Abstract
Primary retroperitoneal serous adenocarcinoma (PRSA) is an extremely rare malignancy, with only seven cases having been previously reported. We report a case of PRSA in a 42-year-old woman treated with surgical resection and adjuvant chemotherapy. The histopathological findings of PRSA resemble those of ovarian serous carcinoma, which indicates that a combination of complete surgical resection with adjuvant chemotherapy may be the best treatment option for PRSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary retroperitoneal tumors are rare, accounting for just 0.2–0.3% of all tumors. The histology of primary retroperitoneal tumors is generally fibrosarcoma, lymphoma, teratoma, etc. However, having epithelial neoplasm occur with retroperitoneum is rare, accounting for just that only 3.1% of all primary retroperitoneum tumors [1]. A primary retroperitoneal serous adenocarcinoma (PRSA) is an extremely rare malignancy, and is a subtype of the primary serous carcinoma of the peritoneum (PSCP), with only seven other cases previously reported. In this study, we report a 42-year-old woman with PRSA who underwent a surgical resection followed by adjuvant chemotherapy consisting of a combination of paclitaxel and carboplatin.
Case report
A 42-year-old woman presented to our department with lower-left back pain. Her family history was not contributory. She had a previous history of a surgical resection of a left ovarian chocolate cyst 10 years ago. Her general appearance was thin and her body mass index (BMI) was 14.6 kg/m2. Abnormal laboratory test values included only a CA (cancer antigen) 19-9 level of 177 IU/ml, along with a CA125 level of 10 IU/ml, which is within the normal limit. Ultrasonography revealed a cystic mass 6 cm in diameter located at the lower pole of the left kidney, where it was possible that there might be some association between the tumor and left low back pain.
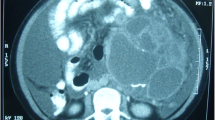
A computed tomography (CT) scan showed the tumor had a mass of 62 × 55 × 55 mm and consisted of cystic lesion and solid component in the cranial side of the tumor. A magnetic resonance imaging (MRI) of the kidney indicated high signal intensity in the T2-weighted images and low signal intensity in the T1-weighted images (Fig. 1). The cystic mass extended into the retroperitoneum and was adjacent to the lower pole of the left kidney. These findings led to a preoperative diagnosis of a malignant retroperitoneal neoplasm.
Surgical resection of the retroperitoneal cystic mass was performed. There appeared to be no involvement of the tumor with ipsilateral kidney or iliopsoas muscle. The tumor was markedly adhesive to the peritoneum and was suspected to have invaded the peritoneum, so, we performed en bloc resection including a part of the peritoneum.
The size of the resected tumor was 80 × 70 × 70 mm (Fig. 2). The resected tumor was cystic and had a well-encapsulated smooth external surface. The content of the cystic tumor was yellowish and serous fluid. The cut surface of the tumor showed a solid component in the cranial side of the tumor. No apparent hemorrhage or necrosis was present. The histopathological examination demonstrated cribriform and small alveolar proliferation of atypical columnar cells at the solid component of the resected tumor (Fig. 3). The internal cyst wall was covered by low polypoid atypical epithelium. The tumor cells were immunohistochemically positive for p53, CEA, CA125, cytokeratin (CK) 7, and Ber-EP4, while being negative for CK20. As there was no evidence of tumor tissue at the surgical margin, the tumor was defined as completely resected. On the basis of these findings, the resected tumor was histologically diagnosed as serous adenocarcinoma arising from retroperitoneum.
Serum level of CA19-9 decreased to 9 IU/ml, within the limit of normal after surgery. Because the pathological findings of the tumor were closely similar to serous adenocarcinoma of the ovary, adjuvant chemotherapy based on the regimen for ovarian cancer was performed (paclitaxel 150 mg/m2 on day 1 plus carboplatin AUC 5 on day 1, every 3 weeks for 2 cycles). After adjuvant chemotherapy, the patient has been followed up for 5 months without clinical evidence of tumor recurrence in imaging study nor the elevation of serum CA19-9 level.
Discussion
Primary serous carcinoma of the peritoneum (PSCP) is rare while serous adenocarcinoma of the ovary is a common subtype of ovarian epithelial neoplasm. Swerdlow et al. were the first to describe serous adenocarcinoma that was found in a patient with peritoneal carcinomatosis with no known primary lesion [2]. The Gynecologic Oncology Group (GOG) developed a concise set of criteria for this diagnostic category as follows [3]: (1) both ovaries must be either physiologically normal in size or enlarged by a benign process. (2) Involvement in the extraovarian sites must be greater than involvement on the surface of either ovary. (3) Microscopically, the ovarian component must be one of the following: (a) nonexistent; (b) confined to the ovarian surface epithelium with no evidence of cortical invasion; (c) involving ovarian surface epithelium and underlying cortical stroma but with any given tumor size less than 5 × 5 mm, or (d) tumor less than 5 × 5 mm within ovarian substance associated with or without surface disease. (4) The histological and cytological characteristics of the tumor must be predominantly of the serous type that is similar or identical to ovarian serous papillary adenocarcinoma, any grade.
PRSA, which is a subtype of PSCP, has been reported in only seven cases (Table 1) [4,5,6,7,8,9,10]. The clinical features and histopathological findings of PRSA resemble those of serous adenocarcinoma of the ovary. All seven of the previously reported cases were women. The details of the tumor origin in PRSA remain unclear. Some possibilities of tumor origin include coelomic metaplasia, extra-ovarian endometriosis, supernumerary ovary, teratoma, and enterogenic cyst (enteric duplication cyst or enteric cyst). However, coelomic metaplasia is the most widely accepted as tumor origin [11,12,13]. The secondary Müllerian duct hypothesis may also be applicable to the development of PRSA.
Previous studies have demonstrated four cases of PRSA completely resected including adjacent organs. The gross appearance of PRSA was cystic in three cases, while a combination of solid components with cystic lesion made up the final case. These findings suggest that PRSA tends to have a cystic pattern and localized growth in the retroperitoneum. The surgical option of a total hysterectomy and bilateral salpingo-oophorectomy is useful to rule out the possibility of the metastasis from primary gynecological malignant lesions. On the other hand, it is important to consider the possibility of excessive invasion in the case with PRSA. There has been a recent report of a subset of serous carcinoma in the ovary that was thought to originate from the fallopian tube [14]. In the present case, such possibility could not be ruled out because of the lack of hysterectomy and bilateral salpingo-oophorectomy.
Adjuvant chemotherapy was considered because PRSA pathologically resembles serous adenocarcinoma of the ovary. Kaku et al. have suggested that a combination chemotherapy consisting of docetaxel with carboplatin may be a good option for primary PRSA as the first-line chemotherapy based on the histologic similarity between retroperitoneal epithelial tumors and ovarian epithelial carcinomas [8]. The histopathological findings of the positive surgical margins, local tumor infiltration, and loco-regional lymph node involvement have been reported as risk factors [8]. In the cases with these findings, immediate chemotherapy might be recommended postoperatively.
The prognosis of PRSA remains unclear due to the rarity of reported cases. Among the seven cases previously reported, one patient died of the PRSA 24 months after initial presentation and two showed a local recurrence within 2 years of surgical treatment.
We treated a 42-year-old woman with PRSA using treatment plan that included both a surgical complete resection and adjuvant chemotherapy, although a definite therapeutic strategy for PRSA has not been established. Accumulation of evidences from further reports is required to clarify the biological characteristics and optimal management of PRSA.
References
Ichiya T, Nomura M, Mitsui S et al (2009) A case of primary retroperitoneal mucinous cystadenocarcinoma. Nihon Shokakibyo Gakkai Zasshi. 106:826–833
Swerdlow M (1959) Mesothelioma of the pelvic peritoneum resembling papillary cystadenocarcinoma of the ovary. Am J Obstet Gynecol 77:197–200
Barda G, Menczer J, Chetrit A et al (2004) National Israel Ovarian Cancer Group. Comparison between primary peritoneal and epithelial ovarian carcinoma: a population-based study. Am J Obset Gynecol 190:1039–1045
Ulbright TM, Morley DJ, Roth LM et al (1983) Papillary serous carcinoma of the retroperitoneum. Am J Clin Pathol 79:633–637
Caruncho M, Pombo F, Arnal-Monreal F (1993) Primary retroperitoneal serous cystadenocarcinoma of “ovarian type”: US and CT findings. Eur J Radiol 17:115–116
Kurosaki Y, Kuramoto K (1998) Case report: serous cystadenocarcinoma of the retroperitoneum: CT and sonographic appearance. Clin Radiol 53:916–918
Fujiwara K, Oda T, Suzuki S et al (1999) Primary serous adenocarcinoma of the retroperitoneum with a response of platinum-based chemotherapy: a case report. Int J Gynecol Cancer 9:170–172
Kaku M, Ohara N, Seima Y et al (2004) A primary retroperitoneal serous cystadenocarcinoma with clinically aggressive behavior. Arch Gynecol Obstet 270:302–306
Iura A, Sasajima Y, Katsumata N et al (2009) Serous adenocarcinoma of the retroperitoneum, as a type of multifocal mullerian carcinoma. Int J Clin Oncol 14:254–257
Arichi N, Yasumoto H, Igawa M et al (2011) A case of primary retroperitoneal serous adenocarcinoma. Int J Urol 18:844–846
Dierickx I, Jacomen G, Schelfhout V et al (2010) Primary retroperitoneal mucinous cystadenocarcinoma: a case report and review of the literature. Gynecol Obstet Invest 70:186–191
Roma AA, Malpica A (2009) Primary retroperitoneal mucinous tumors: a clinicopathologic study of 18 cases. Am J Surg Pathol 33:526–533
Tenti P, Carnevali L, Tateo S et al (1994) Primary mucinous cystadenocarcinoma of the retroperitoneum: two cases. Gynecol Oncol 55:308–312
Przybycin CG, Kurman RJ, Ronnett BM et al (2010) Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol 34:1407–1416
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kohada, Y., Teishima, J., Hattori, Y. et al. Serous adenocarcinoma of retroperitoneum: a case report. Int Canc Conf J 6, 154–157 (2017). https://doi.org/10.1007/s13691-017-0296-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13691-017-0296-8