Abstract
Purpose of Review
As women continue to live longer, physicians adapt to treating well-described diseases such as breast cancer. Here we provide a comprehensive evidence-based review of the treatment of breast cancer in the elderly patient.
Recent Findings
Previous studies conducted to determine optimal treatment for breast cancer have primarily included younger, healthier women. Despite favorable tumor biology, older women are less likely to receive standard of care treatments relative to breast reconstruction, postoperative radiation, and adjuvant chemotherapy. Frailty is cited as a reason for not offering or receiving therapies. There are no strict definitions of frailty for this specific population, but there are general guidelines for older cancer patients, with a lower specificity than desired.
Summary
Treatment recommendations for older women vary little from those for younger women. However, the paucity of data for the older age group within trials raises the question of the basis of evidence. Risks of treatment need to be evaluated relative to life expectancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The past 50 years has ushered in a new era of scientific innovation, leading to improved general health. The US population continues to live longer, with an average life expectancy of 79 years [1]. Today, an 80-year-old woman is expected to live an additional 8.6 years beyond that [2]. The outlook for survival continues to improve for women diagnosed with breast cancer as well. Survival rates among all ages and stages are improving, including those diagnosed after 70 years of age. This is particularly important given that 30% of patients diagnosed with breast cancer are aged 70 years and older and that the greatest incidence of invasive breast cancer can be found in women 80 years and older at roughly 12% [3, 4]. In fact, the risk of developing breast cancer increases from one person in 52 for women younger than 49 years of age, to one woman in 15 for women greater than 70 years of age [5]. Not surprisingly, estimates of mortality rate place older women at the highest rate as compared to the younger group diagnosed with breast cancer [4]. Although age certainly plays a peripheral role, comorbidities bear a stronger influence. Women 80 years of age and older who undergo surgery for invasive breast cancer are more likely to be classified within the American Society of Anesthesiologists (ASA) Physical Classification III or IV and to be diagnosed with diabetes mellitus, hypertension, and coronary artery disease when compared to younger women [6••]. As women live longer, presumably with better health than prior generations, they are considered physiologically younger and potential surgical candidates. They expect treatment of their cancer.
Frailty
The descriptors “fit” and “frail” are labels used to distinguish between elderly patients who function independently in activities of daily living (ADLs) or without minimal comorbidities, and those patients who have severe comorbidities and likely limited functional independence [7]. Conceptually, the person who is regarded as frail is felt to have less physiologic reserve relative to physical and mental stressors. No universally accepted method of assessment currently exists to differentiate between fitness and frailty; however, the Comprehensive Geriatric Assessment (CGA) has been utilized for many years. This tool takes into account comorbidities, nutrition, medications, socioeconomic issues, geriatric syndromes, and independent daily function. This is likely one of the most comprehensive geriatric assessment tools used in clinical practice, although it has not been validated specifically in breast cancer patients, and it can take up to an hour to complete [8]. Shorter geriatric assessment tools exist for cancer patients, for example the G8 [9, 10••], but these are not considered equivalent to the rigor of the CGA. Regardless of how a clinician determines frailty, the decision on optimal breast cancer treatment for elderly women should consider comorbidities, independence, support network, nutritional status, and the patient’s goals of care.
Epidemiology and Tumor Biology
Approximately 40% of women 80 years and older will die from their breast cancer regardless of the stage [11, 12]. Elderly patients diagnosed with stage III or IV disease have worse overall survival compared to the elderly with stage I or II disease. This fact is not surprising, as it is the basis of staging. The fact that nearly 70% of those elderly women with stage III or IV disease will die from the cancer is more alarming, in spite of greater favorable tumor biology [13, 14]. The harsh irony of breast cancer in older women is that although most tumors are considered low risk, elderly women are more likely to die from their breast cancer than are younger women. In fact, 80% of breast cancers in women aged 65 and older are hormone receptor positive, HER-2 receptor negative. Approximately 10% of women aged 65 and older have triple negative breast cancer, a profile of poorer prognosis, and 10% have HER-2-positive disease—another poor prognostic factor [15]. So despite a favorable tumor biology, the overall health of the older patient with breast cancer needs to be considered when considering the optimal treatment course.
Treatment
Current treatment recommendations for elderly women with breast cancer are largely the same as the recommendations for younger women relative to the biology of their tumor. Regrettably, these recommendations are based on clinical trials that seldom include women over the age of 65. Yet, older women with breast cancer are under-treated with a corresponding increase in mortality relative to treatment with the standard of care [16,17,18,19,20], to some degree due to non-compliance [16]. Clinicians cite a higher incidence of comorbidities, shorter life expectancy, and less aggressive biological tumor characteristics as explanations for under-treatment of this group [19, 21,22,23]. Age may be an independent risk factor for not receiving effective cancer therapies, but this has not been demonstrated [17].
Surgical Options
Breast-Conserving Therapy Versus Mastectomy
The current standard of care for those diagnosed with breast cancer advocates locoregional surgery for those with resectable small cancers, followed by adjuvant therapies when indicated. Locally advanced disease is typically approached with neoadjuvant systemic therapy before surgical resection. The details of which patients should receive which adjuvant therapies is beyond the scope of this discussion; however, most practice guidelines advocate a similar approach regardless of the age of the patient [24]. Before embarking on any surgical intervention, each patient should have a detailed conversation with their surgeon regarding the choice of surgical procedure, need for axillary lymph node assessment, reconstruction options, and potential complications. All patients that require surgical resection should have a thorough history and physical performed. In consultation with colleagues in anesthesiology, the patient may not be regarded a safe candidate for general anesthesia but may be considered for local or regional anesthesia (see section to follow). However, breast surgery is generally well tolerated, except for those with a limited life expectancy [25].
Randomized controlled trials comparing breast conservation to mastectomy have demonstrated that both breast-conserving therapy and mastectomy are similar in disease-free and overall survival [26,27,28], with more recent studies demonstrating a survival benefit in those undergoing breast-conserving therapy (BCT) [29,30,31]. Yet these trials largely excluded elderly women [32]. Mamtani et al. [33•] recently reviewed tumor characteristics, treatment choices, and clinical outcomes in women 80 years and older and found that both BCT and total mastectomy were offered and performed in this older cohort. Elderly women were less likely to undergo total mastectomy, axillary lymph node dissection, and reconstruction as compared to younger women with breast cancer. Rates of sentinel lymph node biopsy and return for re-excision due to initial positive margins were similar between the older and younger groups. Locoregional recurrence and distant metastasis rates were also similar between the two age groups [6••, 33•].
Complications in any surgical procedure are never welcome. The comorbidities of the older woman may increase the perioperative systemic risks. A study used the National Surgical Quality Improvement Program (NSQIP) database to compare outcomes for women diagnosed with breast cancer who were 80 years of age and older to those younger than 80 years relative to perioperative outcomes. Older women are more likely to develop pneumonia, urinary tract infections, and cardiac arrest but are less likely to develop wound complications [6••]. Older women also had higher rates of reoperation. Although the older group had a slightly higher 30-day mortality, this was not significantly different from younger women. The mortality in general was low, with only 0.3% dying within 30 days of surgery. Thirty-day mortality was independently associated with prior diagnoses of hypertension, coronary artery disease, ASA Class IV, and older age [6••].
Axillary Node Assessment
Sentinel lymph node biopsy (SLNB) has become the standard of care for the clinically negative axilla [34]. Classically, axillary lymph node dissection (ALND) was used for locoregional disease control, tumor staging, thus guiding decisions relative to adjuvant systemic treatments and radiation. However, with lesser node involvement with time, along with chronic morbidity risk, including lymphedema and chronic pain, several studies have sought to clarify the necessity of ALND versus SLNB [35].
In women older than 65 years of age with tumor that was positive for estrogen or progesterone receptor HER-2 negative, with plans to undergo adjuvant breast radiotherapy, there was no significant difference in overall survival between women randomized to ALND versus not [36]. This mirrors the findings of Rudenstam in 2006 [37], and an Italian group that examined this retrospectively [38]. The use of sentinel lymph node biopsy for axillary assessment applies to the older woman as well, as it has relatively low morbidity, and allows more accurate staging which guides adjuvant management. The American College of Surgeons Oncology Group (ACSOG) Z0011 trial randomized women with stage I breast cancer with a positive intraoperative positive sentinel node on H&E staining to receive either SLNB alone or SLNB followed by completion ALND. There was no significant benefit to completion ALND, both in locoregional control or survival despite the removal of additional tumor-involved lymph nodes [34]. This is important to keep in mind when managing a patient who may have restricted ipsilateral shoulder range of motion, possibly from a cerebrovascular accident or post-polio syndrome, and full access to the axilla may not be possible.
Reconstruction
Breast reconstruction following mastectomy is an excellent option for many women as it offers both cosmetic and psychological benefits. According the National Institute for Health and Care Excellence (NICE) guidelines, all patients should be offered reconstruction regardless of their age, assuming it is oncologically safe [3] and that the patient is a reasonable candidate. However, the data would support that breast reconstruction is offered less often to elderly women compared to younger women [39]. Various reconstruction options have proven to be safe in this population including tissue expanders, muscle flaps, and DIEP flaps. When counseling women regarding reconstruction, it is important to discuss cosmetic outcomes, possible complications, and the recovery period. The National Mastectomy and Breast Reconstruction Audit reported that implant-related complications were higher in the elderly than in the general population. About 3% of those who underwent an implant reconstruction developed a complication, the most common of which was infection, requiring removal of the implant. Complication rates of autologous reconstruction are also noted to be higher in older women compared to the general population [3], with a trend toward high seroma development in the older population [40].
Choice of Anesthetic
The choice of anesthesia for older breast cancer patients should be thought through before the day of surgery. On occasional, certain comorbidities bring consideration of general inhaled anesthetic into question as in they may be unsafe or increase perioperative risk, such as patients with chronic kidney disease, congestive heart failure, and COPD. Consultation with an anesthesiologist prior to surgery should occur for those patients with complicated medical histories. The anesthesiologist could consider the use of a thoracic paravertebral block (TPVB). TPVB produces an ipsilateral somatic and sympathetic nerve blockade that spreads from the site of the injection across several contiguous dermatomes [41]. A meta-analysis of 15 randomized control trials published between 1999 and 2009 looked at the efficacy and adverse events related to TPVB in women undergoing breast surgery. The authors concluded that a considerable amount of evidence exists in support of the use of TPVB alone or with general anesthesia to decrease postoperative pain, as well as the need for postoperative opioids [42].
A second group was interested in TPVB in those undergoing breast surgery with general anesthesia (GA) compared to monitored anesthesia care (MAC). The authors found that the time from post-anesthesia care unit (PACU) admission to the patient taking fluids, walking, and being transferred to the ward was significantly shorter in the MAC group as compared to the GA group [43]. Patients in the GA group were more likely to experience postoperative nausea and sore throat. Incident rates of intraoperative hypotension and ephedrine administration during anesthesia were significantly higher in the GA group. Overall, TPVB appears to be a safe option in those undergoing breast surgery and should be considered for those with multiple comorbidities for whom general anesthesia may be inappropriate.
A local anesthetic with MAC can be a safe and effective option for the management of small tumors with breast conservation, in addition to a sentinel lymph node biopsy when necessary. Furthermore, the use of a general inhaled anesthetic, with a slow induction, can be utilized with minimal blood pressure fluctuations.
Radiation
Radiation remains critical in the treatment of breast cancer and is considered standard of care in the management of locoregional disease along with partial mastectomy. Radiation may also be administered to those with disease in the axilla when the presence of axillary nodal disease is confirmed. Two randomized controlled trials have shown significantly lower rates of locoregional recurrence among elderly women who underwent postoperative radiation following partial mastectomy compared to those who underwent surgery alone [44, 45•, 46]. Despite these findings, the recommendations regarding the use of radiation is different depending on a patient’s age, primarily due to the less aggressive nature of most breast cancers in the elderly. There is evidence to support the selective use of postoperative radiation in elderly women with hormone receptor-positive (HR+) early breast cancers [24]. Still, the postoperative radiation is still the mainstay of therapy in those older patients with higher risk and hormone receptor-negative (HR-) tumors. Again, disparities exist in the delivery of radiotherapy relative to access and socioeconomic status [47].
Relative to the recently published consensus guidelines by the American Society for Radiation Oncology, the older patient may have a greater chance of receiving accelerated partial breast radiation [48]. This then lends itself to a smaller area radiated, usually over a shorter period of time, making for more convenient delivery for the patient.
Low-Risk and Hormone Receptor-Positive Disease
The role of radiotherapy is well accepted in younger women; however, the degree of clinical benefit of routine radiation following partial mastectomy has been questioned in the treatment of elderly breast cancer patients. In women 70 years and older with low-risk and hormone receptor-positive disease, omission of radiation has become an option since many studies have shown a lack of impact on survival [45•, 49, 50]. For low-risk, hormone receptor-positive disease, survival was minimally affected if any component of adjuvant local therapy was withheld. In these studies, the adjuvant therapy that appeared to be the most important for overall and cancer-specific survival was the administration of hormone replacement therapy [44, 45•, 51]. Using the Surveillance, Epidemiology, and End Results (SEER) database, Mogel et al. conducted a retrospective review of the type of locoregional therapy women received and its association with overall survival and cancer-specific survival. Women older than 70 with stage I node-negative disease who underwent lumpectomy alone had similar overall survival and cancer-specific survival compared those who underwent partial mastectomy plus postoperative radiation [52].
High-Risk and Hormone Receptor-Negative Disease
Women with high-risk tumor characteristics or hormone receptor-negative tumors should be considered very seriously for adjuvant radiation. Most hormone receptor-negative tumors recur within 5 years and women with hormone receptor-negative tumors have higher recurrence rates and breast cancer-specific mortality compared to their hormone receptor-positive counterparts [23, 50, 53]. Omission of adjuvant radiation in hormone receptor-negative women over age 70 is associated with an increase in breast cancer-specific mortality [44]. A recent study retrospectively reviewing the use of adjuvant radiation following partial mastectomy in women 70 and older with estrogen receptor-negative T1N0M0 disease found significant differences in 5-year overall survival between those who underwent radiation (81%) and those who did not receive adjuvant radiation (61.7%). The study also demonstrated a significant difference in 5-year cancer-specific survival between those who underwent radiation (93.1%) and those who did not receive radiation (85%) [54]. In general, patients with high-risk disease, such as locally advanced, positive nodes, HER-2-positive or hormone receptor-negative tumors, should be considered by their clinicians for adjuvant radiation therapy.
Systemic Therapies
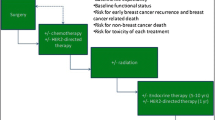
Systemic treatment for breast cancer can be quite complex and current recommendations for systemic protocols continue to evolve. Here, the focus is to identify the patient in whom systemic therapy is considered, based on the patient’s tumor biology and stage. We seek to focus on the current issues facing medical oncologists in their treatment of the elderly patient with breast cancer. Older patients benefit from chemotherapy to the same degree as younger patients. Yet, despite these recommendations, older patients have lower rates of initiation and adherence to adjuvant therapies while also being more likely to receive non-guideline therapies [17, 55,56,57,58]. All types of adjuvant therapy are recommended less frequently to the elderly and are received less frequently by this population [14].
The systemic therapies offered are based on the tumor receptor phenotype, along the lines of estrogen receptor (ER), progesterone receptor (PR) expression, and HER-2 receptor amplification. For those patients without nodal involvement, systemic therapy is still the standard of care if the tumor fails to express both estrogen and progesterone receptors, or if the tumor expresses HER-2 receptor amplification (Table 1). Locally advanced tumors and nodal disease still prompt consideration of systemic therapy, often in the neoadjuvant setting, regardless of the tumor biology. Metastatic disease is generally approached with single-agent therapy, in addition to targeted therapy such as an immunologic (pertuzumab and/or trastuzumab) if HER-2 is amplified, or endocrine therapy if ER or PR is expressed by the breast cancer. There, the focus is on palliation and quality of life, minimizing toxicities.
Infusion Chemotherapy
Standard chemotherapy regimens for breast cancer are usually composed of two or three of the following agents: anthracycline, taxane, 5-fluorouracil, cyclophosphamide, and carboplatin. Newer agents such as capecitabine were previously thought to be useful in the elderly comorbid population due to decreased toxicity; however, they are now falling out of favor. A prospective randomized control trial in women with breast cancer aged 65 years and older compared standard chemotherapy (cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide plus doxorubicin) to single-agent capecitabine. Patients that were assigned to the capecitabine arm were twice as likely to have a relapse and nearly twice as likely to die from their cancer [60]. The authors concluded that even in elderly patients, standard chemotherapy regimens are superior to single-agent capecitabine. The ICE trial (Ibandronate with or without Capecitabine in Elderly Patients with Early Breast Cancer) provided additional evidence against single-agent capecitabine therapy. Overall and disease-free survival was similar between the ibandronate plus capecitabine and single-agent ibandronate arms [61]. The ICE study was followed by a trial that looked at capecitabine plus taxol therapy. Women aged 65 and older with moderate- to high-risk breast cancer were randomized to receive standard regimen chemotherapy (either 4 cycles of epirubicin plus cyclophosphamide versus 6 cycles of cyclophosphamide/methotrexate/5-fluorouracil, aka, CMF) or 6 cycles of paclitaxel plus capecitabine. The authors found that a significantly higher proportion of women discontinued the capecitabine regimen, with a higher rate of non-hematologic toxicities compared to the standard chemotherapy regimens [62].
Trials that evaluate optimal chemotherapy regimens typically do not include women over 70 or have very low rates of inclusion. The current literature supports taxane-based treatment for those women who are deemed fit enough to receive it. However, older patients do have increased rates of toxicity compared to younger women and other chemotherapy regimens. Similarly, anthracycline-based regimens pose an increased risk of cardiac toxicity in elderly patients. The CMF regimen (cyclophosphamide, methotrexate, and 5-fluorouracil) is an option for those elderly patients who cannot tolerate either a taxane- or anthracycline-based regimen [63]. Jones et al. attempted to distinguish survival between different chemotherapy regimens in elderly women and assigned women over age 65 to receive doxorubicin plus cyclophosphamide or docetaxel plus cyclophosphamide. After 7 years, the docetaxel arm had significantly improved overall and disease-free survival compared to the doxorubicin arm; those in the docetaxel arm experienced more febrile neutropenia and anemia [64].
Endocrine Therapy
Most elderly patients with breast cancer will present with ER-positive and PR-positive tumors—about 75–80%. In patients with tumors testing positive for either ER or PR (or both), endocrine therapy should be recommended and maintained for at least 5 years. This recommendation holds true with older women as the efficacy of endocrine therapy has no correlation with age and is generally well tolerated compared to other adjuvant therapies [59, 65,66,67]. The mainstay of endocrine adjuvant therapy includes either tamoxifen or aromatase inhibitors. Aromatase inhibitors are the preferred agent for post-menopausal women, given the more favorable side effect profile and superiority in improving disease-free survival and overall survival compared to tamoxifen [60, 68,69,70]. Surgery remains the first line of therapy and standard of care for elderly women with breast cancer who acceptable surgical candidates. For those women with hormone receptor-positive disease, counseling should include discussion regarding possible bone mineral density loss, arthralgia, myalgia, uterine complications, and potential thromboembolic events prior to beginning adjuvant endocrine therapy.
Trastuzumab and HER-2 Status
The development of trastuzumab represents a major advance in the ability of clinicians to fight breast cancer with newer biologic agents. A monoclonal antibody directed against the HER-2 receptor, trastuzumab given during chemotherapy and then every third week for a year, has significantly increased disease-free survival and overall survival in those with HER-2-amplified breast cancer [71]. In the elderly, there is a 47% relative risk reduction in those receiving trastuzumab plus chemotherapy compared to those who received chemotherapy alone [72]. Trastuzumab does carry with it the risk of a reversible cardiotoxicity. Two separate trials have demonstrated that age is an independent risk factor for developing trastuzumab-related cardiotoxicity, and those with pre-existing cardiac disease are also at increased risk [73, 74]. Unfortunately, due mainly to this toxicity, older women with HER-2-positive disease are less likely to initiate or complete a year of treatment with trastuzumab [75, 76]. Chen et al. attempted to characterize the risk of trastuzumab with different chemotherapy regimens and found that trastuzumab plus anthracycline was associated with the highest rate of cardiotoxicity [77].
The recently published CLEOPATRA trial, in which patients were randomized to docetaxel and trastuzumab, with or without pertuzumab, identified 127 women who participated who were over age 65. Gastrointestinal symptoms were more frequent in the older age group, but neutropenia was less frequent [78•]. Others have found the combination of trastuzumab and pertuzumab highly efficacious when given with taxol, followed by epirubicin plus cyclophosphamide for early HER-2-amplified breast cancers [79]. The combination of immunologic agents, with various single-agent chemotherapeutic agents, has also been successful in the setting of metastatic disease [80,81,82].
Evaluating Toxicity
A common reason cited by clinicians for recommending non-guideline adjuvant therapy is a concern for toxicity, especially in those with advanced age and/or comorbidities. This is understandable, as multiple studies have demonstrated that older patients have higher rates of toxicity and adverse side effects following adjuvant therapies [17, 57, 77]. Unfortunately, though many tools are available for estimating toxicity of chemotherapy in the elderly, there is no single assessment tool that has been validated solely in the breast cancer population. When considering different adjuvant regimens, providers can use other geriatric and chemotoxicity tools to help guide them in their decision-making. Important predictors of chemotoxicity in one study included having fair or poor hearing, at least one fall in the last 6 months, difficulty taking one’s own medications, difficulty walking a city block, and having decreased social activity due to emotional health [83]. In the “CRASH” geriatric assessment tool, the mini-mental status exam, mini-nutritional assessment, and difficulties with ADLs were predictive of toxicity with chemotherapy [84•]. If fine-tuned, these assessments may be useful in decisions regarding initiation and dose management in those with breast cancer.
Conclusions
As the lifespan of the average American extends, physicians need more hard data in order to optimize the medical care for our aging adults. Most research that we base our clinical practice upon has been conducted on young and healthy participants; this holds true for breast cancer. The incorporation of an older population in breast cancer trials has gradually provided evidence on which to base therapeutic decisions. Use of breast-conserving surgery, sentinel lymph node biopsy, and partial breast radiotherapy are techniques that are not limited by age but by extent of disease. Still, the mortality of older women with breast cancer is a sobering statistic; more work needs to be done to help define the optimal adjuvant strategies.
Perhaps a better or refined tool to gauge the physiologic reserve and resilience of the older breast cancer patient will be of use. This begs the question, though, of how best to treat those who qualify as frail. Overall, physicians treating older women with breast cancer have increasing numbers of tools, guidelines, and therapies available to provide individualized and quality care to their patients. With an increasingly older population, we need the trials to direct our care.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
The World Bank, World Development Indicators. Life expectancy at birth, total (years) | Data. 2017. In : Data.worldbank.org. http://data.worldbank.org/indicator/SP.DYN.LE00.IN. Accessed 9 Feb 2017.
Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–92. doi:10.1001/jama.285.7.885.
Hamnett KE, Subramanian A. Breast reconstruction in older patients: a literature review of the decision-making process. J Plast Reconstr Aesthet Surg. 2016;69:1325–34. doi:10.1016/j.bjps.2016.06.003.
American Cancer Society. Cancer facts & figures 2017. 2017. In: Cancer.org. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html. Access 9 Feb 2017.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2017;67:7–30. doi:10.3322/caac.21387.
•• Pettke E, Ilonzo N, Ayewah M, Tsantes S, Estabrook A, Ma AMT. Short-term, postoperative breast cancer outcomes in patients with advanced age. Am J Surg. 2016;212:677–81. doi:10.1016/j.amjsurg.2016.06.007. Thorough overview of the likely postoperative complication risks in the elderly with breast cancer, using the National Surgical Quality Improvement Program database
Balducci L. Management of cancer in the elderly. Oncology, 1 Feb 2006, p 135. Expanded Academic ASAP, go.galegroup.com/ps/i.do?p=EAIM&sw=w&u=ucinc_main&v=2.1&id=GALE%7CA142723166&it=r&asid=43bf8457e578a9a35d77fc26933e3eb4.ist 2000;5:224–237. Accessed 9 Feb 2017.
Struck AE, Siu AL, Weiland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342:1032–6. doi:10.1016/0140-6736(93)92884-V.
Bellera CA, Raingray M, Mathoulin-Pelissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: first evalution of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–72. doi:10.1093/annonc/mdr587.
•• Petit-Monegar A, Rainfray M, Soubeyran P, Bellera CA, Mathoulin-Pelissier S. Detection of frailty in elderly cancer patients: improvement of the G8 screening test. J Geriatric Oncol. 2016;7:99–107. doi:10.1016/j.jgo.2016.01.004. A practical, short assessment of frailty among patients 70 years of age and older
Weiss A, Noorbakhsh A, Tokin C, Chang D, Blair SL. Hormone receptor-negative breast cancer: undertreatment of patients over 80. Ann Surg Oncol. 2013;20:3274–3278. Erratum in: Ann Surg Oncol 2014:21:S785. doi:10.1245/s10434-013-3115-2.
Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;12:e148–60. doi:10.1016/S1470-2045(11)70383-7.
Angarita FA, Chesney T, Elser C, Mulligan AM, McCready DR, Escallon J. Treatment patterns of elderly breast cancer patients at two Canadian cancer centres. Eur J Surg Oncol. 2015;41:625–34. doi:10.1016/j.ejso.2015.01.02.028.
Cyr A, Gillanders WE, Aft RL, Eberlein TJ, Margenthaler JA. Breast cancer in elderly women (≥80 years): variation in standard of care? J Surg Oncol. 2011;103:201–6. doi:10.1002/jso.21799.
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106 doi:10.1093/jnci/dju055.
Hadj P, Jackisch C, Bolten W, Blettner M, Hindenburg HJ, Klein P, et al. Compliance and arthralgia in clinical therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Ann Oncol. 2014;25:372–7. doi:10.1093/annonc/mdt513.
Freedman RA, Vaz-Luis I, Barry WT, Li H, Lin NU, Winer EP, et al. Patterns of chemotherapy, toxicity, and short-term outcomes for older women receiving adjuvant trastuzumab-based therapy. Breast Cancer Res Treat. 2014;145:491–501. doi:10.1007/s10549-014-2968-9.
Van de Water W, Bastiaannet E, Dekkers OM, de Craen AJ, Westendoop RG, Voogd AC, et al. Adherence to treatment guidelines and survival in patients with early-stage breast cancer by age at diagnosis. Br J Surg. 2012;99:813–20. doi:10.1002/bjs.8743.
Wallweiner CW, Hartkopf AD, Grabe E, Wallweiner M, Taran FA, Fehm T, et al. Adjuvant chemotherapy in elderly patients with primary breast cancer: are women ≥65 undertreated? J Cancer Res Clin Oncol. 2016;142:1847–53. doi:10.1007/200432-016-2194-4.
Malik MK, Tartter PI, Belfer R. Undertreated breast cancer in the elderly. J Cancer Epidemiol. 2013;2013:893104. doi:10.1155/2013/893104.
Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–69. doi:10.1200/JCO.2006.10.4208.
Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92:550–6. doi:10.1093/jnci/192.7.550.
Pierga JY, Girre V, Laurence V, Asselain B, Die’ras V, Jouve M, et al. Characteristics and outcome of 1755 operable breast cancers in women over 70 years of age. Breast. 2004;13:369–75. doi:10.1016/j.breast.2004.04.012.
NCCN clinical practice guidelines in oncology (NCCN Guidelines), Breast Cancer. Version 1.2016. J Natl Compr Cancer Network 2016:14:324–354.
Kemeny MM. Surgery in older patients. Semin Oncol. 2004;31:175–84. doi:10.1053/j.seminoncol.2003.12.028.
Fisher B, Anderson S, Bryant J, Margolese R, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy and lumpectomy plus radiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi:10.1056/NEJMoa022152.
Lichter AS, Lippman ME, Danforth DN Jr, d’Angelo T, Steinberg SM, deMoss E, et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the National Cancer Institute. J Clin Oncol. 1992;10:976–83. doi:10.1200/jco.1992.10.6.976.
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32. doi:10.1056/NEJMoa020989.
Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs. mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149:267–74. doi:10.1001/jamasurg.2013.3049.
Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer. 2013;119:1402–11. doi:10.1002/cncr.27795.
Hofvind S, Holen AT, Roman M, Sebuodegard S, Akslen LA. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol. 2015;41:1417–22. doi:10.1016/j.ejso.2015.07.002.
Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi:10.1056/NEJM199120303412706.
• Mamtani A, Gonzalez JJ, Neo D, Slanetz P, Houlihan MJ, Herold C, et al. Early-stage breast cancer in the octagenarian: tumor characteristics, treatment choices, and clinical outcomes. Ann Surg Oncol. 2016;23:3371–8. doi:10.1245/s10434-016-5368-z. Good overview of the tumor types relative to outcomes in the elderly breast cancer patient
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases. Ann Surg. 2010;252:426–33. doi:10.1097/SLA.0b013e3181f08f32.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33. doi:10.1016/s1470-2045(10)70207-2.
Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection vs. axillary dissection. J Surg Oncol. 2010;102:111–8. doi:10.1002/jso.21535.
Martelli G, Borachi P, Ardoino I, Lozza L, Behm S, Vetrella G, et al. Axillary dissection vs. no axillary dissection in older patients with T1N0 breast cancer: 15-year results of a randomized controlled trial. Ann Surg. 2012;256:920–4. doi:10.1097/SLA.0b013e31827660a8.
International Breast Cancer Study Group, Rudenstam C-M, Zahrieh D, Forbes JF, Crivellari D, Holmberg SB, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10-93. J Clin Oncol. 2006;24:337–44. doi:10.1200/JCO.2005.01.5784.
Fenlon D, Frankland J, Foster CL, Brooks C, Coleman P, Payne S, et al. Living into old age with the consequences of breast cancer. Eur J Oncol Nurs. 2013;17:311–6. doi:10.1016/j.ejon.2012.08.004.
Song D, Slater K, Papsdorf M, VanLaeken N, Zhong T, Hazen A, et al. Autologous breast reconstruction in women older than 65 years versus women younger than 65 years: a multi-center analysis. Ann Plast Surg. 2016;76:155–63. doi:10.1097/sap.0000000000000527.
Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth. 2013;111:711–20. doi:10.1093/bja/aet213.
Schnabel A, Reichl SU, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: a meta-analysis of randomized controlled trials. Brit J of Anaesth. 2010;105:842–852. Erratum in: Br J Anaesth 2013:111:522. doi:10.1093/bja/aeq265.
Sato M, Shirakami G, Fukuda K. Comparison of general anesthesia and monitored anesthesia care in patients undergoing breast cancer surgery using a combination of ultrasound-guided thoracic paravertebral block and local infiltration anesthesia: a retrospective study. J Anesth. 2015;30:244–51. doi:10.1007/s00540-015-2111-z.
Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–7. doi:10.1056/NEJMoa040587.
• Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–7. doi:10.1200/JCO.2012.45.2615. Use of radiotherapy in women over the age of 70 with breast cancer resected with lumpectomy and whether radiation improves survival
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–106. doi:10.1016/S0140-6736(05)67887-7.
LeMasters T, Madhavan S, Sambamoorthi U. Comparison of the initial local-regional treatment received for early-stage breast cancer, between elderly women in Appalachia and a United States-based population: good and bad news. Glob J Breast Cancer Res. 2016;4:10–9. doi:10.20941/2309-4419.2016.04.2.
Shaitelman SF, Lin HY, Smith BD, Shen Y, Bedrosian I, Marsh GD, et al. Practical implications of the publication of consensus guidelines by the American Society for Radiation Oncology: accelerated partial breast irradiation and the National Cancer Data Base. Int J Radiat Oncol Biol Phys. 2016;94:338–48. doi:10.1016/j.ijrobp.2015.10.059.
Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98:681–90. doi:10.1093/jnci/djj/86.
Shen X, Anne PR, Keith SW, Wojcieszynski A, Mishra MV, Bar-Ad V, et al. Radiation therapy use and outcomes among older women with ER-positive and ER-negative stage I breast cancer. Am J Clin Oncol. 2014;37:241–7. doi:10.1097/coc.0b013e318271b326.
van de Water W, Bastiaannet E, Scholten AN, Kiderlen M, de Craen AJ, Westendorp RG, et al. Breast-conserving surgery with or without radiotherapy in older breast patients with early stage breast cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21:786–94. doi:10.1245/s10434-013-3374-y.
Mogel HD, Clark C, Dodson R, Fino N, Howard-McNatt M. Outcomes after mastectomy and lumpectomy in elderly patients with early-stage breast cancer. Ann Surg Oncol. 2016:1–8. doi:10.1245/e10434-016-5582-8.
Nagar H, Yan W, Christos P, Chao KS, Nori D, Ravi A. Older patients with early-stage breast cancer: adjuvant radiation therapy and predictive factors for cancer-related death. Am J Clin Oncol. 2014; doi:10.1097/coc.0000000000000144.
Daugherty E, Daugherty M, Bogart J, Shapiro A. Adjuvant radiation improves survival in older women following breast-conserving surgery for estrogen receptor negative breast cancer. Clin Breast Cancer. 2016;16:500–6. doi:10.1016/j.clbc.2016.06.17.
Griggs JJ, Culakova E, Sorbero ME, Poniewierski MS, Wolff DA, Crawford J, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25:2522–7. doi:10.1200/JCO.2006.10.2749.
Elkin EB, Hurria A, Mitra N, Schrag D, Panageas KS. Adjuvant chemotherapy and survival in older women with hormone receptor-negative breast cancer: assessing outcome in a population-based, observational cohort. J Clin Oncol. 2006;24:2757–64. doi:10.1200/JCO.2005.03.6053.
Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24:2750–6. doi:10.1200/JCO.2005.02.3028.
Hassett MJ, Silver SM, Hughes ME, Blayney DW, Edge SB, Herman JG, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30:2218–26. doi:10.1200/JCO.2011.38.5740.
Senkus E, Kyriakides S, Penault-Llorca F, Ohno S, Penault-Llorca F, Poortmans P, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–30. doi:10.1093/annonc/mdu298.
Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. Erratum in N Engl J Med 2009:361:1714. doi:10.1056/NEJMoa0810266.
von Minckwitz G, Conrad B, Reimer T, Decker T, Eidtmann H, Eiermann W, et al. A randomized phase 2 study comparing EC or CMF versus nab-paclitaxel plus capecitabine as adjuvant chemotherapy for nonfrail elderly patients with moderate to high-risk early breast cancer (ICE II-GBG 52). Cancer. 2015;121:3639–48. doi:10.1002/cncr.29506.
von Minckwitz G, Reimer T, Potenberg J, Conrad B, Schurer U, Eidtmann H, et al. The phase III ICE study: adjuvant ibandronate with or without capecitabine in elderly patients with moderate or high risk early breast cancer. Cancer Res. 2015;75:S3–04. doi:10.1158/1538-7445.SABCS14.S3-04.
Sun J, Chia S. Adjuvant chemotherapy and HER-2-directed therapy for early-stage breast cancer in the elderly. Br J Cancer. 2017;116:4–9. doi:10.1038/bjc.2016.360.
Jones S, Holmes FA, O’Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7 year followup of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–83. doi:10.1200/JCO.2008.18.4028.
Owusu C, Lash TL, Silliman RA. Effectiveness of adjuvant tamoxifen therapy among older women with early stage breast cancer. Breast J. 2007;13:374–82. doi:10.1111/j.1524-4741.2007.00445.x.
Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351:963–70. doi:10.1056/NEJMoa040595.
Hind D, Wyld L, Reed MW. Surgery, with or without tamoxifen, vs. tamoxifen alone for older women with operable breast cancer: Cochrane review. Br J Cancer. 2007;96:1025–9. doi:10.1038/sj.bjc.6603600.
Colleoni M, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell’Orto P, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011;29:1117–24. doi:10.1200/JCO.2008.17.0829.
Arimidex T, Alone or in Combination (ATAC) Trialists’ Group, Forbes JF, Cuzick J, Buzdar A, Howell A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi:10.1016/S1470-2045(07)70385-6.
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Livingston RB, et al. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA 17 trial of extended adjuvant letrozole. Ann Oncol. 2013;24:355–61. doi:10.1093/annonc/mds330.
Slamon DJ, Eiermann W, Robert NJ, Giermek J, Martin M, Jasiowka M, et al. Ten year follow-up of the BCIRG-006 trial comparing doxorubicin plus cyclophosphamide followed by docetaxel with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER2 + early breast cancer patients. Cancer Res. 2016;76:S5–04. doi:10.1158/1538-7445.SABCS15-S5-04.
Brollo J, Curigliano G, Disalvatore D, Marrone BF, Criscitiello C, Bagnardi V, et al. Adjuvant trastuzumab in elderly with HER-2 positive breast cancer: a systematic review of randomized controlled trials. Cancer Treat Rev. 2013;39:44–50. doi:10.1016/jctrv.2012.03.009.
Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–8. doi:10.1200/JCO.2013.48.7884.
Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr, Roe MT, Wood F, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant Breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28:3416–21. doi:10.1200/JCO.2009.23.6950.
Freedman RA, Hughes ME, Ottesen RA, Weeks JC, He Y, Wong YN, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119:839–46. doi:10.1002/cncr.27831.
Vaz-Luis I, Keating NL, Lin NU, Lii H, Winer EP, Freedman RA. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: a population-based study. J Clin Oncol. 2014;32:927–34. doi:10.1007/s10549-014-2668-9.
Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Col Cardiol. 2012;60:2504–12. doi:10.1016/j.jacc.2012.07.068.
• Miles D, Baselga J, Amadori D, Sunpaweravong P, Semiglazov V, Knott A, et al. Treatment of older patients with HER@-positive metastatic breast cancer with pertuzumab, trastuzumab, and docetaxel: subgroup analyses from a randomized, double-blind, placebo-controled phase III trial (CLEOPATRA). Breast Cancer Res Treat. 2013;142:89–99. doi:10.1007/s10549-013-2710-z. Analysis of the subset of elderly patients in the pivotal CLEOPATRA trial
Loibl S, Jackisch C, Schneeweiss A, Schmatioch S, Aktas B, Denkert C, et al. Dual HER2-blockade with pertuzumab and trastuzumab in HER2-positive early breast cancer: a subanalysis of data from the randomized phase III GeparSepto trial. Ann Oncol. 2016;
Gianni L, Penkowski T, Im YH, Tseng LM, Liu MC, Lluch AS, et al. 5-year analysis of neodjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicenter, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi:10.1016/S1470-2045(16)00163-7.
Smyth LM, Iyengar NM, Chen MF, Popper SM, Patail S, Wasserheit-Lieblich C, et al. Weekly paclitaxel with trastuzumab and pertuzumab in patients with HER2-overexpressing metastatic breast cancer: overall survival and updated progression-free survival results from a phase II study. Breast Cancer Res Treat. 2016;158:91–7. doi:10.1007/s10549-016-3851-7.
Perez EA, Lopez-Vega JM, Petit T, Zamagni C, Easton V, Kamber J, et al. Safety and efficacy of vinorelbine in combination with pertuzumab and trastuzumab for first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer: VELVET Cohort 1 final results. Breast Cancer Res. 2016;18:126. doi:10.1186/s13058-016-0773-6.
Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–65. doi:10.1200/JCO.2011.34.7625.
• Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–86. doi:10.1002/cncr.26646. Helpful in choosing whether to initiate chemotherapy in the treatment of the elderly patient with breast cancer
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Elizabeth Shaughnessy and Grace Martin declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Surgical Care for the Geriatric Patient
Rights and permissions
About this article
Cite this article
Martin, G., Shaughnessy, E. Breast Cancer Care in the Elderly Patient. Curr Geri Rep 6, 139–148 (2017). https://doi.org/10.1007/s13670-017-0218-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13670-017-0218-9