract connecting the pancreas to the pleural space, which is supported by magnetic resonance cholangiopancreatography or endoscopic retrograde cholangiopancreatography (ERCP). After chest radiograph or CT presenting pleural effusion, thoracentesis is usually performed and laboratory analysis of the pleural fluid shows an amylase level greater than 1000 IU in the absence of malignant cells [7]. The confirmation of elevated pleural fluid amylase is the most important diagnostic test [14].
Patients with PPF typically present respiratory symptoms, such as shortness of breath, cough, and chest pain, which are caused by massive pleural effusion. Therefore, treatments of the pleural effusion and fistula are necessary to relieve the patient’s symptoms and to improve their general condition. Some cases have been reported to receive several treatment types, including conservative medical therapy [2], pancreatic ductal stenting [4], and surgery [6]. However, very few cases have been reported in which a pancreatic tail cyst was involved in the PPF as a complication of chemotherapy for pancreatic head cancer. In the present case, it was difficult to plan the therapeutic strategy. We report a case of PPF associated with a pancreatic tail pseudocyst in a patient with pancreatic head cancer on chemotherapy.
Case report
A 76-year-old man had a past history of type 2 diabetes, hypertension, and hyperlipidemia, and his height, body weight, and body mass index were 161 cm, 60 kg, and 23.1 kg/m2, respectively. He did not smoke or drink alcohol. The patient was diagnosed with resectable pancreatic ductal adenocarcinoma (PDAC) of the pancreatic head with metastatic lymph node of number 8a (#8a) and number 11p (#11p) indicated by abdominal enhancement CT (cT1cN1M0, cStage IIB, UICC, 8 edition). Concurrently, the patient had an approximately 2-cm cystic mass originating from the pancreatic tail, indicating a pseudocyst.
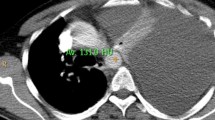
The patient received preoperative chemotherapy; gemcitabine (GEM) and tegafur gimeracil oteracil (S-1), for the resectable PDAC and was scheduled to undergo pancreaticoduodenectomy (PD). The therapy comprised of GEM at 1000 mg/m2 on day 1 and day 8 and S-1 at 100 mg per day for 14 days from day 1 to day 14 followed by 1 week off. After two courses of the preoperative chemotherapy, the patient presented with dyspnea and lower left thoracic pain. Chest X-ray revealed a massive left pleural effusion. The examination of exudative pleural fluid showed a very high amylase level (21,720 U/L). Laboratory examination showed a mild increase in inflammatory response (white blood cells of 11,200 /μL and C-reactive protein of 1.89 mg/dL) and pancreatic enzymes (amylase of 409 U/L and lipase of 803 IU/L). CT revealed a fistula directly connecting the pancreatic tail pseudocyst to the left diaphragm (Fig. 1). Endoscopic retrograde pancreatography depicted a stricture of the pancreatic head duct and a moderate dilation and destruction of the pancreatic body-to-tail duct. In addition to this feature, the fistulogram confirmed leakage from the pseudocyst into the pleural cavity (Fig. 2).
Axial (A) and coronal (B) abdominal enhanced CT views showing the 2-cm PDAC (circle) of the pancreatic head with pseudocyst (arrow head) of the pancreatic tail. The fistula (arrow) directly connected to the pancreatic tail pseudocyst extending into the left diaphragm. CT computed tomography, PDAC pancreatic ductal adenocarcinoma
On the first day of admission, a broad-spectrum antibiotic therapy was introduced, and pleural drainage was performed immediately. On the fourth day, endoscopic nasopancreatic drainage was performed into the pancreatic duct, and on the sixth day, a 5 Fr plastic stent was placed in the pancreatic duct. However, from the ninth day, pleural effusion did not decrease any further, despite continued medical treatment using thoracic drainage, pancreatic ductal drainage, and antibiotics. In addition, we considered drainage of the cyst from the stomach wall, but could not perform it, as the cyst had ruptured and shrunken. The patient’s clinical condition and nutritional state severely worsened. Total pancreatectomy (TP) was necessary for the treatment of pancreatic cancer and PPF; however, it was difficult due to the poor condition of the patient. Therefore, we performed distal pancreatectomy (DP) combined with splenectomy, fistula closure, and thoracoscopic pleural decortication for PPF.
The intraoperative features showed that the pancreas became fibrotic from chronic pancreatitis and the inflammation of pancreatic juice leakage had spread to the tail of the pancreas. The fistula was connected from the dorsal side of the pancreatic tail cyst to the left diaphragm along the retroperitoneum (Fig. 3). The fistula was transected and sutured. The splenic hilum was inflamed and adhered to the tail of the pancreas, forming a mass with the pseudocyst. Adhesive detachment around the spleen was difficult, and combined resection of the spleen was performed. The patient also had encapsulated empyema that did not improve with drainage, and the transthoracic approach was performed after the abdominal approach. From the thoracic side, the fistula extended to the left side of the aorta on the diaphragm. After surgery, the pleural effusion resolved and the symptoms were improved immediately. The patient was discharged on postoperative day 14.
Intraperitoneal surgical view of (A), the fistula, which ran from the dorsal side of the pancreatic tail along the retroperitoneum to the left diaphragm (arrow). The nelaton catheter was placed in the fistula from the thoracic side (B), and the fistula flowed to the left side of the aorta on the diaphragm (arrow head)
Pathological examination of the resected specimen showed acute and chronic pancreatitis with dilation of the pancreatic duct without any tumor, stone, or other lesions. The pancreatic duct epithelium in the pancreatic body had a steep papillary arrangement and each swelling was observed; however, no evidence of carcinoma was identified. Furthermore, necrosis and hemorrhage were found in the pseudocyst through the fistula. Therefore, the increasing pressure in the distal pancreatic duct was due to the stenosis of the pancreatic head duct caused by the tumor.
One month after the DP, radical surgery was scheduled as the patient’s general condition improved. In the abdominal cavity, there were strong adhesions between the stomach and transverse colon and around the stomach, pancreatic stump, and common hepatic artery. After adhesion peeling, we found an invasion of the tumor into the common hepatic artery, which was unresectable. Therefore the excision was called off. The patient resumed chemotherapy: GEM at 80 mg/m2 on day 1 and day 8 and S-1 at 80 mg per day from day 1 to day 14, followed by one week off, after discharge. The primary lesion has not increased, and the patient is still alive one year after last surgery without respiratory symptoms.
Discussion
PPF is a rare condition with only few reported cases. The incidence of PPF has been reported to be as low as 0.4% in patients with pancreatitis [9]. PPF usually occurs in males in their late 40 s with alcohol-induced acute or chronic pancreatitis. The causes of PPF include the rupture of a pancreatic pseudocyst (60%) and pancreatic duct (15%) due to chronic pancreatitis, and pancreatic trauma (10%). In general, chronic inflammation of the pancreas accompanied with the obstruction of the main pancreatic duct leads to the formation of a posterior pseudocyst, eventually leading to the rupture of the pseudocyst and posterior pancreatic duct [3]. In the present case, the tumor did not shrink and grew further despite preoperative chemotherapy. As a result, the stenosis of the main pancreatic duct worsened, and the pancreatic duct pressure increased further. The increasing pressure of the pancreatic duct led to the rupture of the pseudocyst, which caused PPF.
The standard of care is medical treatment, which includes octreotide, somatostatin, total parental nutrition, and antibiotics [7]. However, medical treatment is successful only in 31%–65% of patients [1]. The second line of treatment is endoscopic intervention, such as ERCP-guided stent placement, endoscopic ultrasound–guided rendezvous ERCP, or nasopancreatic drainage with stenting. Early endoscopic intervention is an effective and relatively safe treatment for PPF. Surgical treatment of PPF in patients who have failed all other treatments have a success rate of about 90% and an associated mortality rate of 6%–9%. The most common procedure is DP, followed by pancreaticojejunostomy [14]. However, it was reported that 80% of deaths in patients treated medically for internal pancreatic fistula occurred when the surgical intervention was postponed over three weeks [5]. Therefore, it is important to determine the timing of surgical treatment.
To the best our knowledge, there are only five previous reports of PPF associated with PDAC (Table 1). Our case is the first case of PPF during chemotherapy for pancreatic cancer. In the other cases, pancreatic cancer was found to be triggered by PPF. It is important to consider appropriate and prompt therapeutic strategies for PPF and PDAC. In the case of PDAC in the pancreatic body or tail, PDAC and PPF can be treated during the same surgery. On the other hand, in the case of PDAC in the pancreatic head or body, which require PD for resection, treatment for PPF should be considered carefully because total pancreatectomy (TP) is necessary for radical treatment.
In two cases among five previous reports, PDAC was located in the pancreatic head or body, requiring PD for resection. Shimaoka et al. and Miyamoto et al. reported that endoscopic intervention was successful and PDAC could be resected [12, 13]. In contrast, in the present case, the endoscopic therapeutic strategy was ineffective. TP was required because the PDAC and pseudocyst were separated, but it was impossible due to the poor general condition.
The basic treatment policy for resectable PDAC is surgery after preoperative chemotherapy. Considering that PPF occurred during chemotherapy, there was an option to perform radical resection first without chemotherapy. In light of the present case, there may have been a method of placing pancreatic duct tubes before treatment, considering the possibility of rupture or PPF with a pseudocyst. It is difficult to decide the treatment strategy, but it is necessary to treat the patient according to their condition.
Conclusion
We herein report a case of PPF after preoperative chemotherapy for PDAC. The present report is the first case of PPF during chemotherapy for pancreatic cancer. It is important to consider appropriate and prompt therapeutic strategies for PPF and PDAC for each case.
References
Bąk M, Murawa D (2020) Pancreatic-pleural fistula presenting as epigastric pain. Pol Przegl Chir 92(4):54–57
Bedingfield JA, Anderson MC (1986) Pancreatopleural fistula. Pancreas 1(3):283–290
Bishop JR et al (2003) Pancreaticopleural fistula: a rare cause of massive pleural effusion. J Pediatr Gastroenterol Nutr 36(1):134–137
Boudaya MS et al (2007) Hemothorax as the clinical presentation of a pancreaticopleural fistula: report of a case. Surg Today 37(6):518–520
Cameron JL et al (1976) Internal pancreatic fistulas: pancreatic ascites and pleural effusions. Ann Surg 184(5):587–593
Francisco E et al (2015) Pancreaticopleural fistula: an unusual complication of pancreatitis. BMJ Case Rep. https://doi.org/10.1136/bcr-2014-208814
Kord Valeshabad A et al (2018) Pancreaticopleural fistula: a review of imaging diagnosis and early endoscopic intervention. Case Rep Gastrointest Med 2018:7589451
Miyamoto T et al (2019) Cancer of the pancreatic body associated with a pancreatic pleural effusion-A case report. Suizo 34(2):106–113
Rockey DC, Cello JP (1990) Pancreaticopleural fistula. Report of 7 patients and review of the literature. Medicine (Baltimore) 69(6):332–344
Saito K et al (2020) J Soc Ibaraki Kouseiren Hosp 32:29–31 (Japanese only)
Sankarankutty M et al (1978) Adenocarcinoma of the pancreas with massive pleural effusion. Br J Clin Pract 32(10):294–297
Shimaoka S et al (2003) Minute carcinoma of the pancreas presenting as pancreatic pleural effusion. J Gastroenterol 38(9):900–904
Sugiyama Y et al (2010) A case of pancreatic carcinoma presenting as pancreaticopleural fistula with pancreatic pleural effusion. Nihon Shokakibyo Gakkai Zasshi 107(5):784–791
Tay CM, Chang SK (2013) Diagnosis and management of pancreaticopleural fistula. Singapore Med J 54(4):190–194
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or other potential conflicts of interest related to this manuscript to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Shiraishi, J., Yugawa, K., Nagata, S. et al. Pancreatic-pleural fistula from tail pseudocyst in a patient with pancreatic head cancer: a case report. Int Canc Conf J 11, 261–265 (2022). https://doi.org/10.1007/s13691-022-00555-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13691-022-00555-w