Abstract
Purpose of review
Obesity is a multifactorial disease that is now endemic throughout most of the world. Although addressing proximate causes of obesity (excess energy intake and reduced energy expenditure) have been longstanding global health priorities, the problem has continued to worsen at the global level.
Recent findings
Numerous microbial agents cause obesity in various experimental models—a phenomena known as infectobesity. Several of the same agents alter metabolic function in human cells and are associated with human obesity or metabolic dysfunction in humans. We address the evidence for a role in the genesis of obesity for viral agents in five broad categories: adenoviridae, herpesviridae, phages, transmissible spongiform encephalopathies (slow virus), and other encephalitides and hepatitides. Despite the importance of this topic area, there are many persistent knowledge gaps that need to be resolved.
Summary
We discuss factors motivating further research and recommend that future infectobesity investigation should be more comprehensive, leveraged, interventional, and patient-centered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Simplistic explanations for obesity enjoy popular appeal, but obesity is complex and multifactorial [1]. By contrast, the germ theory of disease has faced considerable scrutiny since it was first proposed, spurring over a century of rigorous infectious disease inquiry. Likewise, the idea that infectious agents cause obesity has also faced criticism and spurred research. Thus far, numerous infectious agents have been shown to cause experimental lab animals to gain body fat (i.e., infectobesity) and serologic studies have demonstrated humans with obesity are often infected with the same agents (Table 1). For example, substantial epidemiologic evidence links adenovirus (Ad) 36 and obesity with over 10,000 subjects in a recent meta-analysis [20]. Further investigation of infectobesity is needed to understand the relevance of obesogenic infectious agents in human obesity pathophysiology, clinical obesity management, and public health.
We provide a focused overview of viruses as causative agents of obesity including overall biological plausibility of infectobesity, discussion of the theoretical selection benefits for a virus that can alter host metabolism, consideration of adipogenesis as a host adaption to infection, discourse of specific agents and mechanisms, and proposed priorities to resolve persistent knowledge gaps. Five viral agent categories with varying evidence of infectobesity causality are considered: adenoviridae, herpesviridae, phages, transmissible spongiform encephalopathies (slow virus), and other encephalitides and hepatitides.
Biological Plausibility
Infectobesity is biologically plausible when considering a pathogen’s fitness, (i.e., how well a pathogen survives and propagates). For instance, cytomegalovirus (CMV) reprograms glucose and lipid metabolism through multiple mechanisms to enhance biosynthesis of the viral envelope and other substrates for viral progeny [21, 22], and interruption of the cellular effect via cholesterol lowering medication inhibits production of substrates and viral particles [23]. Likewise, Ad-5 enhances host glycolytic enzyme activity that increases nucleotide biosynthesis for progeny [24, 25]. Similarly, gut microbes can metabolize dietary fat into acetate, and acetate causes rodents to eat more fat [26], which theoretically should promote survival of microbes best suited for digesting fat.
Symbiotic host selection is also a theoretical possibility: that is, if an obesogenic host phenotype is selected, then a virus conferring this phenotype could be simultaneously selected. Recent livestock domestication includes aggressive selection for metabolic features like efficient fat gain; when an organism (e.g., heaviest cow) is selected, some of its associated microbiota are also selected [9••]. Although there is uncertainty about how artificial selection of livestock impacted livestock microbiota, the proposition is not entirely theoretical. For instance, there is evidence that numerous infectobesity agents have livestock reservoirs [9••]. By similar logic, purposeful selection of microbes associated with obesity (or other traits) could provide an experimental model for selecting infectobesity agents and has been described elsewhere as “pawnobe” evolution [9••].
A third perspective on biological plausibility is that host immune responses could be adaptive in the short term but promote obesity risk over the long term if adipose tissue has multiple biological functions. Although energy storage is the most visible purpose for adipocytes (i.e., fat cells), the immunologic roles of adipose tissue are also noteworthy. For instance, some have proposed adipocytes as the origin of the adaptive immune system in vertebrates [27]. In fact, there is evidence of plasticity between macrophages and adipocytes bearing similar cellular markers, sharing the same stem cell lineage, and executing similar functions [28]. Macrophages enlarge as they collect and engulf pathogens or cellular debris during phagocytosis, and adipocytes sequester excess glucose and lipophilic toxins and engage in antimicrobial functions. For instance, the presence of a pathogen (Staphylococcus aureus) leads to acute expansion of local adipose tissue (increased size and number of adipocytes) and elaboration of an antimicrobial peptide (cathelicidin) and experimentally blocking this function made mice more vulnerable to pathogen invasion [29, 30]. Adipocytes also modulate immunologic cell activity (e.g., CD4+ T cells) via fatty acid release [31]. At the same time, the localized benefit of expanded adipose tissue could be outweighed by obesity risk in a chronic infection with ongoing systemic inflammation. Human studies have shown obesity is independently associated with systemic inflammation [32, 33], and the total burden of multiple chronic infections has been associated with body fat among men [34].
Adenoviridae
Adenoviridae is the most widely investigated adipogenic viral family. Systematic reviews and meta-analyses of human epidemiologic data show a robust association between Ad-36 viral antibodies [20, 35, 36] and human obesity. A systematic review of adipogenesis evidence among Ad-5, Ad-9, Ad-31, and Ad-37 has also been published [2•]. The evidence can be divided into laboratory, animal, and epidemiologic categories [37•]. Adipocyte progenitor 3T3-L1 cell infection with Ad-9, Ad-31, Ad-36, and Ad-37 in vitro enhances adipogenesis with more lipid content, more numerous adipocytes, and/or more commitment to an adipocyte lineage. Ad-36 is adipogenic in Colo-320 cells [38]. Ad-5 is glycolytic in epithelial cells and adipogenic in Colo-320 cells, but not 3T3-L1 cells [2•, 3•, 24, 38].
Animal experiments have demonstrated Ad-5 and Ad-36 infection cause rodents to gain body fat [39, 40], and Ad-36, Ad-37 (compared to Ad-2), and SMAM-1 infections cause chickens to gain body fat, but not Ad-31 infection [41,42,43]. Ad-36-infected chickens also transmitted an obesogenic phenotype to cage mates and to blood transfusion recipients [41]. Observational (natural) Ad-36 infection in non-human primates was positively correlated with weight gain in one study that identified time of infection [44] while another small study comparing primates with any prior exposure to Ad-36 showed non-significant differences in body weight, but persistent differences in glycemia [45]. Non-human primate infection with adenovirus caused substantial increases in body fat shortly after infection [44].
Epidemiologically, the association between adenoviridae and obesity in humans is stronger among children with Ad-5 and Ad-36 [2•, 35, 46] while Ad-8 [2•] also had a similar trend toward obesity among children but no association among adults for Ad-5 or Ad-37; however, association between Ad-36 and obesity is also present in adults in meta-analyses conducted by different international groups including a combined analysis of over 10,000 (mostly adult) subjects [20, 35, 36]. Additionally, Ad-31 has a non-statistically significant association of similar magnitude as Ad-36 in adults, and antibody to a non-human adenovirus SMAM-1 was associated with human body mass index in adults [2•, 47].
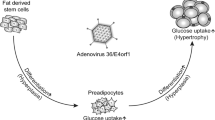
The mechanisms by which Ad-36 promotes adipogenesis are known. The presence of viral protein early 4 open reading frame 1 (E4-ORF1) is necessary and sufficient for acute adipogenic effects [2•]. The virus activates the so-called master-switch of adipocyte development, peroxisome proliferator-activated receptor-γ (PPARγ), signaling adult stem cells to become adipocytes [48, 49]. Ad-36 causes cells to express glucose transporters (Glut4 & Glut1) by activating the Ras pathway (upregulating phosphatidal inostitol 3-kinase) even without insulin signaling [50,51,52]. Glucose transporters bring glucose into the cell and upregulated fatty acid synthase rapidly converts additional glucose to fatty acids [53], meaning adipocytes are larger and more abundant shortly after infection.
Chronic adipogenic mechanisms are also understood as infected cells show lower fat oxidation [2•, 54] and hormone alterations in insulin and leptin occur [53, 55]. Leptin hormone ordinarily provides negative feedback on appetite after lipid accumulation, but infected adipocytes secrete less leptin [53]. Insulin regulates glucose metabolism and humans with a history of Ad-36 infection have a lower concentration of insulin [55]. Additionally, Ad-36 infection causes systemic inflammation; a knock-out study in mice showed that an inflammatory protein, monocyte chemoattractant protein 1 (MCP-1), is necessary for maintaining Ad-36-induced obesity [56]. Studies using mice with genetic deletions have demonstrated the importance of MCP-1 and PPAR-γ for obesity and insulin resistance in mice without infection [2•, 57], so this is also a plausible mechanism for obesity maintenance in Ad-36 infections. This is supported by a study which showed lowering MCP-1 with anti-inflammatory mulberry extract reduced body fat in Ad-36 infected mice [58].
Herpesviridae
At least two herpesviridae, cytomegalovirus (CMV) and herpes simplex virus 1 (HSV-1), appear to be lipogenic. CMV has been connected with several components of metabolic syndrome, including higher blood pressure after infection [4] and metabolic dysfunction among the general population without obesity [5]. In vitro, CMV alters fatty acid synthase and other metabolic enzymes, engorging the host cell with lipid as suggested by the name “cytomegalovirus” [3•, 21, 22]. CMV fitness is influenced by metabolic changes and statin medication reduced the production of progeny [23]. Cross-sectional association between HSV-1 and obesity has been identified in some studies [34, 59, 60], and there are links between total burden of infections, inflammation, and obesity [34, 61, 62]. One study found HSV-1 and CMV were connected to central obesity in women, but not men, suggesting a mechanism of chronic inflammation or other host factors [62]. The genetic polymorphism predisposing individuals to HSV-1 infection is associated with higher body mass, arguing against reverse causation (i.e., genetic susceptibility to herpes necessarily comes before obesity) [59].
In vitro evidence supports an adipogenic role for HSV-1. The HSV60 segment of synthetic HSV-1 DNA enhances proliferation of adipose cells while reducing leptin release [63], similar to AD-36. HSV-1 also alters cellular metabolic processes related to glucose and glycolysis [3•]. It is likely that Kaposi sarcoma herpes virus (HHV8) is also lipogenic [3•] in vitro, but an association with human obesity has not been identified.
Phages
Research has shown that the gut microbiome, including bacteria, viruses, and other microscopic organisms, likely have a causal influence on host body weight. A stool transfer from a heavier human twin caused mice to gain weight [6]. The mice (like Ad-36 infected chickens) also transferred a stool-derived metabolic phenotype to cage mates [6]. In human adults, numerous probiotic formulations cause modest weight loss [64, 65] although the quality and size of the studies were limited. Finally, several Bacillus spp.-derived probiotics cause commercially favorable weight gain in multiple agricultural animals [66,67,68].
Potential mechanisms for the microbiome to influence weight are numerous. Whole-genome analysis at the strain level correlated host obesity with Lactobacillus genes for oxidative stress and glycolysis [69]. Acetate production from microbiota caused hyperphagia and obesity among rodents through a parasympathetic nervous system pathway [26]. Non-specific inflammatory mechanisms similar with adenoviridae and herpesviridae are also possible. Bacterial translocation could contribute to low-grade inflammation: lipopolysaccharide binding protein (translocation marker) serum concentration was associated with overweight and obesity in a population-based study [70]. Additionally, a probiotic has been shown to prevent the inflammatory infiltration of macrophages into adipose tissue that is a feature of the persistence of Ad-36 obesity in mice [56, 71].
Despite rapidly emerging causal evidence, findings are modest, and meta-analysis of observational data shows there are relatively weak correlations between bacterial taxa and obesity across studies [72]. Phages are more diverse than the bacteria they infect and represent the most numerous biologic group known [73], and so, the stool virome could be important in fat gain and obesity. Phage in human stools is capable of shifting the dynamics of the gut microbial ecosystem in vivo [73], and host stress may affect phage composition of gut microbiota [7]. The role of phages in obesity transmission was identified in mice which like humans gain weight after treatment with risperidone, an antipsychotic medicine [8]. Risperidone depresses energy expenditure, inducing weight gain in mice. Stool transplanted from risperidone-treated mice causes transference of both weight gain and depressed energy expenditure. Phage isolated by filtration from among gut microbes was sufficient for lowering energy expenditure and causing weight gain in recipient mice [8].
Transmissible Spongiform Encephalopathies
Numerous transmissible spongiform encephalopathies (TSEs) exist that transmit obesity to at least one host species [9••]. The specific TSE agent is often described as a “prion” or “slow virus.” Controversy exists over whether a misfolded protein or its associated 25-nm viral particle is the transmissible agent [74]. Recent evidence using nuclease and keratinase indicates misfolded proteins are not sufficient to transmit TSEs while nucleic acids are sufficient; therefore, including TSEs in our discussion of viruses is appropriate [75, 76]. This class of transmissible agent also shares features with adenoviridae (Table 1—livestock reservoirs, primate experiments, and human observational evidence). The neurodegenerative disorder Kuru is commonly associated with obesity and bulimia in humans in early stages of disease [10]. A Creutzfeld Jakob disease (CJD) agent variant (263 K-sc) with lower virulence causes “extreme obesity” in mice [11], and several scrapie agents cause hyperphagia, fat gain, and/or weight gain in some mouse strains [12,13,14]. Infection with a high dose of bovine spongiform encephalopathy has a different time course for the metabolic and neurologic manifestations in non-human primates [15, 16]. In a feeding study, the agent initially remained confined in the gut and caused rapid weight gain within 1.5 years that was not seen in controls [15]. The mechanism of weight gain appears related to interaction between the agent and gut endocrine cells [15], but pancreatic involvement has also been observed [16, 77], perhaps related to a type 2 diabetes phenotype, suggesting the possibility of endocrine mechanisms. Further, adrenalectomy prevents mice from gaining weight with another TSE (scrapie) and infection of the hypothalamus augments weight gain, suggesting a hormonal pathway through the hypothalamus-pituitary-adrenal axis [14].
Fatal CJD and kuru are relatively rare in humans, and the possibility of TSE relevance in human obesity at a population level might be dismissed because of the prevalence of human TSEs. However, genetic susceptibility to CJD has shown a modeled penetrance of 0.96 after age 80, which may reflect ubiquitous infection or a form of CJD that is not horizontally transmitted [78]. The prevalence, incidence, and other epidemiologic features of TSE-associated viruses are unknown in humans. Evidence suggests neurologic manifestations are eventually 100% with a very high dose of infectious agent (≥5 g), but infection rates after feeding low dose agent (0.05 and 0.005 g) are less clear, perhaps because of longer incubation [16]. CJD (variant 263 K-sc) causing “extreme obesity” in mice required a 17-times higher dose for GT1 cells to demonstrate prion proteins [11], and the initial stages of primate infection appear confined to the gut [15]. Even if these agents are not relevant for human obesity at a population level, they demonstrate further proof of concept with causal evidence that infectious agents are able to cause rapid weight gain [15] and alter host eating behavior [14].
Other Encephalitides and Hepatitides
Other known examples of viral infectobesity occur in animals with pathogens of the brain or liver [17]. Because these organs have numerous roles in metabolic regulation, identifying human pathogens could be important. Adenoviridae [79, 80] are also known to infect the liver. Hepatitis C virus (HCV) alters cellular metabolism to upregulate fatty acid synthase and glycolosis similar to some adenoviridae and herpesviridae [3•]. Human studies have shown an association between HCV, insulin resistance, and/or metabolic syndrome possibly related to hepatic steatosis (appearing worse with genotype 3) [18]. Other examples include the following: Borna disease virus (BDV) infects the hypothalamus and causes obesity in mice [17, 81]; Rous associated virus 7 (RAV7) causes obesity, ataxia, and liver steatosis in chickens [17, 82]; and canine distemper virus (CDV) alters brain catecholamine pathways in mice [83] and causes enlarged adipocytes [17].
The Importance of the Infectobesity Field in More Effective Obesity Management
Tremendous progress has been made in infectobesity research, but social factors motivating the field are important considerations for appropriately prioritizing future research. Specifically, further understanding of viral etiologies could help counter obesity bias, reinforce public health approaches, and reinforce the value of biological science in the investigation of obesity.
First, public stigma against those afflicted with disease is often pervasive, particularly when there are physical manifestations (e.g., strabismus) [84]. With obesity, there are also behavioral causes, so bias is often more explicit. For instance, one scholar advocated increasing social pressure on people with obesity as a weight loss incentive [85]. However, those reporting perceptions of stigma gain more weight [86], while a socially supportive intervention improves hunger and eating behaviors [87].
Some suggest infectobesity sounds like a “lame excuse” for those who struggle with their weight [88], but biologic insight by itself should not remove the need for healthy behavior. To the contrary, providing information about personal obesity risk appears to increase healthy behavioral intentions regardless of how the causal pathway of the risk is described [89]. On the other hand, if infections were a more widely accepted factor in the development of obesity, an attributional justification for social stigma is reduced and more empathy and greater access to available treatments could follow.
Infectious etiologies of obesity also argue in favor of descriptions of obesity as an “epidemic” (or pandemic/endemic), even though these terms are often applied to characterize infectious diseases. More broadly, infectious etiologies have well established public health approaches for control. An important insight for human immunodeficiency virus treatment was that “treatment is prevention,” suggesting that the whole population benefits when one case is medically treated [90]. Network dynamics also appear relevant for the spread of obesity, and these patterns could be used to prioritize obesity interventions [91].
Researching viral causes of obesity could lead to greater insight about the root causes of obesity within a biological framework related to host and agent adaptations. While obesity is multifactorial, oversimplification based on a physics-based thermodynamic model rather than a biological model has prevailed for over 90 years [92]. Both physics and biology have tremendous utility, but focusing on energy balance alone obscures the complex reasons for these behaviors and could limit the condition to the mathematics of these behaviors.
Some have advocated more tailored treatments for obesity subtypes that might respond differently to different treatments [93], and this also argues in favor of finding all the underlying etiologies, including infectious ones. Yet, current treatment of obesity is not cause-specific [93]. A recent example of an effective cause-specific intervention is the treatment of an obesogenic genetic defect, proopiomelanocortin deficiency, using melanocortin-4 receptor agonist [94] with excellent results.
Developing targeted antimicrobial agents or vaccines against microbes contributing to obesity could also be individualized. Spiramycin, an antibiotic used for toxoplasma in pregnancy [95], reduces adipogenesis in 3T3-L1 cells in vitro by reducing PPARγ and Glut-4 and ameliorating fat gain in mice on a high-fat diet [96]. This same pathway is activated by Ad-36, and this agent could be investigated as a targeted therapy for addressing obesity associated with viral infection.
Frameworks and Future Studies
Future investigation into infectobesity research gaps should carry a high priority. Several frameworks have been proposed to understand the causal role of infectious agents in human disease. Koch’s postulates from 1890 still provide useful criteria for determining causal inferences, and some agents meet some of these postulates. However, in multifactorial diseases like obesity, it is unlikely that a single infectious agent is solely responsible for obesity. Any uninfected group used for comparisons may still develop obesity due to other contributors, including other microbes. Using pathogenic viruses to unequivocally determine causality in humans is generally impermissible from an ethical perspective (vaccine trials could be an exception).
In 2011, Dhurandhar proposed a more accommodating framework for identifying putative obesogen agents based on Ad-36, where three evidence categories (in vitro, animal, and human epidemiology) are used to draw obesogenic inferences [37•]. The framework is a valid starting point, and this review has focused on evidence from each of these three tiers for each category of virus reviewed. Since 2011, it has become clear that human epidemiology studies on this topic are difficult to interpret when considering a single virus in a randomly selected cohort for numerous, empirically supported reasons:
-
Infection with one virus is non-randomly related to infection with other viruses because of co-infections, cross reactivity, host resistance, confounding, and/or other factors
-
Many important adipogenic viruses likely have not been identified; only a handful of over 60 adenoviruses have been investigated, and many of the uninvestigated viruses are genetically similar and common in seroepidemiology studies [2•].
-
Serostatus is not necessarily maintained over time, which has been documented for Ad-36 [97], so those without antibody at a given time point might still have had prior infection.
-
Site and route of infection appear to make a difference in host phenotype for numerous infectious agents, and there is evidence they also matter for adipogenic viruses [14].
-
Host factors appear to play a role in response to infection as empirically demonstrated using meta-regression on the association between Ad-36 and obesity among children and adults [20, 35]. Although significant in both populations, the magnitude of association is stronger in children (odds ratio [OR] = 2.3, p < 0.001 vs. OR = 1.8, p = 0.005) [20]. Insulin is involved in weight gain and weight loss, and a causal change in insulin sensitivity would be expected to promote weight gain in a naturalistic setting while promoting weight loss during a diet. Such an interaction by dieting status would be consistent with several empiric observations in longitudinal Ad-36 studies [55, 98, 99].
-
There is high risk that the “Rose paradox” could play a role in observations at a population level because of host-agent interactions. That is, an individual might experience an inflammatory response to a particular virus and develop obesity even if the particular viral strain is not the most common cause of inflammation (or obesity) throughout the population.
These limitations suggest the need for carefully designed research with probative value [100]. To advance the field at a faster rate, we propose four specific priorities to make infectobesity research more comprehensive, leveraged, interventional, and patient centered.
Several new technologies exist that could enable more comprehensive assessment of potentially adipogenic agents, including metagenomic (agnostic) methods of next-generation sequencing and comprehensive virome characterization based on synthetic proteins [101]. Meta-genomics are necessary to identify phage, and thus far, we are not aware of any comprehensive assessment of phage and obesity correlation. Only within the last 3 years has meta-genomics characterized one of the most abundant biologic agents within the gut microbiome [102]. This tool could be applied to investigate agents in tissues outside the gut (e.g., liver, brain, or adipose tissue). Outcomes should also be assessed comprehensively as there can be beneficial or harmful adipogenic agents. For instance, E4-ORF1 may have beneficial properties for glucose disposal [50].
Other resources could also be leveraged such as emerging bioinformatics tools. We have previously leveraged pattern recognition to prioritize future investigation [2•]. Molecular basic research shows Ad-36 E4-ORF1 is necessary and sufficient for an adipogenic effect, and a public database (e.g., NCBI Blast) can be used to identify other viruses with nucleic acid homology for this gene. Comparison with seroepidemiology of adenoviruses allowed us to identify the agents with a similar gene and an abundant prevalence in population level studies. Likewise, bioinformatic tools may also predict tissue tropism, glycolytic/lipogenic genes, and inflammatory host response among common chronic viruses so that a more comprehensive catalog of potential adipogenic viruses could be identified and empirically validated.
Stronger causal inferences are possible with interventional studies and can be accomplished with emerging observational designs that incorporate an element of randomization [103], fully randomized platform trials [104], or traditional randomized clinical trials of vaccines. Even if many viruses contribute to obesity, recent vaccines have used polyvalent antigens (e.g., 9-valent HPV vaccine [105]) to create broad immunity. DNA viruses like adenoviruses in particular are readily vaccine preventable [106] since they have relative genomic stability [107] and a bivalent oral vaccine induced antibody with some cross protection to multiple adenovirus strains [108]. We proposed iterative selection and transfer of whole compartments of microbes to germ-free hosts in a process called pawnobe evolution [9••], which could allow causal inferences because transfers are interventions. With each transfer, any microbes within the compartment that confer an extreme phenotype should be selected and refined over time, and inferences about the microbial genetic changes responsible for these host traits could also be investigated as the microbes evolve.
Finally, future studies should be more patient centered. Many fields make progress after there is sufficient public interest in at least one application from the research. Some evidence suggests Ad-36 testing could help tailor a diet intervention because the Mediterranean diet is known to be more effective among the infected [98]. Similarly, gut microbe characterization has been used to tailor dietary recommendations with some utility [109]. Anti-inflammatory mulberry extract has been successful in treating mice with Ad-36 obesity, and it could plausibly counteract numerous sources of inflammation [58]. Combining a rigorous interventional design as described above with an anti-inflammatory therapy could provide faster translation to the patient than other novel basic science discoveries.
Conclusions
Overall, there is progress and persistent knowledge gaps in the infectobesity field. The theoretical and empirical support for the biological plausibility of infectobesity has strengthened. There are shared patterns emerging with the five main categories of agents including inflammatory and endocrine pathways in the liver and adipose tissue. Additionally, the priorities for future investigation are becoming clearer. Focusing on early clinical interventions could expand the interest and opportunities for investigating the many questions related to infectobesity, while human epidemiology comes with numerous limitations.
References
Papers of particular interest, published recently, are highlighted as: • Of importance •• Of significant importance
Dhurandhar EJ, Keith SW. The aetiology of obesity beyond eating more and exercising less. Best Pract Res Clin Gastroenterol. 2014;28(4):533–44.
• Voss JD, Atkinson RL, Dhurandhar NV. Role of adenoviruses in obesity. Rev Med Virol. 2015;25(6):379–87. Recent review of several viral etiologies of obesity, including a review of adenoviridea seroprevalence, and homology of E4-ORF-1 with Ad-36.
• Sanchez EL, Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479-480:609–18. Reviews numerous viruses that alter cellular metabolism and are lipogenic.
Cheng J, Ke Q, Jin Z, Wang H, Kocher O, Morgan JP, et al. Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog. 2009;5(5):e1000427.
Hamer M, Batty GD, Kivimäki M. Obesity, metabolic health, and history of cytomegalovirus infection in the general population. J Clin Endocrinol Metab. 2016;101(4):1680–5.
Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1251214.
Mills S, Shanahan F, Stanton C, Hill C, Coffey A, Ross RP. Movers and shakers: influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes. 2013;4(1):4–16.
Bahra SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CM, et al. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine. 2015;2(11):1725–34.
•• Voss JD, Leon JC, Dhurandhar NV, Robb FT. Pawnobiome: manipulation of the hologenome within one host generation and beyond. Front Microbiol. 2015;6:697. Discusses evolutionary perspective on manipulating host-microbe interactions and addresses evidence for the emergence of infectobesity in agriculture.
Collinge J, Whitfield J, McKintosh E, Frosh A, Mead S, Hill AF, et al. A clinical study of kuru patients with long incubation periods at the end of the epidemic in Papua New Guinea. Philos Trans R Soc Lond B Biol Sci. 2008;363(1510):3725–39.
Manuelidis L, Chakrabarty T, Miyazawa K, Nduom NA, Emmerling K. The kuru infectious agent is a unique geographic isolate distinct from Creutzfeldt-Jakob disease and scrapie agents. Proc Natl Acad Sci U S A. 2009;106(32):13529–34.
Kim YS, Carp RI, Callahan SM, Wisniewski HM. Scrapie-induced obesity in mice. J Infect Dis. 1987;156(2):402–5.
Carp RI, Callahan SM, Sersen EA, Moretz RC. Preclinical changes in weight of scrapie-infected mice as a function of scrapie agent-mouse strain combination. Intervirology. 1984;21(2):61–9.
Kim YS, Carp RI, Callahan SM, Wisniewski HM. Adrenal involvement in scrapie-induced obesity. Proc Soc Exp Biol Med. 1988;189(1):21–7.
Strom A, Yutzy B, Kruip C, Ooms M, Schloot NC, Roden M, et al. Foodborne transmission of bovine spongiform encephalopathy to non-human primates results in preclinical rapid-onset obesity. PLoS One. 2014;9(8):e104343.
Holznagel E, Yutzy B, Schulz-Schaeffer W, Kruip C, Hahmann U, Bierke P, et al. Foodborne transmission of bovine spongiform encephalopathy to nonhuman primates. Emerg Infect Dis. 2013;19(5):712–20.
Ponterio E, Gnessi L. Adenovirus 36 and obesity: an overview. Viruses. 2015;7(7):3719–40.
Kralj D, Virović Jukić L, Stojsavljević S, Duvnjak M, Smolić M, Čurčić IB. Hepatitis C virus, insulin resistance, and steatosis. J Clin Transl Hepatol. 2016;4(1):66–75.
Carbone KM. Borna disease virus and human disease. Clin Microbiol Rev. 2001;14(3):513–27.
Xu MY, Cao B, Wang DF, Guo JH, Chen KL, Shi M, et al. Human adenovirus 36 infection increased the risk of obesity: a meta-analysis update. Medicine (Baltimore). 2015;94(51):e2357.
Yu Y, Maguire TG, Alwine JC. ChREBP, a glucose-responsive transcriptional factor, enhances glucose metabolism to support biosynthesis in human cytomegalovirus-infected cells. Proc Natl Acad Sci U S A. 2014;111(5):1951–6.
Yu Y, Maguire TG, Alwine JC. Human cytomegalovirus infection induces adipocyte-like lipogenesis through activation of sterol regulatory element binding protein 1. J Virol. 2012;86(6):2942–9.
Potena L, Frascaroli G, Grigioni F, Lazzarotto T, Magnani G, Tomasi L, et al. Hydroxymethyl-glutaryl coenzyme A reductase inhibition limits cytomegalovirus infection in human endothelial cells. Circulation. 2004;109(4):532–6.
Thai M, Graham NA, Braas D, Nehil M, Komisopoulou E, Kurdistani SK, et al. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19(4):694–701.
Miyake-Stoner SJ, O’Shea CC. Metabolism goes viral. Cell Metab. 2014;19(4):549–50.
Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–7.
van Niekerk G, Engelbrecht AM. On the evolutionary origin of the adaptive immune system—the adipocyte hypothesis. Immunol Lett. 2015;164(2):81–7.
Chazenbalk G, Bertolotto C, Heneidi S, Jumabay M, Trivax B, Aronowitz J, et al. Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity. PLoS One. 2011;6(3):e17834.
Miller LS. Adipocytes armed against Staphylococcus aureus. N Engl J Med. 2015;372(14):1368–70.
Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347(6217):67–71.
Ioan-Facsinay A, Kwekkeboom JC, Westhoff S, Giera M, Rombouts Y, van Harmelen V, et al. Adipocyte-derived lipids modulate CD4+ T-cell function. Eur J Immunol. 2013;43(6):1578–87.
Ndumele CE, Nasir K, Conceiçao RD, Carvalho JA, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31(8):1927–32.
Al Rifai M, Silverman MG, Nasir K, Budoff MJ, Blankstein R, Szklo M, et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239(2):629–33.
Fernández-Real JM, Ferri MJ, Vendrell J, Ricart W. Burden of infection and fat mass in healthy middle-aged men. Obesity (Silver Spring). 2007;15(1):245–52.
Shang Q, Wang H, Song Y, Wei L, Lavebratt C, Zhang F, et al. Serological data analyses show that adenovirus 36 infection is associated with obesity: a meta-analysis involving 5739 subjects. Obesity (Silver Spring). 2014;22(3):895–900.
Yamada T, Hara K, Kadowaki T. Association of adenovirus 36 infection with obesity and metabolic markers in humans: a meta-analysis of observational studies. PLoS One. 2012;7(7):e42031.
• Dhurandhar NV. A framework for identification of infections that contribute to human obesity. Lancet Infect Dis. 2011;11(12):963–9. Provides a framework for identifying and investigating infectobesity.
Sanlidag T, Akcali S, Vatansever S, Cicek C, Sayan M, Cakmakliogullari EK, et al. Investigation of adipogenic effects of human adenovirus serotypes 36 and 5 in a Colo-320 cell line. Futur Virol. 2013;8(6):617–22.
So PW, Herlihy AH, Bell JD. Adiposity induced by adenovirus 5 inoculation. Int J Obes (Lond). 2005;29(6):603–6.
Pasarica M, Shin AC, Yu M, Ou Yang HM, Rathod M, Jen KL, et al. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity (Silver Spring). 2006;14(11):1905–13.
Dhurandhar NV, Israel BA, Kolesar JM, Mayhew G, Cook ME, Atkinson RL. Transmissibility of adenovirus-induced adiposity in a chicken model. Int J Obes Relat Metab Disord. 2001;25(7):990–6.
Whigham LD, Israel BA, Atkinson RL. Adipogenic potential of multiple human adenoviruses in vivo and in vitro in animals. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R190–4.
Dhurandhar NV, Kulkarni P, Ajinkya SM, Sherikar A. Effect of adenovirus infection on adiposity in chicken. Vet Microbiol. 1992;31(2-3):101–7.
Dhurandhar NV, Whigham LD, Abbott DH, Schultz-Darken NJ, Israel BA, Bradley SM, et al. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr. 2002;132(10):3155–60.
Dhurandhar NV, Dhurandhar EJ, Ingram DK, Vaughan K, Mattison JA. Natural infection of human adenovirus 36 in rhesus monkeys is associated with a reduction in fasting glucose 36. J Diabetes. 2014;6(6):614–6.
Cakmakliogullari EK, Sanlidag T, Ersoy B, Akcali S, Var A, Cicek C. Are human adenovirus-5 and 36 associated with obesity in children? J Investig Med. 2014;62(5):821–4.
Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL. Association of adenovirus infection with human obesity. Obes Res. 1997;5(5):464–9.
Pasarica M, Mashtalir N, McAllister EJ, Kilroy GE, Koska J, Permana P, et al. Adipogenic human adenovirus Ad-36 induces commitment, differentiation, and lipid accumulation in human adipose-derived stem cells. Stem Cells. 2008;26(4):969–78.
Floyd ZE, Stephens JM. Controlling a master switch of adipocyte development and insulin sensitivity: covalent modifications of PPARγ. Biochim Biophys Acta. 2012;1822(7):1090–5.
Dhurandhar EJ, Dubuisson O, Mashtalir N, Krishnapuram R, Hegde V, Dhurandhar NV. E4orf1: a novel ligand that improves glucose disposal in cell culture. PLoS One. 2011;6(8):e23394.
Dubuisson O, Dhurandhar EJ, Krishnapuram R, Kirk-Ballard H, Gupta AK, Hegde V, et al. PPARgamma-independent increase in glucose uptake and adiponectin abundance in fat cells. Endocrinology. 2011;152(10):3648–60.
Dhurandhar NV. Insulin sparing action of adenovirus 36 and its E4orf1 protein. J Diabetes Complications. 2013;27(2):191–9.
Vangipuram SD, Yu M, Tian J, Stanhope KL, Pasarica M, Havel PJ, et al. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int J Obes (Lond). 2007;31(1):87–96.
Wang ZQ, Yu Y, Zhang XH, Floyd EZ, Cefalu WT. Human adenovirus 36 decreases fatty acid oxidation and increases de novo lipogenesis in primary cultured human skeletal muscle cells by promoting Cidec/FSP27 expression. Int J Obes (Lond). 2010;34(9):1355–64.
Lin WY, Dubuisson O, Rubicz R, Liu N, Allison DB, Curran JE, et al. Long-term changes in adiposity and glycemic control are associated with past adenovirus infection. Diabetes Care. 2013;36(3):701–7.
Na HN, Nam JH. Adenovirus 36 as an obesity agent maintains the obesity state by increasing MCP-1 and inducing inflammation. J Infect Dis. 2012;205(6):914–22.
Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20.
Na HN, Park S, Jeon HJ, Kim HB, Nam JH. Reduction of adenovirus 36-induced obesity and inflammation by mulberry extract. Microbiol Immunol. 2014;58(5):303–6.
Bassols J, Moreno JM, Ortega F, Ricart W, Fernandez-Real JM. Characterization of herpes virus entry mediator as a factor linked to obesity. Obesity (Silver Spring). 2010;18(2):239–46.
Karjala Z, Neal D, Rohrer J. Association between HSV1 seropositivity and obesity: data from the National Health and Nutritional Examination Survey, 2007-2008. PLoS One. 2011;6(5):e19092.
Vahdat K, Azizi F, Zandi K, Assadi M, Nabipour I. Chronic inflammation is correlated with percentage of body fat independent of the burden of infection. Inflammation. 2012;35(4):1322–9.
Schooling CM, Jones HE, Leung GM. Lifecourse infectious origins of sexual inequalities in central adiposity. Int J Epidemiol. 2011;40(6):1556–64.
Yu L, Liu P, Liu Z, Zhu W, Yan K, Chen Q, et al. p204-mediated innate antiviral responses in mouse adipose cells and their effects on cell functions. Immunol Cell Biol. 2015;93(2):147–57.
Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res. 2015;35(7):566–75.
Dror T, Dickstein Y, Dubourg G, Paul M. Microbiota manipulation for weight change. Microb Pathog. 2016. doi:10.1016/j.micpath.2016.01.002.
Lei X, Piao X, Ru Y, Zhang H, Péron A, Zhang H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian-Australas J Anim Sci. 2015;28(2):239–46.
Le O, Dart P, Harper K, Zhang D, Schofield B, Callaghan M, et al. Effect of probiotic Bacillus amyloliquefaciens strain H57 on productivity and the incidence of diarrhoea in dairy calves. Anim Prod Sci. 2016. doi:10.1071/AN15776. Accessed 22 Aug 2016.
Balasubramanian B, Li T, Kim IH. Effects of supplementing growing-finishing pig diets with Bacillus spp. probiotic on growth performance and meat-carcass grade quality traits. R Bras Zootec. 2016;45(3):93–100.
Drissi F, Merhej V, Angelakis E, El Kaoutari A, Carrière F, Henrissat B, et al. Comparative genomics analysis of Lactobacillus species associated with weight gain or weight protection. Nutr Diabetes. 2014;4:e109.
Gonzalez-Quintela A, Alonso M, Campos J, Vizcaino L, Loidi L, Gude F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS One. 2013;8(1):e54600.
Ukibe K, Miyoshi M, Kadooka Y. Administration of Lactobacillus gasseri SBT2055 suppresses macrophage infiltration into adipose tissue in diet-induced obese mice. Br J Nutr. 2015;114(8):1180–7.
Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. MBio. 2016;7(4):e01018-16.
Reyes A, Wu M, McNulty NP, Rohwer FL, Gordon JI. Gnotobiotic mouse model of phage-bacterial host dynamics in the human gut. Proc Natl Acad Sci U S A. 2013;110(50):20236–41.
Manuelidis L. A 25 nm virion is the likely cause of transmissible spongiform encephalopathies. J Cell Biochem. 2007;100(4):897–915.
Botsios S, Manuelidis L. CJD and scrapie require agent-associated nucleic acids for infection. J Cell Biochem. 2016;117(8):1947–58.
Miyazawa K, Emmerling K, Manuelidis L. High CJD infectivity remains after prion protein is destroyed. J Cell Biochem. 2011;112(12):3630–7.
Strom A, Yutzy B, Kruip C, Völker I, Schloot NC, Roden M, et al. Spontaneous obesity-linked type 2 diabetes in the absence of islet amyloid in a cynomolgus monkey infected with bovine spongiform encephalopathy. Vet Pathol. 2013;50(5):909–13.
Spudich S, Mastrianni JA, Wrensch M, Gabizon R, Meiner Z, Kahana I, et al. Complete penetrance of Creutzfeldt-Jakob disease in Libyan Jews carrying the E200K mutation in the prion protein gene. Mol Med. 1995;1(6):607–13.
Pasarica M, Loiler S, Dhurandhar NV. Acute effect of infection by adipogenic human adenovirus Ad36. Arch Virol. 2008;153(11):2097–102.
Matoq A, Salahuddin A. Acute hepatitis and pancytopenia in healthy infant with adenovirus. Case Rep Pediatr. 2016. doi:10.1155/2016/8648190.
Mitra AK, Clarke K. Viral obesity: fact or fiction? Obes Rev. 2010;11(4):289–96.
Carter JK, Ow CL, Smith RE. Rous-associated virus type 7 induces a syndrome in chickens characterized by stunting and obesity. Infect Immun. 1983;39(1):410–22.
Lyons MJ, Faust IM, Hemmes RB, Buskirk DR, Hirsch J, Zabriskie JB. A virally induced obesity syndrome in mice. Science. 1982;216(4541):82–5.
Satterfield D, Keltner JL, Morrison TL. Psychosocial aspects of strabismus study. Arch Ophthalmol. 1993;111(8):1100–5.
Tomiyama AJ, Mann T. If shaming reduced obesity, there would be no fat people. Hastings Cent Rep. 2013;43(3):4–5.
Jackson SE, Beeken RJ, Wardle J. Perceived weight discrimination and changes in weight, waist circumference, and weight status. Obesity (Silver Spring). 2014;22(12):2485–8.
Provencher V, Bégin C, Tremblay A, Mongeau L, Corneau L, Dodin S, et al. Health-at-every-size and eating behaviors: 1-year follow-up results of a size acceptance intervention. J Am Diet Assoc. 2009;109(11):1854–61.
Coghlan A. Common virus may trigger obesity. New Sci. 2007;195(2618):14.
Sanderson SC, Persky S, Michie S. Psychological and behavioral responses to genetic test results indicating increased risk of obesity: does the causal pathway from gene to obesity matter? Public Health Genomics. 2010;13(1):34–47.
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016. doi:10.1056/NEJMoa1600693.
Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370–9.
Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311(21):2168.
Field AE, Camargo Jr CA, Ogino S. The merits of subtyping obesity: one size does not fit all. JAMA. 2013;310(20):2147–8.
Kühnen P, Clément K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375(3):240–6.
Gratzl R, Sodeck G, Platzer P, Jäger W, Graf J, Pollak A, et al. Treatment of toxoplasmosis in pregnancy: concentrations of spiramycin and neospiramycin in maternal serum and amniotic fluid. Eur J Clin Microbiol Infect Dis. 2002;21(1):12–6.
Kim MO, Ryu HW, Choi JH, Son TH, Oh SR, Lee HS, et al. Anti-obesity effects of spiramycin in vitro and in vivo. PLoS One. 2016;11(7):e0158632.
Sabin MA, Burgner D, Atkinson RL, Pei-Lun Lee Z, Magnussen CG, Cheung M, et al. Longitudinal investigation of adenovirus 36 seropositivity and human obesity: the Cardiovascular Risk in Young Finns study. Int J Obes (Lond). 2015;39(11):1644–50.
Trovato GM, Martines GF, Trovato FM, Pirri C, Pace P, Garozzo A, et al. Adenovirus-36 seropositivity enhances effects of nutritional intervention on obesity, bright liver, and insulin resistance. Dig Dis Sci. 2012;57(2):535–44.
Voss JD, Burnett DG, Olsen CH, Haverkos HW, Atkinson RL. Adenovirus 36 antibodies associated with clinical diagnosis of overweight/obesity but not BMI gain: a military cohort study. J Clin Endocrinol Metab. 2014;99(9):E1708–12.
Brown AW, Bohan Brown MM, Allison DB. Belief beyond the evidence: using the proposed effect of breakfast on obesity to show 2 practices that distort scientific evidence. Am J Clin Nutr. 2013;98(5):1298–308.
Xu GJ, Kula T, Xu Q, Li MZ, Vernon SD, Ndung’u T, et al. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science. 2015;348(6239):aaa0698.
Dutilh BE, Cassman N, McNair K, Sanchez SE, Silva GG, Boling L, et al. A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nat Commun. 2014;5:4498.
Pavela G, Wiener H, Fontaine KR, Fields DA, Voss JD, Allison DB. Packet randomized experiments for eliminating classes of confounders. Eur J Clin Investig. 2015;45(1):45–55.
Berry SM, Connor JT, Lewis RJ. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA. 2015;313(16):1619–20.
Van Damme P, Meijer CJ, Kieninger D, Schuyleman A, Thomas S, Luxembourg A, et al. A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine. 2016;34(35):4205–12.
Na HN, Nam JH. Proof-of-concept for a virus-induced obesity vaccine; vaccination against the obesity agent adenovirus 36. Int J Obes (Lond). 2014;38(11):1470–4.
Nam JH, Na HN, Atkinson RL, Dhurandhar NV. Genomic stability of adipogenic human adenovirus 36. Int J Obes (Lond). 2014;38(2):321–4.
Paris R, Kuschner RA, Binn L, Thomas SJ, Colloca S, Nicosia A, et al. Adenovirus type 4 and 7 vaccination or adenovirus type 4 respiratory infection elicits minimal cross-reactive antibody responses to nonhuman adenovirus vaccine vectors. Clin Vaccine Immunol. 2014;21(5):783–6.
Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–94.
Acknowledgements
The authors thank Dr. Timothy Gill and Dr. Eric Olsen for their helpful feedback on the final manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Air Force, the Department of Defense, or the US Government. Distribution A: 88PA Case no. 2016-4417, 9 Sept. 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jameson D. Voss declares that he has no conflict of interest.
Nikhil V. Dhurandhar has several patents in viral obesity and adenovirus 36 including uses for E1A, E4-ORF1 gene and protein, and AKT1 inhibitor, and has ongoing grant support from Vital Health Interventions for determining anti-diabetic properties of E4-ORF1 protein.
Human and Animal Rights and Informed Consent
This article does not report any original research with human or animal subjects performed by any of the authors.
Funding
No funding was obtained for this work.
Additional information
This article is part of the Topical Collection on Etiology of Obesity
Rights and permissions
About this article
Cite this article
Voss, J.D., Dhurandhar, N.V. Viral Infections and Obesity. Curr Obes Rep 6, 28–37 (2017). https://doi.org/10.1007/s13679-017-0251-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-017-0251-1