Abstract
Placenta Accreta Spectrum (PAS) describes the clinical situation when after delivery of the fetus the placenta does not detach normally from the uterus and cannot be removed without causing potentially catastrophic maternal hemorrhage. The incidence of PAS has increased worldwide, most likely associated with the rising rates of cesarean delivery. PAS is the most common reason for peripartum hysterectomy and contributes to the current failure to reduce the maternal death rate in high-income countries. Antenatal recognition of PAS is important to initiate multidisciplinary planning for a safe delivery in a center of expertise to reduce maternal mortality and morbidity. In this chapter, medical and technical aspects of placental (Doppler) ultrasound are described and related to clinical management. To illustrate the abnormal findings, the ultrasound images and perioperative findings of two patients are visualized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abnormally invasive placenta
- Placenta accreta spectrum
- Caesarean section
- Placenta previa
- Ultrasound
- Doppler
35.1 Introduction, Epidemiology and Risk Factors
Placenta Accreta Spectrum (PAS) also known as abnormally invasive placenta (AIP), describes the clinical situation when after delivery of the fetus the placenta does not detach spontaneously from the uterus and if forcibly removed can cause potentially catastrophic maternal hemorrhage [1,2,3]. This “spectrum disorder” ranges from abnormally adherent to deeply invasive placental tissue, traditionally categorized as placenta accreta, when villi are directly attached to the myometrium without interposing decidua (abnormally adherent); placenta increta, when the villi penetrate the myometrium up to the uterine serosa (abnormally invasive); and placenta percreta, when the villi penetrate through the uterine serosa to invade neighboring tissues and organs, such as the bladder (abnormally invasive); but all grades can co-exist within the same placenta [4, 5]. The incidence of PAS has increased worldwide, most likely associated with the rising rates of cesarean delivery [1]. Cesarean section is the greatest risk factor, and therefore PAS could be seen as an iatrogenic disease [6]. Other surgical risk factors include uterine procedures (such as curettage, myomectomy, endometrial ablation, manual removal of the placenta, or adhesiolysis for Asherman syndrome). Advanced maternal age, increasing parity and In vitro Fertilization (IVF) are additional risk factors. Even though PAS is still rare (0.83.1 per 1000 births after a prior cesarean), PAS is the most common reason for peripartum hysterectomy and contributes to the current failure to reduce maternal death rate in high-income countries.
Antenatal recognition of PAS is vital to initiate multidisciplinary planning for a safe delivery in a center of expertise which has been shown to reduce maternal mortality and morbidity. However, the available evidence is complicated by methodologically flawed study designs and variations in the definitions and diagnostic criteria used. In an attempt to address these problems international groups, including the International Federation of Gynecology and Obstetrics (FIGO) and the International Society for AIP (IS-AIP), have published proposals for standardized diagnostic criteria [7] and clinical classification [8].
35.2 Clinical Implications
Optimal management requires accurate antenatal diagnosis, multidisciplinary teamwork, and a robust perinatal management strategy. During routine care, the mid-pregnancy fetal anomaly scan should include placental localization thereby identifying women at risk of persisting low-lying placenta or who have a placenta previa. Women with significant clinical risk factors for PAS (most notably placenta previa and previous cesarean section) should undergo further diagnostic evaluation by an experienced sonographer [9]. MRI is reported to be of adjunctive value for posterior placenta previa, to assess potential bladder invasion and could be a useful tool for multidisciplinary planning for surgery [10,11,12]. Patients with suspected PAS should be referred to a center of excellence. FIGO recommends the use of a clinical grading system for diagnosis at delivery [9] (Table 35.1).
Although the sensitivity of ultrasound diagnosis of PAS is crucial, there is a price of a false-positive diagnosis. A midline laparotomy proceeding straight to post-cesarean hysterectomy is frequently used when a PAS is anticipated. Further risks for complications include the frequently used prophylactic balloons in the pelvic vasculature or planned preterm delivery, resulting in possible iatrogenic maternal and neonatal morbidity [13]. Therefore, the positive predictive value (PPV) and negative predictive value (NPV) of the ultrasound signs are as important as the sensitivity and specificity. Practically, the PPV indicates the confidence with which clinicians can proceed straight to post-cesarean hysterectomy without removing the placenta. In contrast, the NPV characterizes the confidence with which obstetricians can remove a placenta previa without concerns of severe bleeding [14].
Clinical outcomes of women affected by PAS are related to the depth and location of placental invasions, such as bladder involvement or parametrium invasion, although variability in surgical outcome in women presenting with the same degree of placental invasion has been described. A recent approach to prenatal ultrasound staging of PAS showed a good correlation with surgical outcomes, depth of invasion, and the FIGO clinical grading system [15]. Although prenatal diagnosis by ultrasound is an extremely valuable tool, the absence of ultrasound signs does not preclude the diagnosis of focal PAS especially at the abnormally adherent, accreta end of the spectrum and clinical factors as described above remain important in identifying women at high risk.
35.3 Ultrasound Findings
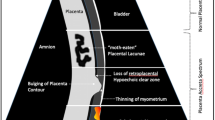
Antenatal ultrasound is the primary tool to identify the location of the placenta and its relation to the uterine scar. The detecting PAS, however, still relies on subjective interpretation of typical sonographic signs of the uterine wall and placenta with two-dimensional (2D) grayscale and color Doppler imaging. Its accuracy depends not only on the experience of the operator, which is limited in many settings by the rarity of the condition but also on technical aspects. Several signs have been reported with varying results in diagnostic accuracy, mainly due to differences in definitions of the ultrasound signs (e.g., how abnormal lacunae are defined), as well as the final clinical diagnosis. To improve consistency and allow for comparison of the ultrasound markers, the IS-AIP proposed a uniform description of ultrasound signs used for the prenatal diagnosis of PAS and an adhoc International Expert Group produced a proforma protocol for the ultrasound assessment (Tables 35.2 and 35.3) [16, 17].
Recent systematic reviews and meta-analysis of ultrasound studies in pregnancies at risk of PAS (women with a prior cesarean delivery, presenting with the anterior low placenta or placenta previa) found that the overall performance of ultrasound, when performed by skilled operators, was very good with a sensitivity and specificity of >95% [18,19,20]. Myometrial thinning, bladder wall interruption, and uterovesical hypervascularity were associated with the most severe types of PAS (percreta) [21]. Placental lacunae and the increased vascularity of the placental bed with large feeder vessels entering the lacunae were the most common ultrasound signs associated with PAS [8]. The highest level of inter-observer agreement for ultrasound signs was found for loss of clear zone, myometrial thinning, the presence of lacunar feeding vessels on 2D color Doppler and crossing vessels and lacunae on 3D color Doppler [4]. Abnormal implantation of the placenta is not always homogeneous, but usually combines areas of abnormal adherence and invasion in the same placenta. This may explain why yet no single sign or combination of markers has been found to be specific for the depth of PAS on histology.
35.4 Color Doppler Ultrasound
The introduction of color Doppler ultrasound has enabled better visualization of the uteroplacental circulation: PAS is often associated with hypervascularization patterns within the placenta and between the placental basal plate and underlying tissues (myometrium, bladder wall). However, despite attempts to standardize these signs, the exact interpretation of color Doppler ultrasound findings remains subjective [22].
The combination of grayscale and color Doppler imaging ultrasound markers is reported to have increased the sensitivity of ultrasound imaging with NPV ranging between 95% and 98%. In a recent study, ultrasound staging had a high correlation with surgical outcome, depth of invasion, and the FIGO classification system [15].
35.5 3D and Power Doppler Imaging
The 3D color Doppler technique has been increasingly used: the placental borders can be manually traced and placental volume can be assessed and measured [22]. In studies to diagnose PAS with three-dimensional (3D) power Doppler, the placenta was assessed in various ways: subjective for abnormal vascularity; intra-placental hypervascularity, inseparable cotyledonal (fetal) and intervillous (maternal) circulations or tortuous confluent vessels across the placental width [14, 23]. In order to quantify hypervascularity, new measurements have been suggested, such as 3D Volume Rendering for placental–bladder interface, the area of confluence or the vascular index [13, 22, 24]. In prospective studies, these measurements differentiated between the presence and absence of PAS and were more predictive of severe cases of PAS than were the 2D ultrasound signs [13]. Although these findings are promising, they need to be confirmed in multicenter trials before appropriate clinical applications can be determined (Figs. 35.6 and 35.6).
35.6 Technical Aspects [16]
35.6.1 Bladder Volume [25]
PAS is most commonly associated with an anterior low-lying placenta or placenta previa. To visualize the anterior lower segment of the uterus, an ultrasound examination must be carried out with a full bladder (around 250–300 mL). Without a full bladder, signs such as bladder wall interruption, placental bulge, and uterovesical hypervascularity cannot be appropriately assessed. On the other hand, if the bladder is overfilled (>500 mL), compression of the placental bed may obscure the vascular architecture and change the appearance of the interface.
35.6.2 Angle of Insonation
The best angle of insonation of the area of interest, the border between the placenta and the myometrium, is 90°; this angle is not easily achieved by either transabdominal or transvaginal scanning. If the angle is close to 0°, artifacts can make the correct analysis of the placental-myometrial interface difficult and result in false-positives in particular regarding “myometrial thinning.”
35.6.3 Transabdominal and Transvaginal Probe Pressure
The required pressure used by the operator on the probe depends on the woman’s body habitus and distribution of adipose tissue. Pressing too hard may result in artifacts such as “loss of the clear zone” and myometrial thinning.
35.6.4 Color Doppler and (3D) Power Doppler
Attention must be paid to using the appropriate blood flow velocity settings (pulse repetition frequency, PRF, usually 1.3 kHz for color Doppler and 0.9 kHz for power Doppler). If the PRF is too low there will be increased aliasing potentially leading to the suggestion of hypervascularization. To select the appropriate Doppler setting the gain should be increased until the image is saturated with color and then slowly reduce the gain until the apparent artifacts disappear (called the Sub-Noise Gain (SNG) setting). Using the SNG ensures that the difference in Doppler signal attenuation caused by differences in the amount and type of tissue being insonated is appropriately corrected for.
35.7 Clinical Examples
35.7.1 Clinical Cases-Imaging-Related to Clinical Findings During Surgery
Case 1
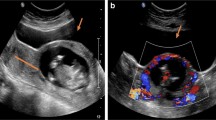
Mrs. A, 36-year-old, Gravida 4, Parity 3, 35 weeks of gestational age with three previous cesarean sections (Fig. 35.7).
(a) Loss of “clear zone” and undetected myometrial wall (arrow), which is seen in (b) 3D volume rendering ultrasound (axial plane), which is predominantly a uterine wall defect in the left lower uterine segment (arrow) in 33 weeks of gestational age and (c) proven during surgery (arrow) at 34 weeks of gestational age
Case 2
Mrs. L, 30-year-old, Gravida 2, Parity 1, 33 weeks gestational age with one previous cesarean section (Fig. 35.8).
All examinations were carried out using a transabdominal probe from 4.0 to 6.0 MHz or transvaginal 5.0–7.0 MHz (GE Voluson® 730, General Electric, and Samsung WS80A Elite, Samsung); when using color Doppler ultrasound, the pulse repetition frequency (PRF) or scale was set at 1.3 KHz, but this was adapted to identify the presence of placental lacunar flow.
References
Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6):1226–32.
Hasegawa J, Tanaka H, Katsuragi S, Sekizawa A, Ishiwata I, Ikeda T. Maternal deaths in Japan due to abnormally invasive placenta. Int J Gynaecol Obstet. 2018;140(3):375–6.
Palacios-Jaraquemada JM, D’Antonio F, Buca D, Fiorillo A, Larraza P. Systematic review on near miss cases of placenta accreta spectrum disorders: correlation with invasion topography, prenatal imaging, and surgical outcome. J Matern Fetal Neonatal Med. 2019:1–8.
Zosmer N, Jauniaux E, Bunce C, Panaiotova J, Shaikh H, Nicholaides KH. Interobserver agreement on standardized ultrasound and histopathologic signs for the prenatal diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140(3):326–31.
Bhide A, Sebire N, Abuhamad A, Acharya G, Silver R. Morbidly adherent placenta: the need for standardization. Ultrasound Obstet Gynecol. 2017;49(5):559–63.
Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33(4):244–51.
Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511–26.
Jauniaux E, Hussein AM, Fox KA, Collins SL. New evidence-based diagnostic and management strategies for placenta accreta spectrum disorders. Best Pract Res Clin Obstet Gynaecol. 2019;61:75–88.
Jauniaux E, Bhide A, Kennedy A, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: prenatal diagnosis and screening. Int J Gynaecol Obstet. 2018;140(3):274–80.
Palacios-Jaraquemada JM, Karoshi M, Keith LG. Uterovaginal blood supply: the S1 and S2 segmental concepts and their clinical relevance. 2nd ed. London: Sapiens Publishing; 2012.
Palacios Jaraquemada JM, Bruno CH. Magnetic resonance imaging in 300 cases of placenta accreta: surgical correlation of new findings. Acta Obstet Gynecol Scand. 2005;84(8):716–24.
Palacios Jaraquemada JM, Bruno CH, Martin E. MRI in the diagnosis and surgical management of abnormal placentation. Acta Obstet Gynecol Scand. 2013;92(4):392–7.
Collins SL, Stevenson GN, Al-Khan A, et al. Three-dimensional power doppler ultrasonography for diagnosing abnormally invasive placenta and quantifying the risk. Obstet Gynecol. 2015;126(3):645–53.
Shih JC, Palacios Jaraquemada JM, Su YN, et al. Role of three-dimensional power Doppler in the antenatal diagnosis of placenta accreta: comparison with gray-scale and color Doppler techniques. Ultrasound Obstet Gynecol. 2009;33(2):193–203.
Cali G, Forlani F, Lees C, et al. Prenatal ultrasound staging system for placenta accreta spectrum disorders. Ultrasound Obstet Gynecol. 2019;53(6):752–60.
Collins SL, Ashcroft A, Braun T, et al. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47(3):271–5.
Alfirevic Z, Tang AW, Collins SL, Robson SC, Palacios-Jaraquemada J. Group obotA-hIAE. Pro forma for ultrasound reporting in suspected abnormally invasive placenta (AIP): an international consensus. Ultrasound Obstet Gynecol. 2016;47(3):276–8.
D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42(5):509–17.
Jauniaux E, Bhide A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217(1):27–36.
Jauniaux E, Alfirevic Z, Bhide A, et al. Placenta praevia and placenta accreta: diagnosis and management. Int J Obstet Gynecol. 2019;126(1):e1–e48.
Pagani G, Cali G, Acharya G, et al. Diagnostic accuracy of ultrasound in detecting the severity of abnormally invasive placentation: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97(1):25–37.
Haidar ZA, Papanna R, Sibai BM, et al. Can 3-dimensional power Doppler indices improve the prenatal diagnosis of a potentially morbidly adherent placenta in patients with placenta previa? Am J Obstet Gynecol. 2017;217(2):202.e201–13.
Cali G, Giambanco L, Puccio G, Forlani F. Morbidly adherent placenta: evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta. Ultrasound Obstet Gynecol. 2013;41(4):406–12.
Aryananda RA, Akbar A, Wardhana MP, et al. New three-dimensional/four-dimensional volume rendering imaging software for detecting the abnormally invasive placenta. J Clin Ultrasound. 2019;47(1):9–13.
Maynard H, Zamudio S, Jauniaux E, Collins SL. The importance of bladder volume in the ultrasound diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2018;140(3):332–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rijken, M.J., Aryananda, R.A., Collins, S. (2023). Role of Ultrasonography in Placenta Accreta Spectrum. In: Maulik, D., Lees, C.C. (eds) Doppler Ultrasound in Obstetrics and Gynecology. Springer, Cham. https://doi.org/10.1007/978-3-031-06189-9_35
Download citation
DOI: https://doi.org/10.1007/978-3-031-06189-9_35
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06188-2
Online ISBN: 978-3-031-06189-9
eBook Packages: MedicineMedicine (R0)