Abstract
Purpose of Review
Drug-resistant epilepsy represents around one-quarter of epilepsies worldwide. Although ketogenic diets (KD) have been used for refractory epilepsy since 1921, the past 15 years have witnessed an explosion of KD use in the management of epilepsy. We aimed to review evidence from randomized controlled trials (RCTs) regarding the efficacy and safety of KD in drug-resistant epilepsy in children and adolescents.
Recent Findings
A literature search was performed in the Pubmed, Cohrane, Scopus, ClinicalTrials.gov, and Google Scholar databases. Predefined criteria were implemented regarding data extraction and study quality. Data were extracted from 14 RCTs in 1114 children and adolescents aged from 6 months to 18 years. Primary outcome was seizure reduction after the intervention. In 6 out of the 14 studies, there was a statistical significant seizure reduction by > 50% in the KD-treated group compared with the control group over a follow-up of 3–4 months. Secondary outcomes were adverse events, seizure severity, quality of life, and behavior. Gastrointestinal symptoms were the most frequent adverse events. Serious adverse events were rare.
Summary
We conclude that the KD is an effective treatment for drug-resistant epilepsy in children and adolescents. Accordingly, RCTs investigating long-term impact, cognitive and behavioral effects, and cost-effectiveness are much anticipated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is the most frequent neurological disease affecting about 1% of the general population. The prevalence of epilepsy in children is consistently higher than in other age groups and ranges from 3.2 to 5.5/1000 in developed countries and 3.6–44/1000 in underdeveloped countries. Prevalence also seems highest in rural areas and during the first year of life and declines during adulthood [1].
Epilepsy is a disease characterized by an enduring predisposition to generate seizures and by numerous neurobiological, cognitive, psychological, and social consequences [2]. There are many causes that contribute to epilepsy, but the most well-studied ones include genetic differences, inherited metabolic and mitochondrial diseases, traumatic brain injuries, cerebrovascular diseases, cerebral palsy, tumors and others including toxic and infectious disorders, neurodegenerative diseases, malformations of cortical development and inflammatory diseases [3].
Although many antiepileptic medications have been developed, there is a significant proportion of non-responsiveness (about 25% of patients). According to the International League Against Epilepsy, “drug resistant epilepsy may be defined as failure of adequate trials of two tolerated and appropriately chosen and used antiepileptic drug (AED) schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.” This condition is also mentioned as “medically refractory/intractable” or “pharmacoresistant” epilepsy [4]. There are only a few options for the management of refractory epilepsy, including surgery, neurostimulation, and the noninvasive ketogenic diet (KD).
Several theories have been proposed to explain the mechanisms leading to anticonvulsant medication resistance: (1) The transporter hypothesis, in which multi-drug resistant transporters are overexpressed and obstruct the entrance of the drugs into the cell, thereby keeping them away from their site of action. (2) The target hypothesis, suggesting that modification in the target cellular regions (ion-channels or receptors) contributes to the development of resistance against the antiepileptic effect of the drugs. (3)The gene variant hypothesis, stating that alterations in genes that regulate either the pharmacokinetic or the pharmacodynamic behavior of the drug cause or show resistance to the antiseizure drugs (ASDs). (4) Τhe neural network hypothesis, which postulates that seizure-induced neuro-modulation also triggers the remodeling of the neuronal networks. As a result, there is a downregulation of the physiological anti-seizure system which hinders the ASDs from reaching the target neuronal region [5, 6]. In general, all of the aforementioned mechanisms result in an energy deficit for the brain which subsequently leads to drug-resistant epilepsy.
Fasting and other dietary interventions as treatments of epilepsy have their roots in ancient Greece since 500 BC. Hippocrates and Galen suggested “complete abstinence from food and water” as treatment for individuals with seizures. There are also references to fasting and prayer as a way to cure epilepsy in the biblical times (Bible, Mark 9:14–29). To mimic the metabolism of fasting, the classic KD was introduced by Dr. Wilder from Mayo Clinic as a treatment for epilepsy in the 1920s. It was on July 1921 in Rochester that Dr. Wilder published the first article about “The effect of ketonemia on the course of epilepsy” in The Clinic Bulletin [7]. After three decades of heightened interest in the ketogenic diet, there was a decline in its use by the 1950s, when new antiepileptic drugs emerged. In 1993, the father of a 2-year-old boy, who was suffering from daily myoclonic seizures resistant to available pharmaceutical treatments, Jim Abrahams, turned to KD and John Hopkins Hospital, for his son Charlie [7–10]. After one week on the diet, Charlie experienced complete seizure reduction, his electroencephalogram returned to normal and was released from anticonvulsant’s side effects. In 1994, Jim Abrahams created the Charlie Foundation, while through many campaigns and donations, the diet gained popularity among the public. Over the past 20 years, there has been an explosion of scientific interest in KD and its use worldwide [7–10].
There are four types of KD, including the Classic KD (CKD), the modified Atkins diet (MAD), the low glycemic index treatment (LGIT), and the medium chain triglyceride diet (MCT). The CKD is based on a ratio of fat to carbohydrate and protein, usually 3:1 or 4:1. Fat is provided as long-chain triglycerides, protein is provided in small quantities to ensure growth, and carbohydrate intake is restricted to few servings of vegetables or fruit. The MAD includes a less extreme dietary macronutrient distribution (60% fat, 30% protein, and 10% carbohydrate by weight), without limitations in daily calorie intake. The LGIT is based on a balanced calorie intake to maintain growth and nutrition. In recent trials, this diet is implemented on an outpatient basis. Fat contributes 60% of all calories while protein represents 20–30%. Carbohydrate intake is limited to 40–60 g per day, which is a greater intake than in the CKD or MAD, but the carbohydrates are restricted to foods with a glycemic index < 50. The glycemic index is a measure that reflects the ability of a carbohydrate source to elevate blood glucose [11] (Fig. 1).
As of September 15, 2021, approximately 7 reviews [12••, 13, 14, 15••, 16–18 ] on KD and drug-resistant epilepsy have been published on specific aspects of the association between dietary treatment and seizure control but have not covered the topic in a comprehensive manner. The objective of this systematic review is to synthesize evidence based on randomized controlled trials (RCTs) about the efficacy and safety of KD therapies in children with drug-resistant epilepsy.
Methodology
Literature Search
A literature search was performed in the databases of Pubmed, Cochrane Library, Scopus, ClinicalTrials.gov, and Google Scholar. The terms used were ((((ketogenic diet) AND (children OR infants)) AND (drug resistant OR refractory)) AND (epilepsy OR convulsion OR seizures)) AND (randomized controlled trial). There were no date limits; the study language was English; and the studies were included in the systematic review according to the relevance of the subject.
Study Selection and Quality
Only randomized controlled trials with children and adolescents up to 18 years were included. Children were eligible if they had experienced at least 1 seizure per week despite receiving two or more antiepileptic drugs. The study quality was assessed according to the Oxford Quality Scoring System [19]. The primary outcome was the proportion of children with seizure reduction > 50% after a follow-up period with diet treatment. Secondary outcomes included seizure severity, side effects, tolerance, rate of withdrawals, quality of life and socio-economic parameters.
Results
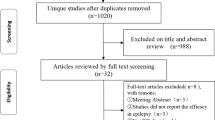
Eighty-two studies were identified through the literature search, and 2 more were identified through cross-referencing; after removal of duplicates, 65 individual records were left. Thirty-eight studies were excluded because they did not meet eligibility criteria (i.e. included adults, compared anti-epileptic drugs, were conducted in animals) and 13 were excluded because they were not RCTs. The search was based on the PRISMA guidelines; the relevant flowchart is presented in Fig. 2.
Study Characteristics
Only two RCTs included children whose seizures were not controlled by at least three antiepileptic drugs (AEDs) [20, 21]. The inclusion criteria regarding seizure frequency varied from > 1 seizure daily [22] to at least 2 seizures per month [23]. The types of KD analyzed in the studies were CKD, MAD, and LGIT. The baseline characteristics of the treatment and control groups were similar (gender, age, nationality, type of epilepsy, epilepsy syndromes, age of diagnosis of epilepsy) in all RCTs. Patients with comorbidities such as diabetes, hyperinsulinemia, hyperlipidemia, metabolic disorders, renal calculi or other medical contraindications, previous treatments with CKD, MAD, or LGIT and surgical remediable epilepsy were excluded. In one study [24], patients with behavioral or motivational problems that affect compliance with the diet were also excluded. In one RCT, the inclusion criteria were strict including patients who achieved seizure-free outcomes and showed improvement in hypsarrythmic patterns [25]. The adverse events were also marked for each type of diet and most frequently included constipation and other gastrointestinal disorders, hunger, anorexia, central nervous system disorders such as headaches or lethargy, and lower respiratory infections. The attrition rate varied between 8 and 33% over 3–6 months. The differential dropout rate at study endpoint (i.e. active group vs control) was lower than 15%. Only in one study [26], the drop out was 42% after 16 months.
The study characteristics are presented in the Table 1. The name, number of participants, age range, seizure type and epilepsy syndromes, duration, retention rate, and the proportion of seizure reduction at 3 and 6 months in both treatment and control groups were analyzed for each study.
Study Quality
Children were randomly assigned to the KD therapy group and the control group receiving the usual therapy, namely, antiepileptic drugs in 6 RCTs [22, 24, 26,27,28, 29•]. In the rest 8 RCTs [20, 21, 23, 25, 30–33 ], comparison was made across KD therapies. In 13 [20–28, 29•, 30–32] out of the 14 RCTs, there was no blinding except in the study by Kim et al. [33], in which participants, providers and investigators were blinded to the treatment group assignments. The seizure records were assessed at baseline and after 3–16 months of treatment. The duration of the follow-up varied between 3–6 months in 12 out of the 14 studies. In the study by Kang et al. [25], patients in the long-term trial group maintained the diet for > 2 years and were followed for 13–18 months after discontinuation of the diet. The primary outcome in all studies was the proportion of reduction in seizure frequency, and, in most studies, secondary outcomes were seizure severity, adverse events, quality of life, and behavioral changes.
Study Outcomes
Primary Outcome
Data were extracted from 14 RCTs including a total of 1114 participants aged from 6 months to 18 years. In 6 [22, 24, 26,27,28, 29•] out of the 14 studies, there was a statistically significant seizure reduction by > 50% in the KD-treated group compared with the control group over a follow-up of 3–4 months. If the follow-up was extended to 16 months, the difference between groups was attenuated and lost significance [26]. Four RCTs [23, 27, 28, 32] revealed that more than half of the patients had > 50% seizure reduction after at least 3 months of dietary intervention. In 6 [22, 24, 26,27,28, 29•] out of the 14 studies comparing KD with usual care, the proportion of KD-treated patients who had > 50% seizure reduction after at least 3 months was 27–56%. In two studies comparing CKD and MAD [32, 33], the first appeared to be slightly (but not statistically significantly) more efficacious than MAD after 6 months. Regarding the lipid ratio of the KD, two RCTs showed the effectiveness of a 2.5:1 or 3:1 ratio instead of a 4:1 ratio. The proportion of patients in the 2.5:1 or the 3:1 diet groups with > 50% reduction in seizures was higher, and the tolerability was better than in the 4:1 diet group.
Secondary Outcomes
Adverse Events
All studies reported gastrointestinal symptoms and a mild increase in fasting total cholesterol concentration as the most frequent side effects of the KD. Constipation, vomiting, anorexia, and diarrhea occurred in the first 3 months of the diet and tended to be reduced in frequency during the follow-up visits. Most of these adverse events were controlled by dietary adjustments or conservative treatment, including H2 blockers and anti-emetics. In two studies with longer follow-up [25, 26], a clinically relevant decrease in height and weight was reported after 6–12 months in children receiving KD than those receiving the usual care. Lower respiratory infections, metabolic acidosis, and symptomatic hypoglycemia were tolerable with conservative treatment. Acute pancreatitis in one child, hyperammonemic encephalopathy in another and frequent chest infections, ureteral stones, aspiration pneumonia and osteoporosis were reasons for discontinuation of KD treatment [21, 28, 33]. Urolithiasis and microscopic hematuria were asymptomatic and were reported in some cases after 3 months and more frequently after 6 months of dietary treatment. In two studies, osteopenia was reported in children after 8 months on the diet. In the study by Sondhi et al. [31], thrombocytopenia was noted during evaluation at 24 weeks at patients receiving KD therapy concomitantly with sodium valproate; ten patients treated with KD and zonisamide experienced hypercalciuria at 24 weeks. Other mild adverse effects were headache and dizziness.
Behavior and Cognitive Outcomes
Four studies [25–28] examined behavioral and cognitive outcomes. The majority of parents reported improvement in their children’s alertness, activity level, social interaction, and behavior [27, 28]. According to the study by Wijnen et al. the KD was associated with significantly fewer side effects regarding behavior (irritability) and helped children experience fewer motor and coordination problems when compared to anti-epileptic drugs [26].
Effectiveness on Seizure Severity
In two studies, the seizure severity was decreased in the KD group. Lambrechts et al. reported that children in the KD group showed a reduction of 50% in seizure severity after 4 months compared with the control group. In the other 12 studies, seizure severity was not reported [24, 26].
Quality of Life
Quality of life was assessed only in 1 study, by using questionnaires for children’s quality of life. The questionnaires were answered by the parents or care-takers to calculate cost per quality-adjusted life year (QALY) for the patients. Due to the high cost of follow-up in the KD group, cost per QALY ratios were inconclusive [26].
Retention Rate
All studies experienced withdrawals. The retention rate varied between 58 and 100%. The lower retention rate was observed in the study by Wijnen et al. where the follow-up was 16 months. The most common reasons for dropping out were adverse events, intolerance, lack of seizure reduction, and negative efficacy. In the study by Karimzadeh et al. the total dropout of patients under 2 years of age was 100% in the CKD group. The use of formula-based KD in this study had a better compliance and tolerability from infants and small children (1–3 years old). Comparing MAD and CKD, one study [32] reported better retention rates in the CKD group, whereas in the study by Kim et al. [33] withdrawals in two groups were similar. One study [21] revealed lower rates of discontinuation in the 4:1 lipid ratio KD group compared to the 3:1 group (Table 1).
Discussion
The results from our systematic review suggest that KD therapies could be an effective treatment option for children and adolescents with drug-resistant epilepsy. The effects of KD appear to be pleiotropic, but the mechanisms responsible have not been fully elucidated. Several biochemical pathways in the brain are affected. One of these is linked to the decrease in glycolysis and the increase in the mitochondrial oxidative metabolism of ketone bodies. Ketones increase ATP production by providing acetyl-CoA units and inhibit production of reactive oxygen species (ROS); at the same time, the rise in acetyl-CoA availability promotes histone and non-histone hyperacetylation and, as a result, increases endogenous antioxidants. Other potential mechanisms of action of ketone bodies include the decrease in glutamate levels in the intrasynaptic space and the increase in GABA levels; the attenuation of inflammatory mediators produced by infiltrating macrophages; the opening of K+-ATP channels; and, consequently, the reduction of neuron exitability (Figs. 3 and 4). In addition, the altered gut microbiota patterns caused by KD treatment may play a beneficial role in the control of the seizures [34, 35].
Simplified schema of glucose and fat metabolism: potential effect on epilepsy pathophysiology. In contrast to energy consuming glycolytic pathways, fat beta oxidation is a high energy producing mechanism which provides high amount of ATP. Epilepsy is a condition associated with cerebral energy deficiency. Ketogenic diet through fat burning could increase energy supply in the brain, replenish the brain energy deficit and, as a result, reduce the epileptic activity
Ketogenic diet effect on GABA metabolism. Increased ketone flow from β-oxidation results in an increased production of acetyl-CoA with consequent increased consumption of oxaloacetate. Increased consumption of oxaloacetic acid requires the consumption of increased amounts of aspartate, and this biochemical pathway leads to increased GABA production. GABA is known to be an inhibitory neurotransmitter with known antiepileptic activity
Epilepsy is a condition associated with decreased energy availability to the brain. KD increases brain energy reserves and contributes to improved brain energy balance and metabolism. This process leads to a reduction of epileptic activity, while simultaneously improves mental functions. Anti-epileptic drugs lead to the reduction of epileptic activity by decreasing the energy needs of the brain. Nevertheless, decreased epileptic activity induced by the latter mechanism deteriorates the epileptic brain energy deficit, thereby contributing to the suppressive side effect of anticonvulsant drugs (Fig. 5).
Ketogenic diet and drug effect on epileptic brain metabolism. Epilepsy is a condition associated with decreased energy balance of the brain. Ketogenic diet by increasing brain energy reserves contributes to the improvement of the energy balance of brain metabolism. This process leads to a reduction of epileptic activity while at the same time improves mental functions. Antiepileptic drugs lead to the reduction of epileptic activity by decreasing the energy needs of the brain. Nevertheless, decreased epileptic activity induced by anticonvulsant drugs deteriorates the epileptic brain energy deficit contributing to the suppressive side effect of antiepileptic drugs
Many studies have suggested that KD is an evidence-based treatment for drug-resistant epilepsy. There have been several systematic reviews and meta-analyses assessing the efficacy of KD on refractory epilepsy, some of which were based on prospective and cohort studies, whereas there is a plethora of studies providing data for adults. This systematic review included only RCTs in children and adolescents aged from 6 months to 18 years. The main outcomes of this systematic review were in accordance with previous studies [12••, 13, 15••, 16, 36].
In the 14 RCTs identified, the proportion of children with > 50% reduction in seizures was higher in the KD group than in the control group. This proportion ranged between 7 and 63% and depended on the duration of the follow-up and the type of KD and was adjusted in most RCTs for baseline characteristics of the patient groups. The above findings are compatible with previous studies. Sourbron et al. [15••] showed that seizure frequency reduction ≥ 50% occurred in 35–56% of participants in the KD group, compared with 6–18% in the control group. In the study by Lyons et al. [12••], the proportion of infants who achieved ≥ 50% seizure reduction was 59% (95% CI, 53–65%) and 33% were seizure-free (95% CI, 26–43%). Another observational study including 29 adult and adolescent patients (mean age: 32 years, range 11–51 years) showed that 45% of patients had ≥ 50% reduction in seizure frequency after KD treatment [36].
With regard to the attrition rate, Wijnen et al. concluded that dropouts ranged between 10 and 26% during 3–6 months, while the higher dropout rate was reported when the follow-up was extended to 16 months [26]. Lyons et al. showed retention rates of 84%, 68%, 43%, and 27% after 3, 6, 12, and 24 months, respectively. The reasons for discontinuation were similar, including inefficacy and adverse events, and included extra intercurrent enterocolitis and being seizure free [12••]. In the study by Nei et al. 62% of patients remained on the diet by 3 months and this declined to 38% by 6 months. The main reasons for discontinuation were intolerance and lack of efficacy.
Overall, the adverse events were similar to those reported in previous studies including mainly gastrointestinal symptoms and dyslipidemia. These side effects were reported within the first 3 months of KD [24, 25]. More serious adverse events included lower respiratory infections, abdominal pain, anorexia, lethargy, and hyperammonemic encephalopathy [28], which in most cases were treated with conservative treatment.
Limitations
In the majority of the retrieved RCTs, the main limitation was lack of treatment blinding; however this is a common problem with diet interventions. Another issue was the follow-up duration that ranged from 3 to 6 months, and only in 2 studies the follow-up continued for more than 12 months [25, 26]. In the study by Wijnen et al. [26], the control group was studied for 4 months, but the results were extrapolated to 16 months, assuming that the findings during these 4 months are representative of long-term CAU treatment. The control group was studied for 4 months and then offered to receive the KD until the end of the follow-up at 16 months, but the specific number of participants who agreed to continue on the KD was not reported. Finally, data about quality of life, behavior, and seizure frequency were reported by the parents or child care-takers, and, therefore, there is a lack of subjectivity in data collection.
Perspectives
KD and its effects have been studied on a short-term basis, but long-term studies in human subjects need to be conducted. Long-term effects of the KD need be clarified not only regarding efficacy but also regarding frequent adverse events such as urolithiasis, dyslipidemia, osteopenia, and poor growth. Alterations in mental state and behavior of the children on the KD should also be further examined. In the study by Ijff et al. [37], which assessed the impact of KD on cognitive and behavioral outcomes of children and adolescents with refractory epilepsy, those in the KD group experienced lower levels of anxiety and tended to be more productive. Also, cognitive test results suggested an improvement of motivation in the KD group. Similar studies of longer duration are required to provide more insight into these possibilities.
Another aspect of the KD treatment that lacks clarity is its cost-effectiveness. In a retrospective review by Kayyali et al. [38], there was a statistically significant (p = 0.038) decline of the charges related to hospitalizations and visits at the emergency departments over a period of 12 months after the initiation of the KD, which is very promising and should be confirmed by future RCTs.
Conclusion
Refractory epilepsy represents about 25–30% of all pediatric epilepsies and is a phenomenon with global socio-economic consequences. Uncontrolled seizures result in cognitive and behavioral problems, brain dysfunction, and are associated with an increase in hospitalizations and high mortality rates among pediatric patients. The KD should no longer be considered as the last treatment option for children and adolescents with drug-resistant epilepsy, but rather be included among the primary treatment options, because many studies have demonstrated its efficacy on seizure reduction with minor adverse events in the majority of cases. Given the established efficacy of the KD in controlling refractory epilepsy in children in the short-term, RCTs investigating long-term efficacy, cognitive and behavioral effects, and cost-effectiveness are much anticipated.
Abbreviations
- AEDs:
-
Anti-Epileptic Drugs
- ASDs:
-
Anti-Seizure Drugs
- BDNF:
-
Βrain-Derived Neurotrophic Factor
- BHB:
-
Beta-Hydroxybutyric Acid
- BMI:
-
Body Mass Index
- CAU:
-
Care As Usual
- CKD:
-
Classic Ketogenic Diet
- GABA:
-
Gamma Aminobutyric Acid
- GI:
-
Gastrointestinal
- KD:
-
Ketogenic Diet
- LGIT:
-
Low Glycemic Index Treatment
- MAD:
-
Modified Atkins Diet
- MCT:
-
Medium Chain Triglycerides
- NADH:
-
Nicotinamide Adenine Dinucleotide Phosphate
- RCTs:
-
Randomized Controlled Trials
- ROS:
-
Reactive Oxygen Species
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Camfield P, Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord. 2015. https://doi.org/10.1684/epd.2015.0736.
Fisher RS, et al. Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005. https://doi.org/10.1111/j.1528-1167.2005.00273_4.x.
Sirven JI. Epilepsy: A Spectrum Disorder. Cold Spring Harb Perspect Med. 2015. https://doi.org/10.1101/cshperspect.a022848.
Kwan P, et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010. https://doi.org/10.1111/j.1528-1167.2009.02397.x.
Sheng J, Liu S, Qin H, Li B, Zhang X. Drug-Resistant Epilepsy and Surgery. Curr Neuropharmacol. 2017. https://doi.org/10.2174/1570159x15666170504123316.
Kwan P, Schachter SC, Brodie MJ. Drug-Resistant Epilepsy. N Engl J Med. 2011. https://doi.org/10.1056/NEJMra1004418.
Wheless JW. History of the ketogenic diet. Epilepsia. 2008. https://doi.org/10.1111/j.1528-1167.2008.01821.x.
Cervenka MC, Doerrer S, Henry BJ, Kossoff EH, Turner Z. The Ketogenic and modified Atkins diets : treatments for epilepsy and other disorders. 6th ed. New York: Demos Health; 2016.
Freeman JM, Kossf EH, Hartman AL. The ketogenic diet: One decade later. Pediatrics. 2007;119:535-43.
Williams E, Abrahams J, Maguire A, Harris G. A parent’s prerspective on dietary treatments for epilepsy. Epilepsy Res. 2012. https://doi.org/10.1016/j.eplepsyres.2011.09.024.
Auvin S. Non-pharmacological medical treatment in pediatric epilepsies. Rev Neurol. 2016. https://doi.org/10.1016/j.neurol.2015.12.009.
•• Lyons L, Schoeler NE, Langan D, Cross JH. Use of ketogenic diet therapy in infants with epilepsy: A systematic review and meta-analysis. Epilepsia. 2020. https://doi.org/10.1111/epi.16543. This systematic review indicates that KD is safe and tolerable and that it can be an effective treatment option for infants with drug-resistant epilepsy. However, there are few studies focusing on infants treated with KD, and high-quality evidence is lacking. High-quality randomized-controlled trials are needed to confirm the effectiveness, safety, and tolerability of dietary treatment in this vulnerable age group.
Cai QY, et al. Safety and tolerability of the ketogenic diet used for the treatment of refractory childhood epilepsy: a systematic review of published prospective studies. World J Pediatr. 2017. https://doi.org/10.1007/s12519-017-0053-2.
Wells J, Swaminathan A, Paseka J, Hanson C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy-a review. Nutrients. 2020. https://doi.org/10.3390/nu12061809.
•• Sourbron J, et al. Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Child’s Nerv Syst. 2020. https://doi.org/10.1007/s00381-020-04578-7. This systematic review and meta-analysis investigated the efficacy of KD in drug-resistant epilepsy in children and adolescents. Given the beneficial clinical results, dietary interventions should be considered for children and adolescents with refractory epilepsy who are not eligible for epilepsy surgery. Future studies should be multi-center and long-term, and evaluate potential biomarkers and adverse effects.
Kj M, Cf J, Bresnahan R, Rg L, Pn C. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev. 2018. https://doi.org/10.1002/14651858.CD001903.pub4.www.cochranelibrary.com.
Prezioso G, Carlone G, Zaccara G, Verrotti A. Efficacy of ketogenic diet for infantile spasms: A systematic review. Acta Neurol Scand. 2018. https://doi.org/10.1111/ane.12830.
Keene DL. A Systematic Review of the Use of the Ketogenic Diet in Childhood Epilepsy. Pediatr Neurol. 2006. https://doi.org/10.1016/j.pediatrneurol.2006.01.005.
Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996. https://doi.org/10.1016/0197-2456(95)00134-4.
Karimzadeh P, Moosavian T, Moosavian HR. Effects of a formula-based ketogenic diet on refractory epilepsy in 1 to 3 year-old patients under classic ketogenic diet. Iran J Child Neurol. 2019. https://doi.org/10.22037/ijcn.v13i4.22876.
Hee Seo J, Mock Lee Y, Soo Lee J, Chul Kang H, Dong Kim H. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios - Comparison of 3:1 with 4:1 diet. Epilepsia. 2007. https://doi.org/10.1111/j.1528-1167.2007.01025.x.
Neal EG, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. 2008. https://doi.org/10.1016/S1474.
Raju KNV, et al. Efficacy of 4:1 (classic) versus 2.5:1 ketogenic ratio diets in refractory epilepsy in young children: A randomized open labeled study. Epilepsy Res. 2011. https://doi.org/10.1016/j.eplepsyres.2011.05.005.
Lambrechts DAJE, de Kinderen RJA, Vles JSH, de Louw AJA, Aldenkamp AP, Majoie HJM. A randomized controlled trial of the ketogenic diet in refractory childhood epilepsy. Acta Neurol Scand. 2017. https://doi.org/10.1111/ane.12592.
Kang HC, et al. Comparison of short-versus long-term ketogenic diet for intractable infantile spasms. Epilepsia. 2011. https://doi.org/10.1111/j.1528-1167.2010.02940.x.
Wijnen BFM, et al. Long-term clinical outcomes and economic evaluation of the ketogenic diet versus care as usual in children and adolescents with intractable epilepsy. Epilepsy Res. 2017. https://doi.org/10.1016/j.eplepsyres.2017.03.002.
Sharma S, Goel S, Jain P, Agarwala A, Aneja S. Evaluation of a simplified modified Atkins diet for use by parents with low levels of literacy in children with refractory epilepsy: A randomized controlled trial. Epilepsy Res. 2016. https://doi.org/10.1016/j.eplepsyres.2016.09.002.
Sharma S, Sankhyan N, Gulati S, Agarwala A. Use of the modified Atkins diet for treatment of refractory childhood epilepsy: A randomized controlled trial. Epilepsia. 2013. https://doi.org/10.1111/epi.12069.
• Lakshminarayanan K, et al. Efficacy of low glycemic index diet therapy (LGIT) in children aged 2–8 years with drug-resistant epilepsy: A randomized controlled trial. Epilepsy Res. 2021. https://doi.org/10.1016/j.eplepsyres.2021.106574. This Randomized Controlled Trial examined the efficacy of LGIT in children aged 2-8 years with drug-resistant epilepsy. The administration of LGIT along with ongoing anti-seizure medications (ASM) is more efficacious in reducing seizure frequency as compared to ASM alone.
Gupta S, Dabla S, Kaushik JS. Modified Atkins Diet versus Low Glycemic Index Treatment for Drug-Resistant Epilepsy in Children: An Open Label, Randomized Controlled Trial. Indian Pediatr. 2021. http://www.ncbi.nlm.nih.gov/pubmed/33634794.
Sondhi V, et al. Efficacy of Ketogenic Diet, Modified Atkins Diet, and Low Glycemic Index Therapy Diet among Children with Drug-Resistant Epilepsy: A Randomized Clinical Trial. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.2282.
Yoon J-R, Lee E, Dong Kim H, Soo Lee J, Chul KH. Comparison of ketogenic diet and modified atkins diet in children with epilepsy. Epilepsy Curr. 2014. https://doi.org/10.1002/central/CN-01010761.
Kim JA, et al. Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia. 2016. https://doi.org/10.1111/epi.13256.
Simeone TA, Simeone KA, Stafstrom CE, Rho JM. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology. 2018. https://doi.org/10.1016/j.neuropharm.2018.01.011.
Dahlin M, Prast-Nielsen S. The gut microbiome and epilepsy. EBioMedicine. 2019. https://doi.org/10.1016/j.ebiom.2019.05.024.
Nei M, Ngo L, Sirven JI, Sperling MR. Ketogenic diet in adolescents and adults with epilepsy. Seizure. 2014. https://doi.org/10.1016/j.seizure.2014.02.015.
IJff DM, et al. Cognitive and behavioral impact of the ketogenic diet in children and adolescents with refractory epilepsy: A randomized controlled trial. Epilepsy Behav. 2016. https://doi.org/10.1016/j.yebeh.2016.04.033.
Kayyali HR, Luniova A, Abdelmoity A. Ketogenic Diet Decreases Emergency Room Visits and Hospitalizations Related to Epilepsy. Epilepsy Res Treat. 2016. https://doi.org/10.1155/2016/5873208.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes and Obesity
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Desli, E., Spilioti, M., Evangeliou, A. et al. The Efficacy and Safety of Ketogenic Diets in Drug-Resistant Epilepsy in Children and Adolescents: a Systematic Review of Randomized Controlled Trials. Curr Nutr Rep 11, 102–116 (2022). https://doi.org/10.1007/s13668-022-00405-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-022-00405-4