Abstract
Purpose of Review
Placenta accreta spectrum (PAS) is a rare pregnancy complication with an increasing incidence worldwide. PAS can cause serious maternal morbidity and even mortality at delivery. Ultrasound (US) is an excellent imaging modality for the diagnosis of PAS, but it must be used correctly and there is little standardised training in placental imaging available. The aim of this paper is to discuss US screening for PAS and provide practical advice on its use for antenatal diagnosis.
Recent Findings
Screening for PAS in a high-risk population (history of previous caesarean delivery (CD) and an anterior low-lying/praevia placenta) is possible in well-trained hands with high sensitivity and specificity. This can be performed in the first trimester but usually occurs around 24–28 weeks after the routine anomaly US. A detailed examination of the placental bed using the US signs defined by EW-AIP with an adequately filled bladder enables the operator to produce a detailed report fully outlining the anticipated findings at delivery. This facilitates an appropriate multi-disciplinary team (MDT) approach which is the goal for optimal PAS management.
Summary
Women with a history of previous CD and an anterior low-lying/praevia placenta need a detailed examination of the placenta by an experienced operator. The US examination should be undertaken systematically, and the risk factors and US signs reported in a way which is useful to the MDT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Placenta accreta spectrum (PAS) or abnormally adherent and invasive placenta (AAIP) describes the clinical conditions where the placenta abnormally adheres to or invades the uterine wall. If the placenta is forcibly removed, massive obstetric haemorrhage (MoH) can ensue. The pathologist differentiates PAS into 3 subgroups: placenta accreta is defined by abnormal attachment to the myometrium with an absent decidua; increta represents invasion of the villous tissue deeply into the myometrium but not reaching the serosa; and percreta has placenta villi which completely invade the myometrium reaching the serosa or beyond [1]. Whilst correlating prenatal ultrasound (US) signs with the eventual histopathological diagnosis is important, just reporting ‘suspected accreta, increta or percreta’ does not fully inform the surgical team. Therefore, prenatal imaging should not only attempt to diagnose presence and grade of PAS, but should also clearly describe important clinical features, such as presence and site of significant neovascularity, posterior bladder wall involvement or cervical invasion. This enables appropriate management (surgical or conservative) to be planned in advance of delivery thereby reducing maternal morbidity [2]. The aim of this paper is to discuss some of the techniques used for US diagnosis of PAS, including potential pitfalls, as well as recommending ways of reporting the findings which are more useful to the multi-disciplinary team (MDT) involved.
Screening for PAS
The reported prevalence of PAS in the general population of pregnant women is highly variable at between 1.7 and 16 per 10,000 pregnancies [3, 4]. As previous caesarean delivery (CD) is the single greatest risk factor, this variation may be partly explained by different CD rates but it may also relate to the criteria used to diagnose PAS. However, irrespective of which estimate is correct, PAS appears to be relatively rare in the general pregnant population so any screening must be targeted to those at highest risk. Whilst PAS can involve scar tissue at any location in the uterus, the scar resulting from a caesarean delivery is particularly problematic as it is usually in the lower segment. This provides a ‘toxic’ combination of PAS and a low-lying/praevia placenta which significantly increases the maternal risks at delivery due to the potential for transecting the placenta with a lower segment incision, the inherent poor contractility of the lower segment and the potential for invasion into the bladder, cervix and/or parametrium. Therefore, most current guidelines, including those from FIGO and the RCOG [5, 8], focus screening on the subset of women with previous CD. These guidelines recommend that the placental location should be assessed in all women presenting for a mid-trimester US scan. If the placenta is low (< 2 cm from the internal os) or a praevia (covering the internal os), she should be asked if she has had a previous CD [5, 7]. If she has, it is likely that the placenta is overlying the CD scar and is at risk of PAS, so she should be referred to an operator experienced in diagnosing PAS for a detailed US examination. Robust prenatal diagnosis of PAS is possible by an operator with appropriate experience using US alone [5]. A systematic review and meta-analysis of US studies involving 3707 pregnancies found that the overall performance of US in diagnosing PAS is excellent, with a sensitivity of 90.72% (95% CI 87.2–93.6), specificity of 96.94% (95% CI 96.3–97.5) and diagnostic odds ratio of 98.59 (95% CI 48.8–199.0) [6]. If the diagnosis is unclear, a second opinion should be sought from an operator with more experience in the prenatal assessment of PAS [5, 7, 8].
Preferably, all sonographers should be aware of the signs of PAS; however, this is not yet a standard part of US training courses and there is no prospective data on the efficacy of screening for PAS at the routine mid-trimester US examination by non-expert operators [9]. Introducing any population-based screening program would require careful consideration, planning and assessment for clinical utility, but the current pragmatic strategy is becoming increasingly necessary owing to the constant rise in the number of CDs [8].
First-Trimester Screening for PAS
Under the right conditions, PAS can be detected at 11–13 weeks with an US scan. Panaiotova et al. [10] screened 22,604 singleton pregnancies by recording the placental location and asking about history of uterine surgery. One thousand two hundred ninety-eight (6%) were considered at high risk for PAS due to a low-lying placenta and a history of uterine surgery. These were all referred to a specialist clinic and re-assessed at 12–16 weeks for the presence of the following signs: non-visible caesarean scar, bladder wall interruption, thin retroplacental myometrium, presence of intra-placental lacunar spaces, presence of retroplacental arterial-trophoblastic blood flow and irregular placental vascularisation demonstrated by Doppler US (Fig. 1). Fourteen women were diagnosed with PAS at 12–16 weeks’ gestation. At delivery, 13 of these women were confirmed to have PAS with one false positive. No false negatives were reported [10].
35 years G3P2 history of one caesarean section, referred at 15 weeks. a Sagittal view of the uterus demonstrating the location of the pregnancy in the lower uterine segment (solid line, empty uterine cavity; arrow, urine in the bladder). b Transverse view with colour Doppler showing the important blood flow around the gestational sac
Caesarean Scar Pregnancy and PAS
Recent evidence suggests that caesarean scar pregnancy (CSP, Figs. 1 and 2) is the precursor of PAS [10, 11]. In 2003, Jurkovic et al. proposed that the diagnosis of CSP by transvaginal US should be made if the following criteria were satisfied: (1) empty uterine cavity, (2) gestational sac located anteriorly at the level of the internal os covering the visible or presumed site of the previous lower uterine segment caesarean section scar, (3) evidence of functional trophoblastic/placental circulation in colour and/or pulsed Doppler examination, and (4) negative ‘sliding organs sign’ (inability to displace the gestational sac from the level of the internal os using gentle pressure on the transvaginal probe) (Fig. 2) [12].
a Caesarean scar pregnancy (CSP) at 7 weeks with positive embryonic/foetal heart activity. Retroverted uterus. (Supplementary video 2A). b CSP at 6 weeks, no foetal heart activity and not viable with low progesterone and decreasing HCG. Anteverted uterus. c Same CSP as image b with colour Doppler. This highlights the extent of blood flow around the gestational sac even in a non-viable pregnancy
More recently reported is the ‘crossover sign’ (COS) which aims to define the location of the CSP more precisely in an attempt to assess the risk of adverse pregnancy outcomes including development of PAS [13]. The authors recommend the following technique: in a sagittal view of the uterus, a straight line is drawn connecting the internal cervical os and the uterine fundus through the endometrium (endometrium line). The gestational sac is identified and its superior-inferior (S-I) diameter, perpendicular to the endometrial line, is drawn. Patients are categorised according to the relationship between the endometrial line and the S-I diameter of the gestational sac into two groups (Fig. 3). A subsequent retrospective study with 68 pregnancies concluded that the COS may identify women who will go on to develop clinically significant PAS [14] but further confirmatory research is needed to confirm clinical utility.
The current issue with the diagnosis of CSP is that many women do not have an US scan before 11 weeks’ gestation unless they experience complications such as vaginal bleeding. This can make the diagnosis difficult due to the large sac size at this gestation. The smaller the gestational sac, the easier it is to define whether it is within the caesarean niche surrounded by myometrium or ‘just’ close to the internal os and in limited contact with the caesarean scar [12]. Another issue complicating any introduction of screening for CSP is the lack of information on the natural history of CSP and exact risks it poses. A recent systematic review and meta-analysis showed that CSP with foetal heart activity managed expectantly is associated with a high rate of maternal morbidities including haemorrhage, early uterine rupture, hysterectomy and severe PAS [15]. A second systematic review of different treatment strategies on CSP reports a hysterectomy rate of 1.5–7% in 3 randomised controlled trials and of 41% in case of expectant management [16]. Out of the 41 pregnancies managed expectantly, 31 live births occurred (74%). The only prospective case series to describe the natural evolution of CSP was by Zosmer et al. [17]. They reported on 10 cases of CSP, five women needed a hysterectomy at delivery for PAS but no foetal nor neonatal complication was described. Therefore, whilst woman should be informed about the risk of pregnancy complications and interruption of pregnancy offered, no clear evidence exists to support systematic termination of pregnancy for all cases of CSP.
It has recently been proposed that an early (6–9 weeks) first-trimester scan should be mandatory in pregnancies following a caesarean delivery [18]. The evidence for such a statement is weak but the hypothesis is logical. Further investigation with prospective studies including a detailed description of the type of CSP, pregnancy follow-up and the outcome for mother and child are required to support this.
Practical Advice for Ultrasound Examination for PAS
Transabdominal or Transvaginal?
The examination is usually started using a transabdominal approach with a standard convex ultrasound probe (3–5 MHz). Improvement in image quality can be obtained by using a higher frequency (5–9 MHz) probe or where possible, a linear transducer. Indeed, in the case of an anterior placenta, depth of penetration is usually less important and better resolution close to the probe may be preferred. This is possible with a high-frequency probe. The placenta should be explored systematically, from one border to the other, in order to examine the entire placental bed. The transabdominal scan generally provides a good overview and many clinicians are happy to rely on just this modality, although it can be limited in women with a high body mass index (BMI). This can often be overcome, however, by asking the woman to lift her pannus and scanning underneath it. Some clinicians prefer the transvaginal approach which generally also employs a high-frequency probe. The advantage is closer proximity to the lower segment, cervix and praevia or low-lying placenta, but the field of view is narrow. It also provides the most accurate assessment of the relationship of the placenta to the internal os of the cervix to define if it is a praevia. If the rationale is explained, the transvaginal approach is usually well accepted by the women. Most clinicians employ a combination of transabdominal and transvaginal US according to operator preference and the individual woman’s circumstances.
Machine Settings
The appropriated setting of the US machine is important for correct 2D grayscale imaging (depth, focus and gain), but it is even more essential when using colour flow mapping and power Doppler. Excessive vascularity of the lower uterine segment is associated with abnormal placental invasion but is an extremely subjective sign. It is even challenging for highly experienced operators to correctly asses the blood flow. Appropriate machine setting includes the correct power Doppler gain setting for the individual woman, often referred to as the sub-noise gain. This is the gain value where any bloom artefact just disappears on reducing the level of the gain. This individual setting allows for optimal visualisation of the flow despite differences in tissue attenuation. Likewise, the correct velocity scale for colour flow is crucial to appropriate visualisation of the vasculature: if it is set too high, low flow will not be seen; if too low, an ‘aliasing’ artefact will appear [8].
Many modern US machines also offer compound imaging (CRI, CrossXBeam, sonoCT etc.). This sends signals at multiple angles, allowing it to ‘see’ ” tissue from multiple angles with the intention of eliminating artefact. This effectively ‘smooths’ the image seen and tends to blur the interface between the placenta and the myometrium, making many of the US signs difficult to interpret. It should be turned off when examining for the grayscale signs of PAS.
Gestation for USS
Although Panaiotova et al. [10] demonstrated that PAS can be diagnosed between 12 and 16 weeks, they relied on placental localisation at 11–13 weeks to assign risk and determine who should be referred to the specialist clinic. Until sonographers are adequately trained to reliably identify placental location in the first trimester, the majority of screening will occur at the mid-pregnancy anomaly scan. The timing of the diagnostic US by an experienced operator will depend on the resources available but should preferably be as soon as possible in women with any risks for preterm delivery or vaginal bleeding to enable a risk assessment to be made in the event of an emergency delivery. Asymptomatic women with no risks for preterm delivery can usually wait until after 24 weeks (when the trophoblast invasion should be complete) but should be seen by 28 weeks at the latest.
Requirement for Bladder Filling
The US scan must be performed with a filled bladder (between 200 and 300 ml). This is vital because the bladder outline enables identification of the lower uterine segment, presumed location of the previous caesarean scar and allows assessment of placental position relative to it. Also, without a full bladder, important signs such as bladder wall interruption, placental bulge and utero-vesical hypervascularity cannot be appropriately assessed [8, 19•, 20••] (Fig. 4).
(MP4 1912 kb)
(MP4 746 kb)
Probe Pressure
If too much pressure is exerted on the transabdominal US probe, the ‘retroplacental clear zone’ can be obliterated and the myometrium appears falsely thinned leading to anomalous results (Supplementary video 1).
Insonation Angle
It is important to ensure that the probe remains as perpendicular to the placental bed as possible to facilitate appropriate examination of the placental-myometrial interface. Failing to do so can lead to significant ‘drop-out’ artefact preventing robust assessment particularly of the lower aspect of the lower segment (Fig. 5).
Standardised Ultrasound Descriptors
In 2016, the European Working Group on Abnormally Invasive Placenta (EW-AIP) published a proposal to standardise the commonly used US signs for PAS. The agreed descriptors were produced by Delphi consensus [21••]. The aim was to produce a clear definition for each commonly cited US sign based on the published descriptions. US images with examples of the presence and absence of each sign are published in this paper [21••].
The EW-AIP-Standardised Ultrasound Signs and Their Physio-Pathology
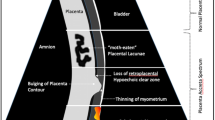
Loss of the ‘Clear Zone’
This term is used when the hypoechoic line normally seen in the myometrium under the placental basal plate (the ‘clear zone’) is not visible on ultrasound. The echolucent area under the placenta is thought to be caused by the thinning of the decidua basalis and development of the fibrinoid layer (Nitabuch’s layer) [19•]. Its absence is thought to be caused by an abnormal extension of the placental villi through the decidua basalis into the myometrium. This sign has been reported in around 70% of cases in series that include information on the depth of invasion [22].
It has been noted that the appearance of this sign changes with advancing gestational age and can be more easily seen in an anterior high placenta. Care must be taken with the US probe pressure as it can be easily obliterated by pressing on the surrounding tissues. It is more readily visualised with a filled bladder [20••].
Myometrial Thinning
This sign is reported as a prenatal diagnostic sign for PAS but is only reported in 50% of cohort studies [22]. In abnormally invasive placenta, the myometrium appears to be vanishingly thin as it cannot be seen (or measured) separately to the placenta. Differential diagnosis includes a ‘uterine window’; here, a normal placenta covers a dehiscence in the myometrium. Therefore, the pathology of a uterine window is that of a scar defect rather than a placental abnormality [19•].
Placental Lacunae
This is the presence of numerous lacunae including some that are large and irregular, often containing turbulent flow visible in grayscale imaging. This is visible on transabdominal and transvaginal US and is the most common US sign described in PAS with around 80% of the authors reporting it prenatally, independently of the depth of invasion [19•]. The differential diagnosis has to be made with placental lakes (echolucent areas in the centre of a cotyledon) which have nothing to do with PAS.
Placental lacunae develop secondary to the distortion of the anatomy of 1 or more cotyledons including in interlobular septa due to the arrival of high-velocity (peak systolic velocity often > 10 cm/s) maternal blood from a radial or arcuate artery [23].
The blood flow inside the lacunae can be detected and observed also in colour and/or pulsed-wave Doppler.
Placental Lacunae Feeder Vessels
These are seen as vessels with high-velocity blood flow arising from the deep arterial vasculature of the myometrium which feed the lacunae. A study found that the total area occupied by vessels in normal and placenta increta placenta beds is similar, but that vessels are significantly sparser and larger in the invasive placenta [24]. This could explain the abnormal haemodynamics underlying the development of the lacunae seen in invasive placentation.
Bladder Wall Interruption
This is the loss of, or interruption to, the bright line representing the bladder wall (the hyperechoic line between the uterine serosa and the bladder lumen). This is potentially caused by villous invasion into the muscle of the posterior wall of the bladder, thereby changing the echogenicity, but is most often an US artefact arising from the massive neovascularity found between the posterior bladder wall and the anterior aspect of the uterus [19•]. Care must be taken with the angle of insonation which can cause artefactual drop-out if the probe is not kept in the correct axis to the placental bed.
Placental Bulge
This is defined as the deviation of the uterine serosa away from the expected plane, caused by an abnormal bulge of placental tissue into a neighbouring organ, typically the bladder. The uterine serosa appears intact, but the outline shape is distorted [21••]. It most likely represents villous invasion deep into and/or through the myometrium resulting in loss of structural integrity of the surrounding uterine muscle. The placenta will then bulge outward into surrounding structures [19•]. This phenomenon is also described at MRI and laparotomy (the ‘snowman’ sign) [25].
Focal Exophytic Mass
Placental tissue is seen breaking through the uterine serosa and extending beyond it usually into a filled urinary bladder. This finding is extremely rare.
Subplacental and/or Utero-Vesical Hypervascularity
This is the observation of a ‘striking’ or abnormally large amount of colour Doppler signal seen in the placental bed or between the myometrium and the posterior wall of the bladder. It is a subjective decision by the operator and therefore requires experience with normal placental beds which can be very vascular. This sign probably indicates numerous, closely packed, tortuous vessels in that region (demonstrating multi-directional flow and aliasing artefact). It results from excessive dilatation of the uteroplacental circulation beyond the spiral arteries, including the radial and arcuate arteries as well as the myometrial arterio-venous anastamoses [26], and it is a prominent feature of PAS on prenatal US [19•]. This can indicate the finding of extensive neovascularisation within the peritoneum, especially between the anterior wall of the uterus and the posterior wall of the bladder at laparotomy.
Bridging Vessels
These vessels appear to ‘bridge’ from the placenta, across the myometrium and beyond the serosa into the bladder or other organs, often running perpendicular to the myometrium [21••]. This ‘bridging’ is an US artefact as these vessels do not traverse between the myometrium and bladder but are actually the contorted vessels of the neovascularity within the peritoneum caught in cross section in a 2-dimensional image [19•].
Reporting an Ultrasound Scan for PAS
An international consortium of experts published a pro forma for US reporting in suspected PAS cases [27]. Reporting all the items contained within this document ensures that the operator has considered many of the important risk factors associated with PAS then examined the woman in a systematic manner, reporting on all of the signs commonly used in the literature. This should ensure that all potential diagnostic factors have been considered and help the operator to come to a decision on the risk of PAS for that particular woman. However, an US report needs to be useful for the MDT involved including the surgeons preparing for delivery. Difficult decisions need to be made in advance before starting the surgery such as whether hysterectomy, partial surgical resection or conservative management may be appropriate [28]. To decide this, the surgical team needs detailed information regarding the invasion and vascularity they will find when they start to reflect the bladder downwards, e.g. into the cervix or parametrium. The US operator is in the perfect position to provide this information and so should consider what clinically relevant information is required.
The US report should therefore contain detailed information on the exact location of the placenta and its relationship to the surrounding structures, the degree of vascularity seen between the bladder and anterior uterus, any vascularity in the cervix/parametrium, and the thickness of the myometrium. US reports should no longer just report the signs and state ‘possible percreta on the left’ but should give a detailed, clinically useful report, e.g. ‘there is an anterio-left lateral placenta praevia covering the internal os and extending posteriorly for approximately 5 cm. This is seen to be bulging into the bladder and possibly the broad ligament on the left. The myometrium is vanishingly thin and cannot be identified on the left where there is suspicion of invasion into the posterior bladder wall. There is significant hypervascularity anteriorly and extending into the cervix on the left side’. Such a detailed report will enable the surgical team to plan appropriately and ensure that the correct decision on management strategy is taken before the surgery is started. To ensure that this occurs, it is vital that the US operators understand the complexity of surgery and the surgeons understand the difficulties and potential limitations of the US. A truly well-functioning MDT will have the US operators who have been present in theatre and surgeons who are familiar with the US room.
Clinical Grading at Delivery
Comparison of US description with clinical observation at delivery and with the pathological report if available is of great importance. FIGO has recently produced a clinical classification for PAS determined by the findings at delivery [29]. This should be reported for all cases of PAS and the exact intra-partum findings fed back to the US operator and compared with the original US report and images to ensure ongoing continual professional education. An MDT debriefing after delivery should be considered to improve the understanding of PAS by each team member.
Conclusion
Whilst PAS remains relatively rare, the incidence is increasing significantly with the rising rate of caesarean delivery. Women with a history of previous CD and an anterior low-lying/praevia placenta need a detailed examination of the placenta by an experienced operator. The US examination should be undertaken systematically and the risk factors and US signs reported as per the recommended pro forma. The sonographer should also endeavour to report the clinically relevant findings which will be useful to the surgical team to plan the delivery. If the US examination is inconclusive or PAS is suspected, the patient should be referred to a specialist centre of excellence [28] to get second opinion and/or expert MDT management of delivery.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Luke RK, Sharpe JW, Greene RR. Placenta accreta: the adherent or invasive placenta. Am J Obstet Gynecol. 1966;95:660–8.
Chantraine F, Braun T, Gonser M, Henrich W, Tutschek B. Prenatal diagnosis of abnormally invasive placenta reduces maternal peripartum hemorrhage and morbidity. Acta Obstet Gynecol Scand. 2013;92(4):439–44.
Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case-control study. PLoS One. 2012;7(12):e52893.
Morlando M, Sarno L, Napolitano R, Capone A, Tessitore G, Maruotti GM, et al. Placenta accreta: incidence and risk factors in an area with a particularly high rate of cesarean section. Acta Obstet Gynecol Scand. 2013;92(4):457–60.
Jauniaux E, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, et al. Placenta praevia and placenta accreta: diagnosis and management: green-top guideline no. 27a. BJOG. 2019;126(1):e1–e48.
D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42(5):509–17.
Society of Gynecologic O, American College of O, Gynecologists, the Society for Maternal-Fetal M, Cahill AG, Beigi R, et al. Placenta accreta spectrum. Am J Obstet Gynecol. 2018;219(6):B2–B16.
Jauniaux E, Bhide A, Kennedy A, Woodward P, Hubinont C, Collins S, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: prenatal diagnosis and screening. Int J Gynaecol Obstet. 2018;140(3):274–80.
Jauniaux E, Bhide A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217(1):27–36.
Panaiotova J, Tokunaka M, Krajewska K, Zosmer N, Nicolaides KH. Screening for morbidly adherent placenta in early pregnancy. Ultrasound Obstet Gynecol. 2019;53(1):101–6.
D’Antonio F, Timor-Tritsch IE, Palacios-Jaraquemada J, Monteagudo A, Buca D, Forlani F, et al. First-trimester detection of abnormally invasive placenta in high-risk women: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(2):176–83.
Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol. 2003;21(3):220–7.
Cali G, Forlani F, Timor-Tritsch IE, Palacios-Jaraquemada J, Minneci G, D’Antonio F. Natural history of Cesarean scar pregnancy on prenatal ultrasound: the crossover sign. Ultrasound Obstet Gynecol. 2017;50(1):100–4.
Cali G, Forlani F, Minneci G, Foti F, Di Liberto S, Familiari A, et al. First-trimester prediction of surgical outcome in abnormally invasive placenta using the cross-over sign. Ultrasound Obstet Gynecol. 2018;51(2):184–8.
Cali G, Timor-Tritsch IE, Palacios-Jaraquemada J, Monteaugudo A, Buca D, Forlani F, et al. Outcome of Cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(2):169–75.
Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Svarre Nielsen H. Cesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril. 2016;105(4):958–67.
Zosmer N, Fuller J, Shaikh H, Johns J, Ross JA. Natural history of early first-trimester pregnancies implanted in Cesarean scars. Ultrasound Obstet Gynecol. 2015;46(3):367–75.
Timor-Tritsch IE, D'Antonio F, Calí G, Palacios-Jaraquemada J, Meyer J, Monteagudo A. Early first-trimester transvaginal ultrasound is indicated in pregnancy after previous Cesarean delivery: should it be mandatory? Ultrasound Obstet Gynecol. 2019. https://doi.org/10.1002/uog.20225.
• Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218(1):75–87 A very good comparison between the pathophysiology of PAS and the corresponding US signs.
•• Maynard H, Zamudio S, Jauniaux E, Collins SL. The importance of bladder volume in the ultrasound diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140(3):332–7 This study clearly demonstrates the importance of performing US with a filled bladder when there is suspicion of PAS.
•• Collins SL, Ashcroft A, Braun T, Calda P, Langhoff-Roos J, Morel O, et al. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47(3):271–5 An international group of experts defined the US signs evident in PAS allowing a more standardised approach for clinical management and research, making studies comparable. It provides clear pictures of each sign.
Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016;215(6):712–21.
Cramer SF, Heller DS. Placenta accreta and placenta increta: an approach to pathogenesis based on the trophoblastic differentiation pathway. Pediatr Dev Pathol. 2016;19(4):320–33.
Chantraine F, Blacher S, Berndt S, Palacios Jaraquemada JM, Sarioglu N, Nisolle M, et al. Abnormal vascular architecture at the placental-maternal interface in placenta increta. Am J Obstet Gynecol. 2012;207:188.e1–9 Available olnline 06/Jul/2012.
Matsuo K, Conturie CL, Lee RH. Snowman sign: a possible predictor of catastrophic abnormal placentation. Eur J Obstet Gynecol Reprod Biol. 2014;181:341–2.
Schaaps JP, Tsatsaris V, Goffin F, Brichant JF, Delbecque K, Tebache M, et al. Shunting the intervillous space: new concepts in human uteroplacental vascularization. Am J Obstet Gynecol. 2005;192(1):323–32.
Alfirevic Z, Tang AW, Collins SL, Robson SC, Palacios-Jaraquemada J, Ad-hoc International AIPEG. Pro forma for ultrasound reporting in suspected abnormally invasive placenta (AIP): an international consensus. Ultrasound Obstet Gynecol. 2016;47(3):276–8.
Collins SL, Alemdar B, van Beekhuizen HJ, Bertholdt C, Braun T, Calda P, et al. Evidence-based guidelines for the management of abnormally-invasive placenta (AIP): recommendations from the International Society for AIP. Am J Obstet Gynecol. 2019;220:511–26.
Jauniaux E, Ayres-De-Campos D, Langhoff- Roos J, Fox K, Collins S. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynecol Obstet. 2019;146:20–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Frederic Chantraine and Sally L. Collins declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Aberrant Placentation: Contemporary Management of Placenta Accreta
Electronic Supplementary Material
Supplementary Video 1
Example of excessive probe pressure (MP4 522 kb)
Supplementary Video 2A
To accompany Fig. 2 (MP4 1600 kb)
Supplementary Video 4A
To accompany Fig. 4 (MP4 1476 kb)
Rights and permissions
About this article
Cite this article
Chantraine, F., Collins, S.L. Prenatal Ultrasound Imaging for Placenta Accreta Spectrum (PAS): a Practical Guide. Curr Obstet Gynecol Rep 8, 86–93 (2019). https://doi.org/10.1007/s13669-019-00267-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-019-00267-8