Abstract
Purpose of Review
Contact urticaria syndrome includes contact urticaria and protein contact dermatitis. Underreport, underdiagnosis, or misdiagnosis of entities within the contact urticaria syndrome is believed to be common, especially in the occupational setting. This review provides a structured overview of the entities comprised in this syndrome as well as the diagnostic work-up and management strategies.
Recent Findings
Contact urticaria syndrome has been increasingly described due to personal protective equipment and hand sanitizers in the context of the COVID-19 pandemic. The use of legal cannabis products has led to a rise in occupational cases of contact urticaria to cannabis. A declining trend in the evolution of contact urticaria has been described for natural rubber latex allergy due to the use of synthetic gloves. Prick test has been proposed as a screening method, particularly if multiple products are to be tested, instead of the classical sequential scheme.
Summary
Physicians should be aware of the growing number of culprit agents leading to contact urticaria syndrome. Clinical presentation may be challenging since it includes immediate urticaria and/or eczema and even more generalized reactions. Diagnosis requires a high degree of suspicion, detailed occupational history, and complementary tests, including skin testing. The best treatment is to avoid contact with the culprit agent and to implement preventive measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The contact urticaria syndrome (CUS) includes several forms of immediate contact skin reactions (ICSR) to eliciting substances that can be accompanied with systemic involvement. Since its description by Maibach and Johnson in 1975, growing evidence has showed multiple triggering factors and varying clinical pictures [1,2,3]. CUS can present with CoU and/or protein contact dermatitis (PCD) after contact with the substance. CoU can even present with severe generalized symptoms. Early detection and prevention remain essential in its management.
Due to a lack of awareness by patients and providers, it is suspected that CUS is regularly underdiagnosed and/or misdiagnosed [2]. Previous publications have highlighted the importance of spreading knowledge on this entity among dermatologists and occupational health doctors [2, 4].
Epidemiology and Occupational Relevance
To date, no precise data on CUS prevalence in the general population are available and current evidence is frequently obtained from occupational registers. Occupational CoU cases in European healthcare workers are estimated to have a prevalence between 5 and 10%, whereas in the general population data vary between 1 and 3% [2, 5]. Due to the high frequency of immediate skin reactions in real dermatology practice, it has been hypothesized that CoU is more common than the data indicate outside the occupational setting [2, 5].
CUS is well established in the occupational setting [6]. The classification of occupational dermatosis of the International Classification of Diseases-11 includes CoU. There are some occupational screening questionnaires including specific symptoms of CoU, such as the Nordic Occupational Skin Questionnaire (NOSQ-2002) [7]. Both CoU and PCD are considered to have a favorable prognosis in comparison to occupational allergic contact dermatitis and occupational irritant dermatitis [8]. However, in some reports, patients with PCD presented worse and more severe symptoms than patients with other diagnoses [9].
The Finnish Register of Occupational Diseases showed that CoU was the second most common skin occupational condition (29.5%) after contact dermatitis. The three most common responsible agents reported in this register were cow dander, flour and grains, and natural rubber latex (NRL) [10•]. In two German cohorts, the most frequent elicitors of CoU were cosmetics and NRL, respectively [11]. In an Australian study, the three most common occupations involved were health workers, food handlers and hairdressers due to NRL, foodstuffs and ammonium persulfate, respectively [12].
A favorable trend has been observed with NRL allergy, considered to be the most common form of CUS among health workers [13]. Healthcare workers with latex allergy produce IgE specific for a 20-kDa latex peptide (prohevein), which can be found in latex gloves, powder and even in the air due to local transmission. The introduction of non-NRL or nonpowdered synthetic gloves, and progressive elimination of powdered gloves, has enabled workers to avoid the air contamination. Consequently, the lowering of latex release has prevented new cases of sensitization, with subsequent observation of a declining trend in the evolution of CoU in different studies [13,14,15].
An Insight into Pathophysiology
The precise mechanisms that lie behind CUS remain unknown. An initial approach to improve its understanding may be to divide this entity into immunologic and non-immunologic urticaria.
Immunologic CoU is a type I hypersensitivity reaction which occurs in patients with specific IgE against a specific agent. Thus, immunologic CoU needs prior sensitization and only after repeated contact with the culprit agent will the patients present symptoms. The clinical translation of this mechanism is evidenced when skin testing is performed, as positive tests will be seen in the affected patients, and will be negative in controls. Immunologic CoU may be caused by two types of agents. The former group includes high molecular weight proteins (10,000 kD or more), whereas the second includes hapten chemicals of low molecular weight (less than 10 kD) [5]. A classification of the agents leading to immunologic CoU has been proposed and can be found in Table 1.
The main example of immunologic CoU is NRL, for which thirteen different allergenic proteins have been described, named hevein (Hev) b1 to b13 [2]. Allergy to NRL has broader implications for patients since latex-allergic patients show a high degree of cross-reactivity to other antigens, particularly present in fruits (banana, kiwi, avocado, chestnut), sometimes referred to as “latex-fruit syndrome” [16]. Latex Hev b 6.02 and class I chitinase (Hev b11) with an N-terminal Hev-like domain have been described as the main allergens responsible for cross-reactivity [17].
In contrast, non-immunologic CoU occurs without prior sensitization. Therefore, a solitary contact with the agent may directly trigger the reaction. It is seen with a higher frequency than immunologic CoU but is not accompanied by systemic manifestations [5]. Among the substances that can induce non-immunologic CoU, cinnamaldehyde, benzoic acid, sorbic acid, and nicotinic acid esters are to be emphasized [2]. However, the best example remains contact urticaria due to the stinging nettle (Urtica dioica). Histamine is not believed to play a key role due to the therapeutic inefficacy of antihistamines. Contrary to immunologic CoU, skin testing may be positive in all individuals.
The pathogenesis of PCD is thought to be a co-occurrence of type I and IV hypersensitivity reactions against proteins, normally with a high molecular weight or even low molecular weight haptens, as described for immunologic CoU. Various foods such as fruits, vegetables, meats, and seafood or non-food proteins have been reported as responsible for PCD [18].
Risk factors and association of immunological CoU and PCD also provide insight for CUS. Concomitant history of allergic or atopic disorders like asthma, eczema, or hay fever is a risk factor for the former [5], whereas the history of atopy can be found in up to half of the cases of PCD [19].
Clinical Manifestations of CUS
CUS clinical symptoms are determined by the nature of exposure (form, duration, and extent), properties of the allergen and the individual susceptibility [2].
CoU mainly occurs within 10 to 30 min after skin contact with the eliciting agent and disappears within minutes or hours (< 24 h). It affects areas of the body interacting with the sensitizer, normally exposed areas [2, 4]. Delayed onset of CoU has been occasionally described after repeated applications of the trigger substance [20]. It consists of erythema and swelling, rarely angioedema, associated with itch, sting, burning sensation, and/or pain, at the site of the contact with the eliciting agent. The clinical appearance of the primary lesions does not differ from that of other types of urticaria.
Volatile proteins (e.g., flour) may cause conjunctivitis, rhinitis, or asthma if there is contact with conjunctival mucosa or respiratory tract. Systemic symptoms like abdominal pain, oral itching after ingestion (oral allergy syndrome), and diarrhea may develop if there is contact with the mucosa of the gastrointestinal tract [5]. Oral allergy syndrome is a form of contact urticaria that occurs within minutes of ingestion and presents as itching, burning, and swelling of lips, tongue, roof of the mouth, or throat, and it is particularly linked to hypersensitivity to fresh fruits [21].
Spreading of urticarial lesions to generalized urticaria or to extracutaneous symptoms is possible and the progression of symptoms in CUS has been summarized in four stages, which can be seen in Table 2.
It is important to highlight that CoU may present as transient erythema, swelling, and discomfort in the face. This presentation has been described with the use of cosmetics. Before diagnosing “sensitive skin syndrome,” “cosmetic intolerance syndrome,” or “status cosmeticus,” clinicians should assess the possibility of CoU to the products used by the patient [22].
In contrast, PCD shows predilection for involvement of hands (especially the fingertips) and sometimes extends to the wrists and arms. Rarely, the face and other locations have been reported to be involved [23]. Pruritus, erythema, wheals, or angioedema are characteristic of the acute phase, occurring in a matter of minutes, followed by vesicles in the subacute phase. Examination after the acute phase shows chronic hand dermatitis (erythema, lichenification, fissures, or sometimes residual scaling), chronic paronychia, or fingertip dermatitis [18]. In the chronic phase, excoriations and lichenification are seen.
Agents Responsible for CoU and CUS
A vast myriad of compounds is thought to be responsible for both occupational and non-occupational CUS including animal products, plants and plant derivatives, foods, fragrances, cosmetics, flavorings, medications, preservatives, disinfectants, enzymes, or metals.
Both proteins of high molecular weight and low molecular weight haptens can induce immediate contact skin reactions in the setting of immunologic CUS. These substances may be found in plants or animal proteins, chemicals, metals, etc. Non-immunologic CUS may also be seen due to preservatives, fragrances, chemicals, and food products, among others.
Interestingly, there have been some emerging sources of CoU the past years in the context of the COVID-19 pandemic and the increasing use of hand sanitizers and personal protective equipment [24•, 25, 26]. Contact urticaria due to polypropylene in surgical masks and ethanol in hand sanitizers have been reported. Another source of CoU to highlight is cannabis and its derivatives. It has been suggested that the increasing use of legal cannabis products such as hemp and cannabidiol oil may increase exposure and hence sensitization, and recently, some cases of occupational CoU due to cannabis have been reported [27].
In contrast with the previous entities, PCD is mostly seen due to proteins from animal or vegetal origin.
Tables 3, 4, and 5 show the most common causes of immunologic CoU, non-immunologic CoU, and PCD cited in the literature.
Diagnosis
Diagnosis of ICSR requires a full medical history and examination, followed by skin testing of the suspected substances. It is the authors’ belief that a reason why both CoU and PCD are underdiagnosed could be that physicians may not ascertain the presence of immediate sting, itch, or burning sensation–which leads to the attribution of immediate symptoms to diagnoses of the spectrum of sensitive skin syndrome. In terms of medical history, it is also of utmost importance to include the occupational history and habits. Some dermatologists may not take it into account and this may be a cause of underdiagnosing PCD, which is specially seen in this setting.
Physical examination is important to assess the nature of lesions (if present). Dermatologists need to be aware of the fact that in the case of PCD, eczema may be a manifestation of a previous type I hypersensitivity. Therefore, certain clinical scenarios (e.g., food handlers with chronic hand eczema who also complain of immediate symptoms) may prompt the need to interrogate for previous hives and erythema in the area in order to improve the diagnosis of PCD.
In vitro techniques may be available for a few selected allergens. For instance, NRL allergy may be studied with the use of basophil histamine release, radioallergosorbent test (RAST), enzyme-linked immunosorbent assay (ELISA), and IgE immunoblots of peptides present in natural rubber [29].
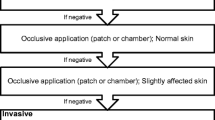
The investigation with in vivo procedures has to be performed with caution since severe systemic responses have been rarely described after testing [2]. A sequential order for skin testing procedures has been proposed, shown in Table 6. In the case of a positive reaction at any step, further studies are discouraged. Positive and negative controls are recommended [30••].
The initial cutaneous provocation test for ICSR is the open test, which entails rubbing the substance on normal looking or slightly affected skin, either on the upper back or the extensor side of the upper arm. A positive result is defined as edema and/or erythema (typical of CoU), or tiny intraepidermal spongiotic vesicles (typical of acute eczema). Positive reactions will generally appear within 15–20 min. Immunologic CoU may show a delayed onset, although this is rare [2, 4].
If the open test results are negative, repetition with occlusion during 24–48 h of the suspected products should be performed. If again negative, prick testing of the suspected allergen or prick-by-prick testing are often the method of choice for immediate contact reactions. Prick-by-prick testing may be a good option, particularly if the studied subject has experienced anaphylactic symptoms, as the amount of allergen is lower than in a prick test performed with the usual technique. In addition, it may also be used with low-molecular weight allergens [31]. Prick-by-prick technique consists of pricking the skin first and then rubbing a piece of the suspected product (e.g., fruit, vegetable) to the area or rubbing the product first and then pricking the area. In our experience, normally the first procedure is more commonly used for prick-by-prick.
Scratch test and chamber scratch test (contact with a small aluminum chamber for 15 min) are less standardized than prick tests, but are useful when a non-standard allergen needs to be studied. When testing with poorly or non-standardized substances, control tests should be assessed on at least 20 people to avoid false positive interpretations [2, 4].
It is important to emphasize that prick testing has been proposed as a screening method for immediate hypersensitivity. Instead of the classical sequential scheme, prick testing may be a better diagnostic technique if there is a need to screen a large number of substances at the same time. If a patient needs skin and respiratory provocation tests, the open tests are preferred [31]. However, to prevent systemic reactions, prick testing technique is to be commenced at very low concentrations. The open testing sequence remains the main diagnostic tool in the USA and Canada, as many physicians are not trained in prick testing.
It is important to note that the diagnosis of PCD lies in prick tests and/or scratch tests, as patch tests are rarely positive [32]. The diagnosis of PCD has historically been associated to positive prick reactions to protein-containing materials. Further studies expanded our understanding of PCD to cases showing an additional type IV contact allergy to proteins. In this sense, isolated reports of non-occupational PCD and cases showing both positive prick-by-prick and patch tests have been reported [33, 34]. To date, the most helpful test to investigate PCD is considered to be the prick test [28•].
Figures 1 and 2 depict positive prick-by-prick tests in two different patients.
Immediate prick-by-prick to eggplant and tomato. Immediate reading at 15 min with two subtle papular wheals for tomato and eggplant in the center can be seen, accompanied by histamine and saline serum control. Subsequent reading confirmed a positive result for eggplant, also in the setting of an occupational protein contact dermatitis
Many cases found in daily practice involve a differential diagnosis approach that may include other tests such as patch testing or photo patch testing [35].
Treatment and Prevention
Discovering the responsible agent is a requirement to correctly avoid the eliciting trigger of CUS. Primary and secondary preventions are highly recommended. Considering their good safety profile, second-generation antihistamines should be considered the preferred first-line symptomatic treatment of immunologic CoU. Before considering alternative treatments, two- to fourfold increase of the licensed dose of antihistamines should be used. If dermatitis is present in PCD, topical steroids are the first-line treatment. Severe cases of CUS may require a short course of oral steroids [2, 4].
Avoidance of further exposure improves occupational CUS. Occupational CoU seems to have a better prognosis than other occupational dermatitis, although previous studies have shown that affected workers may still lose their job. A Danish study on occupational CoU indicated a risk of prolonged sick leave [36]. In addition, job shift may occur during the first years after the recognition of occupational CoU and more often among patients with a severe condition [37].
Some guidelines advise that employers should remove or reduce the exposure to the agent causing occupational CUS, promote the use of after-work creams, refer workers with occupational CUS to specialists, and provide appropriate gloves and cotton liners when it is not possible to remove the inciting agent [38]. In addition, health practitioners need to advise atopic workers to maximize safety measures, have a detailed study of history on the job and materials used at work when a worker is affected by CUS, and confirm it with skin testing.
Additional treatment options may become available for CUS in the future, particularly if there is an improvement in understanding of the pathophysiology lying behind these underreported conditions. It could be hypothesized that selected cases of immunologic CoU with difficulty in avoiding the allergen and without improvement with regular treatments may benefit from anti-IgE therapies. There is rationale for this, as IgE is considered to be one of the key mediators of immunologic CoU. In fact, there has been a case of occupational wheat protein contact dermatitis treated with omalizumab, with clear improvement [39], which probably indicates common pathogenic mechanisms with CoU. On the other hand, taking into account the eczematous nature of PCD, and the common pathways with allergic contact dermatitis, PCD could potentially benefit from anti-IL-4 and IL-13 therapies [40]. Two patients with atopic dermatitis and PCD in the BioDay Registry have already been treated with dupilumab [41].
Conclusions
Immediate contact skin reactions pose an important challenge, as their occupational relevance has been seriously considered in very few selected countries. ICSR may present as urticaria and/or dermatitis. The identification of CUS requires a high level of clinical suspicion, detailed occupational history, physical examination, and complementary tests (e.g., prick testing). Cosmetics, plants, vegetables, and foods are the most common agents. Avoiding the trigger factor is the best treatment. After symptom control, a global approach is required to treat ICSR. This includes appropriate and early diagnosis, occupational reporting, and the development of preventive measures.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Maibach HI, Johnson HL. Contact urticaria syndrome: contact urticaria to diethyltoluamide (immediate type hypersensitivity). Arch Dermatol. 1975;111:726–30.
Gimenez-Arnau AM, Maibach H. Contact Urticaria. Immunol Allergy Clin North Am. 2021;41:467–80.
Fisher AA. Contact dermatitis. 2nd ed. Philadelphia: Lea & Febiger; 1973. p. 283–6.
Gimenez-Arnau A, Maurer M, De La Cuadra J, Maibach H. Immediate contact skin reactions, an update of contact urticaria, contact urticaria syndrome and protein contact dermatitis – “a never ending story.” Eur J Dermatol. 2010;20:552–62.
Vethachalam S, Persaud Y. Contact urticaria. StatPearls, Treasure Island (FL): StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK549890/. Updated 3 Aug 2021.
Ale SI, Maibach HI. Occupational contact urticaria. In: Kanerva L, Maibach HI, Wahlberg J, editors. Occupational dermatology. Berlin/Heidelberg: Saunders; 2000. p. 200–16.
Susitaival P, Flyvholm MA, Meding B, Kanerva L, Lindberg M, Svensson A, et al. Nordic Occupational Skin Questionnaire (NOSQ-2002): a new tool for surveying occupational skin diseases and exposure. Contact Dermatitis. 2003;49:70–6.
Mälkönen T, Jolanki R, Alanko K, Luukkonen R, Aalto-Korte K, Lauerma A, et al. A 6-month follow-up study of 1048 patients diagnosed with an occupational skin disease. Contact Dermatitis. 2009;61:261–8.
Vester L, Thyssen JP, Menné T, Johansen JD. Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermatitis. 2012;67:328–33.
• Pesonen M, Koskela K, Aalto-Korte K. Contact urticaria and protein contact dermatitis in the Finnish Register of Occupational Diseases in a period of 12 years. Contact Dermatitis. 2020;83:1–7. An analysis of over 500 contact urticaria syndrome (CUS) cases in Finland over a twelve-year period. This particular article focuses on real-life data of causative agents and risk occupations in a national register.
Süß H, Dölle-Bierke S, Geier J, Kreft B, Oppel E, Pföhler C, et al. Contact urticaria: frequency, elicitors and cofactors in three cohorts (Information Network of Departments of Dermatology; Network of Anaphylaxis; and Department of Dermatology, University Hospital Erlangen, Germany). Contact Dermatitis. 2019;81:341–53.
Williams JD, Lee AY, Matheson MC, Frowen KE, Noonan AM, Nixon RL. Occupational contact urticaria: Australian data. Br J Dermatol. 2008;159:125–31.
Bensefa-Colas L, Telle-Lamberton M, Faye S, Bourrain JL, Crépy MN, Lasfargues G, et al. Occupational contact urticaria: lessons from the French National Network for Occupational Disease Vigilance and Prevention (RNV3P). Br J Dermatol. 2015;173:1453–61.
Filon FL, Radman G. Latex allergy: a follow up study of 1040 healthcare workers. Occup Environ Med. 2006;63:121–5.
Chowdhury MM. Occupational contact urticaria: a diagnosis not to be missed. Br J Dermatol. 2015;173:1364–5.
Cox AL, Eigenmann PA, Sicherer SH. Clinical relevance of cross-reactivity in food allergy. J Allergy Clin Immunol Pract. 2021;9:82–99.
Raulf-Heimsoth M, Kespohl S, Crespo JF, Rodriguez J, Feliu A, Brüning T, et al. Natural rubber latex and chestnut allergy: cross-reactivity or co-sensitization? Allergy. 2007;62:1277–81.
Amaro C, Goossens A. Immunological occupational contact urticaria and contact dermatitis from proteins: a review. Contact Dermatitis. 2008;58:67–75.
Hernández-Bel P, de la Cuadra J, García R, Alegre V. Protein contact dermatitis: review of 27 cases. Actas Dermosifiliogr. 2011;102:336–43.
Andersen KE, Maibach HI. Multiple application delayed onset contact urticaria: possible relation to certain unusual formalin and textile reactions? Contact Dermatitis. 1984;10:227–34.
Chang YC, George SJ, Hsu S. Oral allergy syndrome and contact urticaria to apples. J Am Acad Dermatol. 2005;53:736–7.
Maibach HI, Engasser P. Management of cosmetic intolerance syndrome. Clin Dermatol. 1988;6:102–7.
Pesqué D, Canal-Garcia E, Rozas-Muñoz E, Pujol RM, Giménez-Arnau AM. Non-occupational protein contact dermatitis induced by mango fruit. Contact Dermatitis. 2021;84:458–60.
• Gondé H, Tedbirt B, Chabrolle P, Hamwi S, Hervouët C, Tétart F. Contact urticaria to ethanol contained in a hand sanitizer. Contact Dermatitis. 2022. https://doi.org/10.1111/cod.14192. Clinical case study that depicts that hand sanitizers used in the context of COVID-19 pandemic may also trigger contact urticaria (CoU).
Corazza M, Bencivelli D, Zedde P, Monti A, Zampino MR, Borghi A. Severe contact urticaria, mimicking allergic contact dermatitis, due to a surgical mask worn during the COVID-19 pandemic. Contact Dermatitis. 2021;84:466–7.
Goller M, Dickel H, Nicolay JP. A case of immediate-type allergy from polypropylene in a particle filter mask in a nurse. Contact Dermatitis. 2022;87:294–6.
Yeo L, Debusscher C, White JML. Occupational contact urticaria to cannabis sativa. Occup Med. 2022;72:273–5.
• Barbaud A. Mechanism and diagnosis of protein contact dermatitis. Curr Opin Allergy Clin Immunol. 2020;20:117–21. The mechanisms underlying contact urticaria syndrome (CUS) remain widely unknown. This review highlights current knowledge on protein contact dermatitis (PCD) pathophysiology.
Palosuo T, Mäkinen-Kiljunen S, Alenius H, Reunala T, Yip E, Turjanmaa K. Measurement of natural rubber latex allergen levels in medical gloves by allergen-specific IgE-ELISA inhibition, RAST inhibition, and skin prick test. Allergy. 1998;53:59–67.
•• Li BS, Ale IS, Maibach HI. Contact urticaria syndrome: occupational aspects. In: John SM, Johansen JD, Rustemeyer T, Elsner P, Maibach HI, editors. Kanerva’s occupational dermatology. Springer; 2020; p. 2595–628. Book chapter focusing on the occupational features of contact urticaria syndrome (CUS) and its management.
Aalto-Korte K, Kuuliala O, Helaskoski E. Skin tests and specific IgE determinations in the diagnosis of contact urticaria and respiratory disease caused by low-molecular-weight chemicals. In: Giménez-Arnau AM, Maibach H, editors. Contact urticaria syndrome. Boca Raton: CRC Press; 2015. p. 129–34.
Walter A, Seegräber M, Wollenberg A. Food-related contact dermatitis, contact urticaria, and atopy patch test with food. Clin Rev Allergy Immunol. 2019;56:19–31.
Perez-Carral C, Garcia-Abujeta JL, Vidal C. Non-occupational protein contact dermatitis due to crayfish. Contact Dermatitis. 2001;44:50–1.
Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321–7.
Nishiwaki K, Matsumoto Y, Kishida K, Kaku M, Tsuboi R, Okubo Y. A case of contact dermatitis and contact urticaria syndrome due to multiple allergens observed in a professional baseball player. Allergol Int. 2018;67:417–8.
Cvetkovski RS, Zachariae R, Jensen H, Olsen J, Johansen JD, Agner T. Prognosis of occupational hand eczema: a follow-up study. Arch Dermatol. 2006;142:305–11.
Carøe TK, Ebbehøj NE, Bonde JP, Agner T. Occupational hand eczema and/or contact urticaria: factors associated with change of profession or not remaining in the workforce. Contact Dermatitis. 2018;78:55–63.
Nicholson PJ, Llewellyn D, English JS, Guidelines Development Group. Evidence-based guidelines for the prevention, identification and management of occupational contact dermatitis and urticaria. Contact Dermatitis. 2010;63:177–86.
Mur Gimeno P, Martín Iglesias A, Lombardero Vega M, Bautista Martínez P, Ventura LP. Occupational wheat contact dermatitis and treatment with omalizumab. J Investig Allergol Clin Immunol. 2013;23:287–8.
Napolitano M, Di Guida A, Nocerino M, Fabbrocini G, Patruno C. The emerging role of dupilumab in dermatological indications. Expert Opin Biol Ther. 2021;21:1461–71.
Voorberg AN, Romeijn GLE, de Bruin-Weller MS, Schuttelaar MLA. The long-term effect of dupilumab on chronic hand eczema in patients with moderate to severe atopic dermatitis -52-week results from the Dutch BioDay Registry. Contact Dermatitis. 2022;87:185–91.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Dr. Ana M Giménez-Arnau has participated as medical advisor for Uriach Pharma/Neucor, Genentech, Novartis, FAES, GSK, Sanofi–Regeneron, Amgen, Thermo Fisher Scientific, Almirall, Celldex, Leo Pharma. She has also received research Grants supported by Uriach Pharma, Novartis, Grants from Instituto Carlos III- FEDER, and participated in educational activities for Uriach Pharma, Novartis, Genentech, Menarini, LEO-PHARMA, GSK, MSD, Almirall, Sanofi, Avene. All other authors declare no relevant conflicts of interest to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Contact Dermatitis
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giménez-Arnau, A.M., Pesqué, D. & Maibach, H.I. Contact Urticaria Syndrome: a Comprehensive Review. Curr Derm Rep 11, 194–201 (2022). https://doi.org/10.1007/s13671-022-00379-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-022-00379-0