Abstract
Although present in the leaves of Mimosa bimucronata (DC.) and many other medicinal plants commonly used to augment urinary volume excretion, the effects of gallic acid as a diuretic agent remain to be studied. Wistar rats were orally treated with vehicle, hydrochlorothiazide, or gallic acid. The effects of gallic acid in the presence of hydrochlorothiazide, furosemide, amiloride, L-NAME, atropine, and indomethacin were also investigated. Diuretic index, pH, conductivity, and electrolyte excretion were evaluated at the end of the experiment (after 8 or 24 h). Gallic acid induced diuretic and saluretic (Na+ and Cl−) effects, without interfering with K+ excretion, when orally given to female and male rats at a dose of 3 mg/kg. These effects were associated with increased creatinine and conductivity values while pH was unaffected by any of the treatments. Plasma Na+, K+, and Cl− levels were not affected by any of the acute treatments. The combination with hydrochlorothiazide or furosemide was unable to intensify the effects of gallic acid when compared with the response obtained with each drug alone. On the other hand, the treatment with amiloride plus gallic acid amplified both diuresis and saluresis, besides to a marked potassium-sparing effect. Its diuretic action was significantly prevented in the presence of indomethacin, a cyclooxygenase inhibitor, but not with the pretreatments with L-NAME or atropine. Although several biological activities have already been described for gallic acid, this is the first study demonstrating its potential as a diuretic agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gallic acid, also known as 3,4,5-trihydroxybenzoic acid, is a naturally phenolic acid found widely distributed in fruits and plants, either in its regular form or as derivatives, including in wine and green tea (Blanco et al. 1998; Ow and Stupans 2003). Gallic acid and its derivatives are known to display a variety of biological functions, in addition to their primary antioxidant activity (Govea-Salas et al. 2016; Omobowale et al. 2017; Phonsatta et al. 2017), like antiproliferative (Subramanian et al. 2016), hypoglycemic (Huang et al. 2016), and anti-inflammatory (Lin et al. 2015) effects.
A previous study has demonstrated that the phenolic compounds are the majorities of the specie M. bimucronata, including methyl gallate and gallic acid, and the extracts and methyl gallate present diuretic, natriuretic, and kaliuretic properties when orally given to rats (Schlickmann et al. 2017). Although gallic acid was identified as one of the main components of M. bimucronata and in many others plants with diuretic activity indication, no study has been conducted so far evaluating its effects on experimental trials. Moreover, gallic acid already revealed several biological properties in the cardiovascular and renal system, demonstrating its therapeutic potential for conditions that affect these systems, which stands out a cardioprotective action against advanced glycation end product-induced cell proliferation (Umadevi et al. 2012) and cardiac remodeling (Umadevi et al. 2014), a vasorelaxant (de Oliveira et al. 2016) and antihypertensive (Jin et al. 2017; Kang et al. 2015) effects and cardio and renoprotection (Ajibade et al. 2016).
This study therefore aimed to explore the diuretic and saluretic effects of gallic acid in rats, including its properties in combination with standard diuretic agents commonly used in clinical practice as an attempt to evaluate its main mode of action.
Material and methods
Plant material and isolation of gallic acid
The leaves of M. bimucronata were used to prepare the extract and fractions and to isolate gallic acid as previously described [9]. Briefly, the methanolic extract was partitioned to obtain the dichloromethane (DCM) and ethyl acetate (EA) fractions. The latter was subsequently submitted to chromatography approaches, which made possible the purification and isolation of gallic acid (≥ 96% purity grade). All methodological details, as well as yield percentages, chromatographic techniques, and HPLC analysis, are available in Schlickmann et al. (2017).
Animals
Male and female Wistar rats, 3–4 months old, provided by Universidade do Vale do Itajaí (UNIVALI) were used in the experiments. The animals were kept under regular laboratory environments, 12-h light/dark cycle, and constant temperature (22 ± 2 °C), with water and food ad libitum. All the protocols described here were submitted and approved by institutional ethics committee from UNIVALI (license no. 045/16) and were performed following all the international standards and ethical guidelines on animal welfare.
Evaluation of diuretic activity
One day before the start of the tests, all animals were acclimated in metabolic cages for 2 h. The animals were then kept fasted, with free access to water, 12 h before the beginning of the treatments. The rats (n = 6–8) received orally the treatments including vehicle (CT; water plus 1% tween; 1 ml/kg), hydrochlorothiazide (HCTZ; 10 mg/kg), or gallic acid (GA; 0.3, 1, or 3 mg/kg). Right after the treatments, the animals were separated and allocated in metabolic cages. The volume of urine excreted was measured in the first and each 2 h, for a total of 8 h. For the groups treated with CT, HCTZ, and GA (3 mg/kg), the urine was collected for a total of 24 h, besides to obtaining blood samples for evaluation of plasma electrolyte content and determination of creatinine clearance. Urine volume was expressed as ml/100 g. The values of pH, conductivity, and electrolyte excretion (Na+, K+, and Cl−) were measured in total urine at the end of collections. The description of the analytical procedures adopted in this study can be found with all the details in de Souza et al. (2017a).
In another experimental approach, different groups of rats were orally treated with HCTZ (10 mg/kg), furosemide (10 mg/kg), amiloride (3 mg/kg), L-NAME (60 mg/kg), indomethacin (5 mg/kg), atropine (1.5 mg/kg), or vehicle (1 ml/kg; water plus 1% tween). After 1 h, these groups were treated with gallic acid (3 mg/kg) or just vehicle (1 ml/kg; water plus 1% tween) and were submitted to the same protocols described above. All drugs as well as the doses used in these experiments were selected from previous studies (Boeing et al. 2017; De Souza et al. 2013, 2017a, b; Gasparotto et al. 2012; Schlickmann et al. 2017).
Statistical analysis
The results were expressed as mean ± standard error of mean (SEM) of 6–8 animals each group. One- or two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test were performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA, USA). A p < 0.05 was considered statistically significant.
Results
Diuretic and saluretic effect of gallic acid in rats
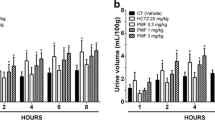
It was first performed an initial screening for biological activity to determine if gallic acid had diuretic effect, by using both female and male rats. As shown in Fig. 1a, gallic acid at a dose of 3 mg/kg, but not 0.3 and 1 mg/kg, was able to efficiently augment the urinary volume when orally given to female rats, similar to the clinical reference thiazide-type diuretic hydrochlorothiazide (used both as a positive control and as an internal control of the experiments). In the same way, Fig. 1b displays the effect of gallic acid-treated male rats, at dose of 3 mg/kg, on cumulative urine volume, which revealed the ability of gallic acid to induce diuresis also in male rats.
Cumulative urine volume of female and male rats treated with gallic acid. a Urine volume of female rats after the treatment with different doses (0.3–3 mg/kg) of gallic acid. b Urine volume of male rats treated with gallic acid (3 mg/kg). The control (CT) group received vehicle (1 ml/kg). The values show the mean ± SEM. Statistical analysis was performed by means of two-way ANOVA followed by Dunnett’s multiple comparisons test. *p < 0.05 when compared with the CT (vehicle-treated only group). GA = gallic acid; HCTZ = hydrochlorothiazide
Moreover, as presented in Table 1, the treatment with gallic acid (female groups) also promoted increased urinary Na+ and Cl− excretion without altering the levels of K+, which is particularly interesting since it shows that the compound has diuretic and saluretic, but not kaliuretic, effect. The urinary pH values were not altered by any of the treatments, while conductivity was increased to the groups treated with hydrochlorothiazide or gallic acid (Table 1). Supporting these findings, creatinine values were also significantly higher in the groups treated with HCTZ and gallic acid, respectively, 1.78 ± 0.28 and 1.56 ± 0.17 mg/dl, while in the vehicle-treated group, it was 1.15 ± 0.06 mg/dl.
Similarly, the levels of Na+ and Cl− excreted in urine samples collected from male rats were also significantly higher, with values of 147.20 ± 14.62 and 185.30 ± 11.47 mmol/L of Na+ for gallic acid and hydrochlorothiazide-treated groups, respectively, against 99.55 ± 13.57 mmol/l in the vehicle-treated group (p < 0.05); and 171.50 ± 10.36 and 257.00 ± 16.27 mmol/l of Cl+ for gallic acid and hydrochlorothiazide-treated groups, respectively, against 128.00 ± 10.71 mmol/l in the urines from the vehicle-treated group (p < 0.05). Such as in female rats, the urinary K+ content was not altered after the treatment with gallic acid (data not shown).
Additionally, the biological properties of gallic acid were also investigated in 24-h assay, as shown in Table 2. Gallic acid was able to induce a sustained diuretic and saluretic, but not kaliuretic, effect when compared with the vehicle-treated group. Although significant changes in urinary electrolyte excretion were found, plasma levels of Na+, K+, and Cl− were not affected by any of the acute treatments. Moreover, both HCTZ and gallic acid significantly augmented creatinine clearance when compared with CT group.
Gallic acid-induced diuresis and saluresis and its combination with diuretics drugs
We then evaluated the effects of gallic acid in combination with other clinical reference diuretics, hydrochlorothiazide, furosemide (one of the most commonly used loop diuretics), and amiloride (a representative of the class of potassium-sparing diuretics), as represented in Fig. 2a–c, respectively. In this way, we can observe that gallic acid plus hydrochlorothiazide treatment was not more effective in inducing diuresis than the effect of each drug administered alone (Fig. 2a). In contrast, as showed in Table 1, the electrolyte excretion was shown to be significantly higher with the use of drugs in combination. Similarly, Fig. 2b shows that when gallic acid was given together with furosemide, the diuretic effect, as well as the electrolytes excretion (Table 1), remained at levels close to those obtained with treatment with only one of the drugs. On the other hand, the group that received gallic acid plus amiloride treatment had a significant improvement in urine output versus the gallic acid or amiloride-only treated group, as represented in Fig. 2c. Moreover, while the electrolyte (Na+ and Cl−) excretion profile was also significantly raised in the urine collected from the group receiving both treatments, the potassium-sparing effect characteristic of amiloride administration prevailed (Table 1).
Diuretic effect of gallic acid in combination with reference diuretic drugs. Cumulative urine volume of female rats treated with a hydrochlorothiazide (HCTZ; 10 mg/kg), b furosemide (10 mg/kg), and c amiloride (3 mg/kg) in combination with vehicle-only or gallic acid (GA; 3 mg/kg). The values show the mean ± SEM. Statistical analysis was performed by means of two-way ANOVA followed by Dunnett’s multiple comparison test. *p < 0.05 when compared with the CT (vehicle-treated only group). #p < 0.05 when compared with GA-treated group
Mechanisms involved in the diuretic effect induced by gallic acid in rats
As an attempt to elucidate the main mode of action of gallic acid-induced diuresis and saluresis, we next explored the role of nitric oxide, muscarinic acetylcholine receptors, and renal endogenous prostanoid generation. Like this, different groups of rats were pre-treated with L-NAME, atropine and indomethacin and after were given gallic acid or just vehicle, as shown in Fig. 3. The pretreatment with L-NAME, a non-selective nitric oxide synthase inhibitor, was able to intensify the diuretic effect of gallic acid (Fig. 3a). With the use of atropine, a drug that acts by non-selectively blocking muscarinic acetylcholine receptors, we also did not detect a significant attenuation in the amount of urine excreted at the end of 8 h (Fig. 3b), suggesting that the activation of muscarinic receptors is probably not pivotal to the diuretic effect of gallic acid. On the other hand, the pretreatment with indomethacin, a non-selective cyclooxygenase inhibitor, fully precluded galic acid-induced diuresis (Fig. 3c) and saluresis (Table 1), which suggests that endogenous prostanoids generation seems to be important to gallic acid that exerts its diuretic effect.
Effect of pretreatment with L-NAME, atropine, and indomethacin on gallic acid-induced diuresis. Panels a L-NAME, b atropine, and c indomethacin pre-treated female rats in combination with vehicle-only or gallic acid (3 mg/kg). The control (CT) group received vehicle (1 ml/kg). The values show the mean ± SEM. Statistical analysis was performed by means of two-way ANOVA followed by Dunnett’s multiple comparisons test. *p < 0.05 when compared with the CT (vehicle-treated only group); #p < 0.05 when compared with gallic acid (GA) plus vehicle-treated group
Discussion
There is great interest in the search for secondary metabolites obtained from vegetal species for the treatment of pathologies that affect humans in the clinic, with particular attention to those that disturb the cardiovascular and renal systems. In this context, the capacity to prompt negative fluid stability has made diuretics useful in the treatment of a variety of conditions, which include cardiac failure, hypertension, hepatic cirrhosis, nephrotic syndrome, nephrogenic diabetes insipidus, and other edematous disease states (Plant 2003; Rose 1991). They are often used as the first line management for mild to moderate hypertension and are a central part of the treatment of symptomatic heart failure (Sarafidis et al. 2010a, b). Considering the therapeutic importance of diuretics, their extensive clinical application and taking into account the abundant use of plants for this purpose in folk medicine, becomes important to elucidate the potential efficacy and security of these plants as well as of their active constituents.
As already mentioned in the introduction section, our previous study demonstrated both chemical characterization and the biological effect of the specie M. bimucronata and its main constituent methyl gallate (Schlickmann et al. 2017). In this way, we give continuity to these investigations now extending the effects of gallic acid, another well-known phenolic compound. When given to female or male rats, gallic acid was able to induce both diuresis and saluresis, 8 or 24 h after treatments. It is worth noting that despite the similar efficacy between the positive control hydrochlorothiazide and gallic acid, the effective dose of gallic acid was about 3 times lower than the dose of hydrochlorothiazide. In addition, unlike hydrochlorothiazide, the renal properties of gallic acid were unassociated with changes in urinary K+ excretion. Considering electrolyte disturbance, especially those related to an excessive loss of potassium, are one of the main side effects associated with diuretics of the thiazide and loop classes (Legroux-Gerot et al. 2004; Sarafidis et al. 2010a, b; Singh et al. 2014), this result suggests a potential for future investigations either with this molecule given alone or in combination with other drugs, as we will discuss in the next paragraphs.
Diuretics are classified according to their mode of action along the nephron, which allows them to have unique effects on the organism (Ferrari and Frey 2000; Wargo and Banta 2009). They act by reducing sodium reabsorption at different sites in the nephron, thereby increasing urinary excretion of sodium and water. Combination diuretic therapy using drugs with different mechanisms of action is commonly used in clinical practice, either as a strategy to amplify their renal effects or to minimize the incidence of adverse events. Therefore, the data described in this study point for a beneficial effect of gallic acid in the promotion of diuresis and saluresis especially when evaluated their effects in combination with amiloride (a potassium-sparing diuretic), which showed an intense water and salt loss, preserving the antikaliuretic characteristic of amiloride. On the other hand, the combination of gallic acid with the loop diuretic furosemide did not result in any additional gains in total electrolyte content and volume of urine excreted, which may either be related to competition for the site of action or even an event of pharmacological antagonism. Finally, the combination of gallic acid plus the thiazide-type diuretic hydrochlorothiazide, although it has not significantly enhanced the diuretic index, was very effective in potentiating electrolytes excretion, which may be particularly interesting in severe cases of associated edema. However, because of the risk of inducing severe hypokalemia and hyponatremia, these combinations should always be closely monitored and their risks and benefits evaluated on a case-by-case.
There are several evidences in the literature supporting vasodilatory effects of diuretics. For instance, both loop and thiazide diuretics are described able to induce vasodilation. The vasculature action of these two classes of diuretics is possibly correlated with either the loss of Na+ and water from the vessel wall (Greenberg et al. 1994; Qavi et al. 2015; Raftery 1994; Silke 1994), and through the release of arachidonic acid metabolites, endothelin derived relaxing factor and potassium channel activation (Pickkers et al. 1998; Wiemer et al. 1994). In addition, there is also data showing that loop diuretics induces venodilation, an effect sustained by the release of nitric oxide, which might contribute for changes in venous return impacting on cardiac output and in this way on blood pressure (de Berrazueta et al. 2007). Applying these evidences for the findings described in this study, we detected that the pre-treatment with L-NAME, a non-selective nitric oxide synthase inhibitor, was able to significantly strengthen the diuretic index induced by gallic acid. This ability of L-NAME to potentiate the diuresis of isolated compounds, although not well understood, has already been described in the literature (Boeing et al. 2017; De Souza et al. 2017b; Schlickmann et al. 2017). On the other hand, while the blockade of muscarinic acetylcholine receptors by atropine was unable to prevent gallic acid-induced diuresis, the pretreatment with indomethacin, a non-selective cyclooxygenase inhibitor, significantly precluded the diuretic effect of gallic acid. Considering cyclooxygenase is an enzyme that is responsible for formation of prostanoids, it is possible to infer that, at least in part, the diuretic and saluretic properties of gallic acid described in this study are due to the greater release of these vasodilator mediators.
Taking together, the results presented herein revealed the diuretic and saluretic effect of a single dose of gallic acid in rats. This study also showed the beneficial effects of the combination of gallic acid with reference diuretics (following a common practice in the clinic) and disclosed about the involvement of endogenous prostanoids on gallic acid-induced diuresis and saluresis.
References
Ajibade TO, Oyagbemi AA, Omobowale TO, Asenuga ER, Afolabi JM, Adedapo AA (2016) Mitigation of diazinon-induced cardiovascular and renal dysfunction by gallic acid. Interdiscip Toxicol 9(2):66–77
Blanco VZ, Auw JM, Sims CA, O'Keefe SF (1998) Effect of processing on phenolics of wines. Adv Exp Med Biol 434:327–340
Boeing T, da Silva LM, Mariott M, Andrade SF, de Souza P (2017) Diuretic and natriuretic effect of luteolin in normotensive and hypertensive rats: Role of muscarinic acetylcholine receptors. Pharmacol Rep 69(6):1121–1124
de Berrazueta JR, González JP, de Mier I, Poveda JJ, García-Unzueta MT (2007) Vasodilatory action of loop diuretics: a plethysmography study of endothelial function in forearm arteries and dorsal hand veins in hypertensive patients and controls. J Cardiovasc Pharmacol 49(2):90–95
de Oliveira LM, de Oliveira TS, da Costa RM, de Souza Gil E, Costa EA, Passaglia R de C, Filgueira FP, Ghedini PC (2016) The vasorelaxant effect of gallic acid involves endothelium-dependent and -independent mechanisms. Vasc Pharmacol 81:69–74
De Souza P, Crestani S, da Silva Rde C, Gasparotto F, Kassuya CA, da Silva-Santos JE, Gasparotto A Jr (2013) Involvement of bradykinin and prostaglandins in the diuretic effects of Achillea millefolium L. (Asteraceae). J Ethnopharmacol 149(1):157–161
De Souza P, Boeing T, Somensi LB, Cechinel-Zanchett CC, Bastos JK, Petreanu M, Niero R, Cechinel-Filho V, Da Silva LM, De Andrade SF (2017a) Diuretic effect of extracts, fractions and two compounds 2α,3β,19α-trihydroxy-urs-12-en-28-oicacid and 5-hydroxy-3,6,7,8,4′-pentamethoxyflavone from Rubus rosaefolius Sm. (Rosaceae) leaves in rats. Naunyn Schmiedeberg's Arch Pharmacol 390(4):351–360
De Souza P, da Silva LM, Boeing T, Somensi LB, Cechinel-Zanchett CC, Campos A, Krueger CMA, Bastos JK, Cechinel-Filho V, Andrade SF (2017b) Influence of Prostanoids in the Diuretic and Natriuretic Effects of Extracts and Kaempferitrin from Bauhinia forficata Link Leaves in Rats. Phytother Res 31(10):1521–1528
Ferrari P, Frey FJ (2000) Pharmacologic action of diuretics in the kidney. Ther Umsch 57(6):345–350
Gasparotto Junior A, Prando TB, Leme Tdos S, Gasparotto FM, Lourenço EL, Rattmann YD, Da Silva-Santos JE, Kassuya CA, Marques MC (2012) Mechanisms underlying the diuretic effects of Tropaeolum majus L. extracts and its main component isoquercitrin. J Ethnopharmacol 141(1):501–509
Govea-Salas M, Rivas-Estilla AM, Rodríguez-Herrera R, Lozano-Sepúlveda SA, Aguilar-Gonzalez CN, Zugasti-Cruz A, Salas-Villalobos TB, Morlett-Chávez JA (2016) Gallic acid decreases hepatitis C virus expression through its antioxidant capacity. Exp Ther Med 11(2):619–624
Greenberg S, McGowan C, Xie J, Summer WR (1994) Selective pulmonary and venous smooth muscle relaxation by furosemide: a comparison with morphine. J Pharmacol Exp Ther 270:1077–1085
Huang DW, Chang WC, Wu JS, Shih RW, Shen SC (2016) Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr Res 36(2):150–160
Jin L, Lin MQ, Piao ZH, Cho JY, Kim GR, Choi SY, Ryu Y, Sun S, Kee HJ, Jeong MH (2017) Gallic acid attenuates hypertension, cardiac remodeling, and fibrosis in mice with NG-nitro-L-arginine methyl ester-induced hypertension via regulation of histone deacetylase 1 or histone deacetylase 2. J Hypertens 35(7):1502–1512
Kang N, Lee JH, Lee W, Ko JY, Kim EA, Kim JS, Heu MS, Kim GH, Jeon YJ (2015) Gallic acid isolated from Spirogyra sp. improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ Toxicol Pharmacol 39(2):764–772
Legroux-Gerot I, Catanzariti L, Marchandise X, Duquesnoy B, Cortet B (2004) Bone mineral density changes in hypercalciuretic osteoporotic men treated with thiazide diuretics. Joint Bone Spine 71:51–55
Lin WH, Kuo HH, Ho LH, Tseng ML, Siao AC, Hung CT, Jeng KC, Hou CW (2015) Gardenia jasminoides extracts and gallic acid inhibit lipopolysaccharide-induced inflammation by suppression of JNK2/1 signaling pathways in BV-2 cells. Iran J Basic Med Sci 18(6):555–562
Omobowale TO, Oyagbemi AA, Ajufo UE, Adejumobi OA, Ola-Davies OE, Adedapo AA, Yakubu MA (2017) Ameliorative effect of Gallic acid in doxorubicin-induced hepatotoxicity in Wistar rats through antioxidant defense system. J Diet Suppl 18:1–14
Ow YY, Stupans I (2003) Gallic acid and gallic acid derivatives: effects on drug metabolizing enzymes. Curr Drug Metab 4(3):241–248
Phonsatta N, Deetae P, Luangpituksa P, Grajeda-Iglesias C, Figueroa-Espinoza MC, Le Comte J, Villeneuve P, Decker EA, Visessanguan W, Panya A (2017) Comparison of antioxidant evaluation assays for investigating Antioxidative activity of Gallic acid and its alkyl esters in different food matrices. J Agric Food Chem 65(34):7509–7518
Pickkers P, Hughes AD, Russel FG, Thien T, Smits P (1998) Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension 32(6):1071–1076
Plant L (2003) Clinical use of diuretics. Clin Med 3:517–520
Qavi AH, Kamal R, Schrier RW (2015) Clinical Use of Diuretics in Heart Failure, Cirrhosis, and Nephrotic Syndrome. Int J Nephrol Article ID 975934, 9 pages
Raftery EB (1994) Hemodynamic effects of diuretics in heart failure. Br Heart J 72(suppl 2):44–7S
Rose BD (1991) Diuretics. Kidney Int 39(2):336–352
Sarafidis PA, Georgianos PI, Lasaridis AN (2010a) Diuretics in clinical practice. Part II: electrolyte and acid-base disorders complicating diuretic therapy. Expert Opin Drug Saf 9:259–273
Sarafidis PA, Georgianos PI, Lasaridis AN (2010b) Diuretics in clinical practice. Part I: mechanisms of action, pharmacological effects and clinical indications of diuretic compounds. Expert Opin Drug Saf 9:243–257
Schlickmann F, de Souza P, Boeing T, Mariano LNB, Steimbach VMB, Krueger CMA, da Silva LM, de Andrade SF, Cechinel-Filho V (2017) Chemical composition and diuretic, natriuretic and kaliuretic effects of extracts of Mimosa bimucronata (DC.) Kuntze leaves and its majority constituent methyl gallate in rats. J Pharm Pharmacol 69(11):1615–1624
Silke B (1994) Hemodynamic impact of diuretic therapy in chronic heart failure. Cardiology 84:115–123
Singh P, Knoedler JJ, Krambeck AE, Lieske JC, Bergstralh EJ, Rule AD (2014) Thiazide diuretic prophylaxis for kidney stones and the risk of diabetes mellitus. J Urol 192(6):1700–1704
Subramanian AP, Jaganathan SK, Mandal M, Supriyanto E, Muhamad II (2016) Gallic acid induced apoptotic events in HCT-15 colon cancer cells. World J Gastroenterol 22(15):3952–3961
Umadevi S, Gopi V, Simna SP, Parthasarathy A, Yousuf SM, Elangovan V (2012) Studies on the cardioprotective role of gallic acid against AGE-induced cell proliferation and oxidative stress in H9C2 (2-1) cells. Cardiovasc Toxicol 12(4):304–311
Umadevi S, Gopi V, Elangovan V (2014) Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chem Biol Interact 208:28–36
Wargo KA, Banta WM (2009) A comprehensive review of the loop diuretics: should furosemide be first line? Ann Pharmacother 43(11):1836–1847
Wiemer G, Fink E, Linz W, Hropot M, Schölkens BE, Wohlfart P (1994) Furosemide enhances the release of endothelial kinins, nitric oxide and prostacyclin. J Pharmacol Exp Ther 271:1611–1615
Acknowledgements
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Universidade do Vale do Itajaí (UNIVALI). Dr. Rita de Cássia Melo Vilhena de Andrade Fonseca da Silva is grateful for the Postdoctoral scholarship from PNPD/CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the protocols described here were submitted and approved by institutional ethics committee from UNIVALI (license no. 045/16) and were performed following all the international standards and ethical guidelines on animal welfare.
Rights and permissions
About this article
Cite this article
Schlickmann, F., Boeing, T., Mariano, L.N.B. et al. Gallic acid, a phenolic compound isolated from Mimosa bimucronata (DC.) Kuntze leaves, induces diuresis and saluresis in rats. Naunyn-Schmiedeberg's Arch Pharmacol 391, 649–655 (2018). https://doi.org/10.1007/s00210-018-1502-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1502-8