Abstract
The ketogenic diet (KD), containing high levels of fat and low levels of carbohydrates, has been used to treat refractory epilepsy since the 1920s. In the past few decades, there has been more interest in less restrictive KDs such as the modified Atkins diet (MAD).
Purpose
Our aim was to review all evidence regarding the efficacy and tolerability of the KD and MAD from randomized controlled trials (RCTs) in children and adolescents with refractory epilepsy.
Methods
We reviewed the current literature using Cochrane, EMBASE, and MEDLINE (using PubMed). We implemented predefined criteria regarding dataextraction and study quality.
Results
We identified five RCTs that generated seven publications and recruited 472 children and adolescents with refractory epilepsy (≤ 18 years). The primary outcome (seizure frequency reduction (SFR) ≥ 50%) was attained in 35–56.1% of the participants in the intervention group, compared with 6–18.2% in the control group. Our meta-analysis underlined the significant efficacy of the KD compared with the control group: RR = 5.1 (95% CI 3.18–8.21, p < 0.001). Additionally, only two studies mentioned possible biomarkers to objectively evaluate the efficacy. Secondary outcomes, such as seizure severity and quality of life, were studied in three trials, leading to indecisive generalization of these findings. Gastro-intestinal adverse effects were the most prevalent, and no severe adverse effects were reported.
Conclusion
Despite the heterogeneity between all studies, the beneficial results underline that dietary interventions should be considered for children and adolescents with refractory epilepsy who are not eligible for epilepsy surgery. Future studies should be multi-center and long-term, and evaluate potential biomarkers and adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a neurological disorder characterized by seizures, which affects 65–70 million people worldwide [1]. Achieving complete seizure control, preserving quality of life (QoL), and avoidance of adverse events are three major goals of epilepsy treatment [2]. Nonetheless, more than 30% of the patients with epilepsy do not reach seizure control with the currently available anti-epileptic drugs (AEDs) [3]. In addition, AEDs cause significant adverse effects affecting the QoL [4]. Therefore, one should first consider surgical therapies if a patient is eligible [5, 6].
If epilepsy surgery is not an option, other therapy modalities are possible, such as non-pharmacological treatments (e.g., nervus vagus stimulation [7]) and dietary treatments like the ketogenic diet (KD) [8]. The KD, a strict diet high in fat and low in carbohydrates, increases the ketone body concentrations that could lead to an enhancement of inhibitory neurotransmission and thereby possibly reducing the seizure frequency [9].
The exact mechanism of the KD is under investigation, and ketone bodies could exert anti-oxidative, anti-inflammatory, cellular, epigenetic, and gut-microbiome alterations [10,11,12].
Initial studies report the classical KD that consists out of long-chain triglycerides (LCTs) in a fat:carbohydrate and protein ratio ranging from 2:1 to 5:1 [13]. A KD using medium-chain triglycerides (MCTs), such as triglycerides of octanoic and decanoic acids, yields relatively more ketones per kilocalorie of energy and leads to a more efficient absorption. As a result, the MCT has a lower need of total fat and enables a higher intake of carbohydrate and protein (less restrictive diet) [14]. The modified Atkins diet (MAD) is another variation to the KD, which mimics the effect on ketosis but is less restrictive [15]. Another liberalized alternative of the KD is the low-glycemic index treatment (LGIT), with a ratio of 1:1 fat:carbohydrate and protein. The LGIT also specifies a limit of carbohydrates (10% a day) and has shown similar efficacy in reducing the seizure frequency as other dietary interventions [16, 17]. LGIT is the most liberalized type of KD, although patients are restricted to carbohydrates with a glycemic index below 50 [18].
In a clinical setting, seizure frequency reduction (SFR) is at least 50% (≥ 50%) in half of the patients on the KD or MAD, i.e., somewhat higher compared with vagal nerve stimulation (VNS) [19]. Even as the KD and the MAD are non-invasive compared with neurostimulation, its use is limited due to bad tolerability, feasibility by caregivers (complexity of diet), and a relatively low compliance. Nonetheless, dietary treatment options remain a valid option for patients with epilepsy, and the absence of neurotoxic effects, compared with the standard AED treatment, should be taken into account [8]. Due to the limited number of studies and small sample sizes, we wanted to review all high-quality studies to generate an overview of the efficacy of dietary interventions for the treatment of pediatric epilepsy.
We reviewed the evidence from randomized controlled trials (RCTs) concerning the efficacy (SFR) of the KD and the MAD in children and adolescents with epilepsy. The primary outcome measure to evaluate efficacy of treatment in refractory epilepsy is usually an SFR ≥ 50% [19] and will therefore be the main outcome measure of this review. Other secondary outcome measures, such as QoL, adverse effects, and effects on cognition and behavior, will be included. In addition, potential biomarkers will be discussed.
The efficacy of the KD (classical or MCT) and the MAD will be compared with the standard treatment and monitoring according to the good clinical practice [20], i.e., the control group (care as usual, CAU).
Materials and methods
Protocol
We built up this review according to the PRISMA guidelines, which imply the statement for reporting systematic reviews of studies evaluating health care interventions [21]. No ethics board was needed since we reviewed already published trials.
Eligibility criteria
We included all RCTs of the KD and the MAD for children and/or adolescents with refractory epilepsy. There have been no randomized controlled trials (RCTs) regarding the LGIT, and therefore, LGIT was not within the scope of this review.
Studies were included if they described children and/or adolescents (age 1–18 years) with refractory epilepsy irrespective of etiology and seizure type. Refractory epilepsy refers to the fact that seizure freedom was not achieved by two trials of adequately dosed AEDs (in monotherapy or combination) [3].
Dietary interventions of interest were the KD and the MAD. The classical KD consists of LCTs, though more recent studies report the use of MCTs as a fat source due to its better absorption and higher yield of ketones (ketosis) [22].
The MAD is an alternative of the KD that mimics the effects of ketosis but is less restrictive [23]. These diets result in the production of ketones, which is believed to be involved for the anti-epileptic effects [24].
The control group received a placebo diet, believed to have no impact on epilepsy, or received no dietary intervention at all, i.e., CAU. There was a continuation of the AED regime in both groups (intervention and control group).
The primary outcome was an SFR of at least 50% (SFR ≥ 50%), which is clinically relevant and defined as success [25].
Secondary outcomes were seizure severity, adverse effects, cognitive and behavior outcomes, QoL, and attrition rate. A severe adverse effect was defined as an event that led to the withdrawal of the dietary intervention and/or the need for immediate intervention. Studies were included if they incorporated the primary and/or secondary outcomes.
Information sources
We reviewed the current literature using Cochrane, EMBASE, and MEDLINE (using PubMed) up to September 2019.
Search
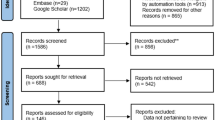
The literature search is summarized in Fig. 1. PubMed was used as primary search engine by using free and medical subject heading (MeSH) terms since the other sources did not result in any additional studies (supplementary information, online only).
Flow chart of study identification, screening, eligibility screening, and inclusion (adjusted from the PRISMA guidelines [49])
Study selection
Articles of interest were examined by two investigators (JS and MM). If the analysis of title and abstract was not sufficient to decide if the article should be included, the full text was reviewed. The following studies were excluded: (1) no RCT, (2) no mention of the KD (classical or MCT) or the MAD, (3) no control group without a dietary intervention, and (4) insufficient information regarding the primary outcome (SFR) and/or secondary outcomes. Subsequently, full-text articles in English or Dutch were checked to determine eligibility. Furthermore, reference lists were checked to identify possible relevant articles, and a final list of studies was generated to be included in the review.
Data extraction and management
Two investigators (JS and SK) extracted the data of the relevant articles by using a standardized Excel datasheet: study characteristics (sex, age, number of participants in each group, study design), diet intervention (KD and/or MAD), length of follow-up, seizure severity, adverse effects, reasons for drop-out, limitations of each study, and risk of bias.
Risk of bias in individual studies
Two investigators (JS and SK) assessed the risk of bias (selection, performance, attrition, reporting, and other biases) by using the different domains of the Cochrane Collaboration tool for assessing risk of bias [26]. An overall summary of the risk of bias was made.
Summary measures
Study characteristics were presented in detail. Overall, we presented the data as a percentage (number of patients with a certain event/condition in the intervention or control group), and computed the risk ratio (RR) for the MAD/KD group compared with the control group (CAU). In addition, the proportion of patients with an SFR of ≥ 50% for the MAD or KD group was calculated. Statistical heterogeneity between studies was quantified as the I-squared (I2) statistic. The I2 denotes the percentage variability in RR that is due to heterogeneity rather than sampling variance. A pooled estimate including 95% confidence interval was computed using either a random effect (RE) or fixed effect (FE) meta-analysis model. In case the I2 statistic would be over 50%, a RE model was used. If not, the FE model was used. Regarding the sensitivity analysis, the pooled effect measure was computed on eligible studies. Studies were eligible if they reported the SFR during a follow-up period that was equal in the intervention and control group.
Results
Study selection
Our search strategy has resulted in 28 studies. Three studies were excluded due to the focus on an intervention distinct from a dietary intervention (VNS, vigabatrin, or lacosamide). Five studies did not include a control group (CAU), and five other studies were no RCT. Six studies were reviews and two articles described a study protocol. Therefore, our current review resulted in the inclusion of seven studies [15, 20, 27,28,29,30,31] (Fig. 1). Three studies described the KD, three studies described the MAD, and no studies evaluated the LGIT (Table 1).
Study characteristics
All included studies are RCTs (Table 1). The number of participants ranged from 40 to 145. The follow-up duration varied between three and 16 months; in six out of seven studies, follow-up ranged between three and 6 months.
All studies included children or adolescents (age 1–18 years) who had not responded to two or more AEDs, described as refractory epilepsy [3]. Furthermore, all studies excluded patients with suspected or known metabolic disorders (e.g., diabetes, hyperinsulinism, fatty acid oxidation disorders).
Two studies excluded patients if they were not dietary-therapy-naïve [27, 29], and two studies excluded patients if there were motivational issues within the family [28, 29].
Only one RCT, resulting in three different articles [20, 30, 31], had more restrictive exclusion criteria; i.e., prolonged QT-syndrome, severe liver, kidney or pancreas disease, hypertriglyceridemia, hypercholesterolemia, malnutrition, treatment with acetazolamide or topiramate, presence of risk factors, or positive family history for kidney stones or acidosis. This RCT also excluded patients if they had a severe behavioral disorder. Nonetheless, patients with AD(H)D and autism were included in this RCT.
Even if patients with focal epilepsy were not excluded, most RCTs report a relatively low percentage of patients with focal epilepsy (< 25%), except two RCTs (42% and 50% for Neal et al. [27] and the Dutch RCT [30, 31], respectively).
Risk of bias within studies
We assessed the risk of bias according to the Cochrane Collaboration tool for assessing risk of bias [26], which is summarized in Table S1 (online only). The risk of bias is also visualized by Fig. 2, made by RevMan (version 5.0).
There were five RCTs that generated seven publications reviewing the use of the KD and/or MAD for patients with refractory epilepsy.
Results of individual studies
Primary outcome
Regarding the primary outcome, i.e., an SFR ≥ 50%, all five trials reported a statistically significant difference between the intervention group (group I, KD and/or MAD) and the control group (group II, CAU), except at 16-month follow-up (Table 2).
The meta-analysis of studies focusing on the KD [15, 27, 31] showed that the proportion of patients with ≥ 50% SFR is 0.52 (95% CI 0.29–0.74; RE model) (Fig. 3). Studies regarding the MAD [15, 28, 29] showed that the proportion of patients with ≥ 50% SFR is 0.52 (95% CI 0.42–0.61; FE model) (Fig. 4).
For the KD and MAD combined, more than 35% of the participants of the intervention group (KD and MAD) showed an SFR of ≥ 50%, compared with 6–18.2% in the control group. Our overall meta-analysis underlined the significant efficacy (SFR in the dietary intervention vs. control group; RR = 5.1 (95% CI 3.18–8.21), p < 0.001; Fig. 5).
Forest plot of the RR of dietary interventions (KD and/or MAD) regarding seizure frequency reduction (SFR) of at least 50%. RR, relative risk; 95%-CI, 95% confidence interval; I2, statistical heterogeneity. Pooled analysis of the individual studies of patients with an SFR of at least 50% leading to a RR = 5.1 (95% CI 3.18–8.21), p < 0.001. This finding indicates that the chance to obtain an SFR of at least 50% is about 5.1 times higher in the dietary intervention group compared with the care as usual (CAU). Since Ijff et al. 2016 did not report an SFR and Wijnen et al. 2017 did not state an equal follow-up duration in intervention compared with control group, we excluded these two studies in our meta-analysis
Secondary outcomes
Adverse effects
All studies reported adverse effects due to the dietary interventions. The RCTs investigating the KD reported mainly gastro-intestinal (GI) adverse effects in 30% of the participants, such as vomiting, diarrhea, and constipation [15, 27, 30, 31]. Three trials, investigating the MAD, also reported GI adverse effects to be the most common; e.g., 15–46% of the participants experienced constipation [15, 28, 29].
Overall, most of the adverse effects could be treated by dietary adjustments and/or drugs (anti-emetics and H2-blockers), and thereby did not lead to discontinuation of treatment in all RCTs [15, 27,28,29, 31]. However, persistence of adverse effects but also the lack of efficacy were the main reasons for participants to drop out of trials.
Interestingly, the only trial that lasted longer than 12 months [30] did not show a statistically significant increase of GI adverse effects due to the KD, even though this was the case at 4-month follow-up [31]. Moreover, they reported significantly fewer adverse effects regarding behavior/irritability, cosmetic/dermatological, and motor problems [30].
Severe adverse effects such as kidney stones, gallstones, fatty liver, nephrocalcinosis, acute pancreatitis, and QTc-prolongation were not reported. In contrast, biochemical parameter alterations were documented but were interpreted as clinically irrelevant [15, 29].
Other adverse effects documented by the trials in lower numbers were lower respiratory tract infections, abdominal pain, anorexia, lethargy, and hyperammonemic encephalopathy.
Cognitive and behavior outcomes
One RCT evaluated the effects of the KD on psychosocial impact [20]. Higher productivity, reduced tension/anxiety/hostility, and better cognitive functioning were reported at 4-month follow-up. These improvements were irrespective of seizure control, in contrast to the improvement of activation. In addition, Sharma et al. reported improvements in alertness, behavior, social interaction, and sleep [29].
Quality of life
Only a minimal difference was reported regarding the QoL by one RCT [30]. Utilities were measured by validated instruments, TAPQOL and TACQOL, to determine the quality-adjusted life years (QALYs) for the participants. Due to the relatively higher costs in the KD group, inconclusive cost per QALY ratios was reported [30]. Moreover, the authors did not investigate a relationship between the level of functioning and the QoL.
Attrition rate
All the RCTs experienced dropouts for various reasons, ranging from 10 to 26% for the trials with the KD during a follow-up period of 3–6 months [15, 27, 31]. The trial in the Netherlands documented a higher dropout (42%) when the follow-up extended to 16 months [30]. This dropout was due to various reasons (Table S2, online only). Overall, reasons for dropout were mostly intolerance of the diet or adverse effects (mostly GI tract related), change of seizures (increase or decrease), and change of mind. In the trials regarding the MAD, dropout rates ranged from 2 to 14% [15, 28, 29]. Reasons for dropping out were intolerance of the diet, weight loss, parental unhappiness, and adverse effects (hyperammonemic encephalopathy and lower respiratory tract infections).
Seizure severity
Three out of seven studies did not report changes in seizure severity. Two out three RCTs reported a statistically significant decrease in seizure severity. El-Rashidy et al. reported a mean decrease of seizure severity of 37.63% (MAD) and 35.89% (KD), compared with 1.79% (CAU) at 6-month follow-up (p < 0.0001 for both dietary interventions compared with the CAU) by using the Chalfont Seizure Severity Scale (CSSS) [15]. In line with these findings, Lambrechts et al. reported a mean decrease of seizure severity of 65.2% (KD), compared with 36.8% (CAU) at 4-month follow-up (p = 0.007 for the KD compared with the CAU) by using the National Hospital Seizure Severity Scale (NHS3) [31]. Consistently, Ijff et al. reported a significant reduction in seizure severity in the KD group, compared with the CAU (p = 0.038) by using a different scoring scale (Hague Restrictions in Childhood Epilepsy Scale, HARCES) [20]. Caution is warranted since the aforementioned studies used different seizure severity scales, i.e., the CSSS [15], NHS3, and HARCES [20, 30, 31]. Moreover, seizure severity was assessed by an experienced clinician at 6 weeks and 4 months in the study of Lambrechts et al. [31] and Ijff et al. [20], while El-Rashidy et al. [15] did not provide any details regarding the assessment of the seizure severity.
In addition, we were unable to determine if the long-term data, i.e., 16-month follow-up of the RCT published by Lambrechts et al. [30], also revealed a statistically significant difference. They only reported a decrease in seizure severity of 46.2% in the KD group, compared with 32.0% in the CAU group.
Biomarkers for efficacy of individual studies
Lambrechts et al. correlated seizure control to ketosis (beta-hydroxybutyrate (BHB) concentrations in the blood) at 3 months [31]. This significant difference disappeared at six and 12 months [30]. Moreover, the other RCTs did not investigate possible biomarkers to objectively evaluate the KD.
Discussion
Summary of main results
Miranda and colleagues described the KD, the MAD, and the LGIT as dietary interventions for the treatment of pediatric epilepsy [18]. There have been several reviews regarding observational studies on the efficacy of these dietary interventions [32, 33]. In addition, four Cochrane systematic reviews of dietary interventions for epilepsy are published [8, 34,35,36], of which some quite recently. However, we complement these studies by focusing on RCTs and applying statistical methods in an attempt to quantify the efficacy of dietary interventions. We also concentrated on those RCTs that included a true placebo group, i.e., care as usual (CAU) to evaluate the effect of a dietary intervention compared with no intervention at all. Hence, studies comparing two types of KDs (classical vs. MCT) [37] or glucose supplements to the MAD [38] were beyond the scope of this review.
Our review identified five RCTs, resulting in seven publications that assessed the efficacy of dietary interventions (KD and MAD) for children and adolescents with refractory epilepsy. To date, there have been no RCTs regarding the LGIT. We show promising, though limited, evidence since the primary outcome (SFR ≥ 50%) was attained in the 35–56.1% of the participants in the intervention group, compared with 6–18.2% in the control group. Our meta-analysis underlined this significant difference (SFR ≥ 50% in the intervention vs. control group; RR = 5.1 (95% CI 3.18–8.21), p < 0.001). In addition, we evaluated the primary outcome in the KD and MAD group separately. Our calculations show that the proportion of patients with ≥ 50% SFR is equal for both groups (KD 0.52 (95% CI 0.29–0.74) and MAD 0.52 (95% CI 0.42–0.61)). These findings are in line with a recent review that elaborated on pro- and retrospective studies regarding the KD and the MAD [39]. Herein, the authors directly compared the responder rate (SFR ≥ 50%) between the KD and MAD, which did not differ substantially after 3 months of treatment.
Adverse effects were monitored by all RCTs. Overall, the KD and MAD were well tolerated, although the only long-term study failed to agree on this statement. Therefore, the major reason of dropout during the study was an intolerance to the diet, and GI adverse effects were common in at least 15% of the participants. However, fine-tuning of the diet was often sufficient to reduce the GI adverse effects.
Four out of seven trials [15, 20, 30, 31] adequately assessed seizure severity and reported statistically significant decreases within the intervention group, although different scales were used and differences were not apparent at longer follow-up (16 months) [30]. Two studies reported changes in cognition and behavior. First, the trial in the Netherlands focused on the cognitive and behavioral functioning, as well as on the QoL. They reported higher productivity, reduced tension/anxiety/hostility, and better cognitive functioning at 40-month follow-up [20] and a lack of QoL improvements [30]. This latter finding could be explained by the fact that the generic instruments for measuring the QoL were not sensitive enough or that being a responder is not sufficient to have an improvement in the QoL. Second, Sharma et al. reported improvements in (social) behavior [29]. Interestingly, these positive effects on cognition and behavior are in line with other studies [40,41,42].
Overall, the validity of each study can be questioned since various biases were present in the different trials (internal validity), and all trials involved a single-center study (not clear if extrapolation to other settings is plausible; external validity). Therefore, future trials should be multi-center, with a sufficiently long follow-up (preferably of at least 1 year) and should aim to keep the performance bias as low as possible (blinding of the personnel and analysts, whenever possible).
Limitations
The follow-up duration was relatively short (three to 6 months) for most trials, which made long-term results difficult to predict. The only long-term RCT is the study by Wijnen et al.; however, the control group (CAU) was only followed for 4 months, and data were extrapolated to 16 months (last observation carried forward) [30]. From an ethical point of view, participants of the CAU group were offered to receive the KD after the first period of 4 months, but no data were available to determine the exact number of participants who agreed on this offer. In addition, there was a relatively low retention rate in the long-term trial (15/26 completed the FU at 16 months, i.e., 58%). Overall, dropouts vary between 2 and 26%, which could be the result of the experienced adverse effects by the patient (e.g., GI effects) and/or the time-intensive nature of dietary interventions, experienced by the caregiver(s). Although, the exact reasons for low retention rates during longer FU were not specified.
The primary and some of the secondary outcomes (e.g., seizure severity) were measured by instruments that are prone to subjectivity, i.e., by seizure diaries. This method is accepted in general clinical research since no alternative is available. Nevertheless, some important limitations should be considered regarding this method: (1) self-recording may affect the observation by causing the subject(s) to be more vigilant regarding the occurrence of seizures; (2) reports could be false positive (events which are not seizures) or no compliance regarding the diary maintenance; (3) subtle (e.g., absence epilepsy), nocturnal, or high-frequent seizures could be missed by the parent/caregiver.
The other secondary outcomes (e.g., QoL, cognition, and behavior) were analyzed by two trials [20, 29] of which Sharma et al. used subjective, parent-proxy reports (dichotomous, poorly defined, and not standardized) [29]. These reports are prone to more positive results than objective measurements [43] and are partially dependent on the reactions and emotions of the parents [44]. This latter finding underlines the need to assess the patients directly, instead of their parents. In contrast, Ijff et al. used objective assessments (well-defined, standardized) [20], which allow a better interpretation and generalization [43]. However, most objective psychometric scales do not report the timing of behavior disturbances, which could be a peri-ictal phenomenon rather than an underlying disorder. In addition, there is a significant tendency of comorbidities before and after epilepsy diagnosis, even though none of the RCTs elaborated on behavioral and psychiatric comorbidities [44].
Therefore, future RCTs should use objective measurement tools, include the assessment of comorbidities, and use self-reports whenever possible.
In general, our review underlines the promising effects of the KD and MAD in the treatment of refractory epilepsy (age 1–18 years). However, the small sample sizes and the limited amount of studies resulted in a relatively low quality of evidence.
Implications for research
The anti-epileptic mechanism of the KD and other dietary interventions is currently unknown, and evidence exists regarding the anti-epileptic and neuroprotective characteristics of ketone bodies [10]. Consistently, Lambrechts et al. were able to correlate seizure control to ketosis (by the ketones in blood, rather than urinary ketones) at 3-month follow-up [31], but not at six and 12 months [30]. The available animal studies underline the cellular and biochemical alterations by ketones (such as BHB, acetone, and acetoacetate) and its role in seizure reduction. These alterations could increase inhibitory neurotransmission (e.g., by enhancing GABAergic or ATP-sensitive potassium channels), decrease excitatory neurotransmission (e.g., by affecting vesicular glutamate transporters), or affect mitochondrial processes.
Novel pathways are reviewed elsewhere [12], and current data indicate the aforementioned plausible role of the ketones; however, most of them are not directly linked to epileptogenesis or seizure reduction. Of interest, recent preclinical data underline the possible role of the gut microbiome since in two different mouse models of different epilepsy types, anti-seizure effects of the KD were mediated by enrichment of Akkermansia muciniphila and Parabacteroides populations in the gut microbiome [45]. In addition, clinical data show that Bacteroidetes [11, 46] and proteobacteria [47] are more prominent after KD, which could be related to the anti-seizure effect.
Thus, research should further elucidate the complex neurobiology of the KD to discover novel targets for therapeutics, to create clinical formulations of the KD, and to determine if certain types of fat and/or ketogenic ratios relate to the clinical efficacy [48]. In addition, future trials should validate the aforementioned potential biomarkers, including the assessment of serum parameters (adenosine, ketones) and/or the gut microbiome. Moreover, these clinical trials should be adequately powered RCTs with large number of patients and well-defined outcomes (SFR, QoL, and neurocognitive improvement).
Conclusions
Our review identified seven studies (five RCTs) with a total sample size of 472 participants with refractory epilepsy (age 1–18 years). The primary outcome (SFR ≥ 50%) was attained in 35–56.1% of the participants in the intervention group, compared with 6–18.2% in the control group. Our meta-analysis underlined this significant difference (SFR ≥ 50% in the intervention vs. control group; RR = 5.1 (95% CI 3.18–8.21), p < 0.001). Objective evaluation of the efficacy, by biomarkers, has not yet been clinically validated, although two studies mention the possible correlation between the concentration BHB (ketones in the blood) and the responder rate.
Only a limited number of participants on the KD and/or MAD experienced severe adverse effects. In addition, the most prominent adverse effects were affecting the GI tract and were reversible by small adjustments of the dietary treatment. Therefore, the KD or MAD has a major benefit compared with standard epilepsy treatment with AEDs, which is related to long-term adverse effects. However, there was a significant methodological and clinical heterogeneity between all studies, and more research is needed regarding the long-term effects of dietary interventions. In addition, future trials should investigate objective methods to evaluate the efficacy, e.g. by biomarkers such as the concentration of ketones (BHB) in the blood and/or by analyzing the gut microbiome.
Nevertheless, given the beneficial clinical results regarding efficacy and safety, the KD and variations of this diet (e.g., MAD) should be considered as a treatment option for children and adolescents with refractory epilepsy who are not eligible for epilepsy surgery.
References
Singh A, Trevick S (2016) The epidemiology of global epilepsy. Neurol Clin 34:837–847. https://doi.org/10.1016/j.ncl.2016.06.015
Loscher W, Klitgaard H, Twyman RE, Schmidt D (2013) New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov 12:757–776. https://doi.org/10.1038/nrd4126
Kwan P, Arzimanoglou A, Berg AT et al (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia 51:1069–1077. https://doi.org/10.1111/j.1528-1167.2009.02397.x
Schmidt D (2009) Drug treatment of epilepsy: options and limitations. Epilepsy Behav 15:56–65. https://doi.org/10.1016/j.yebeh.2009.02.030
Ramey WL, Martirosyan NL, Lieu CM, Hasham HA, Lemole GM Jr, Weinand ME (2013) Current management and surgical outcomes of medically intractable epilepsy. Clin Neurol Neurosurg 115:2411–2418. https://doi.org/10.1016/j.clineuro.2013.09.035
Schijns OEMG, Hoogland G, Kubben PL, Koehler PJ (2015) The start and development of epilepsy surgery in Europe: a historical review. Neurosurg Rev 38:447–461. https://doi.org/10.1007/s10143-015-0641-3
Sourbron J, Klinkenberg S, Kessels A, Schelhaas HJ, Lagae L, Majoie M (2017) Vagus nerve stimulation in children: a focus on intellectual disability. Eur J Paediatr Neurol 21:427–440. https://doi.org/10.1016/j.ejpn.2017.01.011
Martin K, Jackson CF, Levy RG, Cooper PN (2016) Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev 2:CD001903. https://doi.org/10.1002/14651858.CD001903.pub3
Seo JH, Lee YM, Lee JS, Kang HC, Kim HD (2007) Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios--comparison of 3:1 with 4:1 diet. Epilepsia 48:801–805. https://doi.org/10.1111/j.1528-1167.2007.01025.x
Simeone TA, Simeone KA, Stafstrom CE, Rho JM (2018) Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 133:233–241. https://doi.org/10.1016/j.neuropharm.2018.01.011
Zhang Y, Zhou S, Zhou Y, Yu L, Zhang L, Wang Y (2018) Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res 145:163–168. https://doi.org/10.1016/j.eplepsyres.2018.06.015
Boison D (2017) New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol 30:187–192. https://doi.org/10.1097/WCO.0000000000000432
Vining EP (1999) Clinical efficacy of the ketogenic diet. Epilepsy Res 37:181–190
Huttenlocher PR, Wilbourn AJ, Signore JM (1971) Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 21:1097–1103
El-Rashidy OF, Nassar MF, Abdel-Hamid IA et al (2013) Modified Atkins diet vs classic ketogenic formula in intractable epilepsy. Acta Neurol Scand 128:402–408. https://doi.org/10.1111/ane.12137
Larson AM, Pfeifer HH, Thiele EA (2012) Low glycemic index treatment for epilepsy in tuberous sclerosis complex. Epilepsy Res 99:180–182. https://doi.org/10.1016/j.eplepsyres.2011.10.021
Rezaei S, Harsini S, Kavoosi M, Badv RS, Mahmoudi M (2018) Efficacy of low glycemic index treatment in epileptic patients: a systematic review. Acta Neurol Belg 118:339–349. https://doi.org/10.1007/s13760-018-0881-4
Miranda MJ, Turner Z, Magrath G (2012) Alternative diets to the classical ketogenic diet--can we be more liberal? Epilepsy Res 100:278–285. https://doi.org/10.1016/j.eplepsyres.2012.06.007
Wilmshurst JM, Gaillard WD, Vinayan KP, Tsuchida TN, Plouin P, van Bogaert P, Carrizosa J, Elia M, Craiu D, Jovic NJ, Nordli D, Hirtz D, Wong V, Glauser T, Mizrahi EM, Cross JH (2015) Summary of recommendations for the management of infantile seizures: Task Force report for the ILAE Commission of Pediatrics. Epilepsia 56:1185–1197. https://doi.org/10.1111/epi.13057
IJff DM, Postulart D, Lambrechts DAJE, Majoie MHJM, de Kinderen RJA, Hendriksen JGM, Evers SMAA, Aldenkamp AP (2016) Cognitive and behavioral impact of the ketogenic diet in children and adolescents with refractory epilepsy: a randomized controlled trial. Epilepsy Behav 60:153–157. https://doi.org/10.1016/j.yebeh.2016.04.033
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Liu YC (2008) Medium-chain triglyceride (MCT) ketogenic therapy. Epilepsia 49(Suppl 8):33–36. https://doi.org/10.1111/j.1528-1167.2008.01830.x
Kossoff EH, Dorward JL (2008) The modified Atkins diet. Epilepsia 49(Suppl 8):37–41. https://doi.org/10.1111/j.1528-1167.2008.01831.x
Masino SA, Rho JM (2012) Mechanisms of ketogenic diet action. In: Noebels JL, Avoli M, Rogawski MA et al (eds) Jasper’s basic mechanisms of the epilepsies [Internet], 4th edn. National Center for Biotechnology Information (US), Bethesda
de Kinderen RJA, Lambrechts DAJE, Postulart D, Kessels AG, Hendriksen JG, Aldenkamp AP, Evers SM, Majoie MH (2011) Research into the (cost-) effectiveness of the ketogenic diet among children and adolescents with intractable epilepsy: design of a randomized controlled trial. BMC Neurol 11:10. https://doi.org/10.1186/1471-2377-11-10
Higgins JPT, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH (2008) The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol 7:500–506. https://doi.org/10.1016/S1474-4422(08)70092-9
Sharma S, Sankhyan N, Gulati S, Agarwala A (2013) Use of the modified Atkins diet for treatment of refractory childhood epilepsy: a randomized controlled trial. Epilepsia 54:481–486. https://doi.org/10.1111/epi.12069
Sharma S, Goel S, Jain P, Agarwala A, Aneja S (2016) Evaluation of a simplified modified Atkins diet for use by parents with low levels of literacy in children with refractory epilepsy: a randomized controlled trial. Epilepsy Res 127:152–159. https://doi.org/10.1016/j.eplepsyres.2016.09.002
Wijnen BFM, de Kinderen RJA, Lambrechts DAJE, Postulart D, Aldenkamp AP, Majoie MHJM, Evers SMAA (2017) Long-term clinical outcomes and economic evaluation of the ketogenic diet versus care as usual in children and adolescents with intractable epilepsy. Epilepsy Res 132:91–99. https://doi.org/10.1016/j.eplepsyres.2017.03.002
Lambrechts DAJE, de Kinderen RJA, Vles JSH, de Louw AJ, Aldenkamp AP, Majoie HJ (2017) A randomized controlled trial of the ketogenic diet in refractory childhood epilepsy. Acta Neurol Scand 135:231–239. https://doi.org/10.1111/ane.12592
Henderson CB, Filloux FM, Alder SC, Lyon JL, Caplin DA (2006) Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol 21:193–198. https://doi.org/10.2310/7010.2006.00044
Zupec-Kania BA, Spellman E (2008) An overview of the ketogenic diet for pediatric epilepsy. Nutr Clin Pract 23:589–596. https://doi.org/10.1177/0884533608326138
Martin-McGill KJ, Jackson CF, Bresnahan R, Levy RG, Cooper PN (2018) Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev 11:CD001903. https://doi.org/10.1002/14651858.CD001903.pub4
Levy RG, Cooper PN, Giri P, Weston J (2012) Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001903.pub2
Levy RG, Cooper PP (2003) Ketogenic diet for epilepsy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001903
Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH (2009) A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 50:1109–1117. https://doi.org/10.1111/j.1528-1167.2008.01870.x
Kossoff EH, Dorward JL, Turner Z, Pyzik PL (2011) Prospective study of the modified Atkins diet in combination with a ketogenic liquid supplement during the initial month. J Child Neurol 26:147–151. https://doi.org/10.1177/0883073810375718
Rezaei S, Abdurahman AA, Saghazadeh A et al (2019) Short-term and long-term efficacy of classical ketogenic diet and modified Atkins diet in children and adolescents with epilepsy: a systematic review and meta-analysis. Nutr Neurosci 22:317–334. https://doi.org/10.1080/1028415X.2017.1387721
Pulsifer MB, Gordon JM, Brandt J, Vining EP, Freeman JM (2001) Effects of ketogenic diet on development and behavior: preliminary report of a prospective study. Dev Med Child Neurol 43:301–306
Nordli DRJ, Kuroda MM, Carroll J et al (2001) Experience with the ketogenic diet in infants. Pediatrics 108:129–133
Lambrechts DAJE, Bovens MJM, de la Parra NM, Hendriksen JG, Aldenkamp AP, Majoie MJ (2013) Ketogenic diet effects on cognition, mood, and psychosocial adjustment in children. Acta Neurol Scand 127:103–108. https://doi.org/10.1111/j.1600-0404.2012.01686.x
van Berkel AA, IJff DM, Verkuyl JM (2018) Cognitive benefits of the ketogenic diet in patients with epilepsy: a systematic overview. Epilepsy Behav 87:69–77. https://doi.org/10.1016/j.yebeh.2018.06.004
Berg AT, Altalib HH, Devinsky O (2017) Psychiatric and behavioral comorbidities in epilepsy: a critical reappraisal. Epilepsia 58:1123–1130. https://doi.org/10.1111/epi.13766
Olson CA, Vuong HE, Yano JM et al (2018) The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 174:497
Xie G, Zhou Q, Qiu C-Z, Dai WK, Wang HP, Li YH, Liao JX, Lu XG, Lin SF, Ye JH, Ma ZY, Wang WJ (2017) Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J Gastroenterol 23:6164–6171. https://doi.org/10.3748/wjg.v23.i33.6164
Lindefeldt M, Eng A, Darban H, Bjerkner A, Zetterström CK, Allander T, Andersson B, Borenstein E, Dahlin M, Prast-Nielsen S (2019) The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 5:5. https://doi.org/10.1038/s41522-018-0073-2
Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, Buchhalter JR, Caraballo RH, Cross JH, Dahlin MG, Donner EJ, Guzel O, Jehle RS, Klepper J, Kang HC, Lambrechts DA, Liu YMC, Nathan JK, Nordli DR Jr, Pfeifer HH, Rho JM, Scheffer IE, Sharma S, Stafstrom CE, Thiele EA, Turner Z, Vaccarezza MM, van der Louw E, Veggiotti P, Wheless JW, Wirrell EC, Charlie Foundation, Matthew’s Friends, Practice Committee of the Child Neurology Society (2018) Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 3:175–192. https://doi.org/10.1002/epi4.12225
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Acknowledgments
We would like to thank Dr. Adri Voogd for his supportive comments on the manuscript.
Funding information
LL receives research funding, speakers, and advisory board honoraria: Zogenix, UCB, Shire, Novartis, Eisai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JS, MM, HB, SK, SvK, and DL declare no conflict of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Sourbron, J., Klinkenberg, S., van Kuijk, S.M.J. et al. Ketogenic diet for the treatment of pediatric epilepsy: review and meta-analysis. Childs Nerv Syst 36, 1099–1109 (2020). https://doi.org/10.1007/s00381-020-04578-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04578-7