Abstract
Jujube fruits contain high levels of ascorbic acid (AsA). However, the AsA contents of fruits differ significantly in various jujube germplasm resources, and the factors responsible for these differences remain unknown. To explore the metabolic mechanism of AsA accumulation, we investigated the AsA content in fruits at different developmental stages of two jujube species, Ziziphus acidojujuba cv. Yuanxingxiaosuanzao (SZ) and Ziziphus jujuba Mill. cv. Luojiangtiaoyuanzao (LZ). The AsA accumulation pattern in the fruits of the two jujube species was similar during fruit development, and AsA content was negatively correlated with titratable acid. We also analysed the expression patterns of AsA metabolism genes. The results showed that AsA biosynthesis in jujube fruits was mainly related to the L-galactose pathway, as well as the inositol pathway. Furthermore, higher expression levels of genes involved in AsA biosynthesis (GME1, GMP1, GPP, GGP, and MIOX1) and regeneration (MDHAR1 and MDHAR2) were associated with higher AsA concentrations in SZ fruit compared with LZ fruit. In summary, we concluded that more efficient AsA biosynthesis and regeneration was responsible for the higher AsA accumulation in SZ fruit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ascorbic acid (AsA), also known as vitamin C (VC), is an essential metabolite in plants and animals (Magwaza et al. 2017). AsA participates in cell wall synthesis, cell division and growth, photosynthesis, senescence hormone synthesis and other metabolic processes in plants, and also plays an important role in the antioxidant protection under biotic and abiotic stresses (Akram et al. 2017; Chen et al. 2021; Wang et al. 2010). Furthermore, AsA is crucial to maintain human health, such as reducing the risk of cardiovascular diseases, cancer, cataracts, senescence, and other diseases related to oxidative stress (Davey et al. 2000). Humans cannot synthesize AsA owing to a lack of L-gulonolactone oxidase (GulLO) (Huang et al. 2014; Fitzpatrick et al. 2012), therefore fruits and vegetables are the best contributor of AsA in human nutrition.
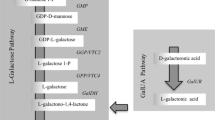
Four AsA biosynthesis pathways have been identified in plants, namely, L-galactose, D-galacturonate, L-gulose, and myo-inositol pathways (Fenech et al. 2019). Among these, the L-galactose pathway is generally considered the major AsA synthesis pathway in plants (Wheeler et al. 1998), in which AsA is synthesized from D-mannose-1-phosphate through a series of enzymatic reactions. In addition, the regeneration and degradation of AsA is essential for maintaining AsA accumulation in plants (Cruz-Rus et al. 2011). AsA concentration is controlled by a balance between biosynthesis, regeneration and degradation (Ishikawa et al. 2006; Yang et al. 2011) (Fig. 1).
The pathway of AsA metabolism in plants. Note: 1, GDP-D-mannose pyrophosphorylase (GMP); 2, GDP-D-mannose-30,50-epimerase (GME); 3, GDP-L- galactose phosphorylase (GGP); 4, L-galactose-1-phosphate phosphatase (GPP); 5, L-galactose dehydrogenase (GalDH); 6, L-galactono-1,4-lactone dehydrogenase (GalLDH); 7, Inositol oxygenase (MIOX); 8, D-galacturonate reductase (GalUR); 9, ascorbate oxidase (AO); 10, ascorbate peroxidase (APX); 11, Dehydroascorbate reductase (DHAR); 12, Monodehydroascorbate reductase (MDHAR)
Chinese jujube is the most important species in the large cosmopolitan family Rhamnaceae in terms of its economic, ecological, and social importance (Liu et al. 2020). Jujube fruit is reputed as a “natural vitamin pill” due to its high AsA concentration (Liu et al. 2013). There are abundant germplasm resources of jujube in the world, but their fruits differ significantly in AsA content (Wojdyło et al. 2016; Huang et al. 2017). Although AsA levels in jujube fruit have been preliminarily researched, there is very little and contradictory information about the regulation mechanism. The presence of two AsA biosynthetic pathways has been confirmed in cultivar ‘Dongzao’, L-galactose and myo-inositol pathways, using de novo genome sequencing (Liu et al. 2014). Moreover, GMP1, GMP2, GME1, GME2, GGP and GalDH were involved in AsA accumulation during fruit development and in different genotypes in cultivar ‘Junzao’ (Zhang et al. 2016). GalLDH, APX and MDHAR were closely associated with high AsA content in ‘Jinsixiaozao’ and ‘Qingjiansuanzao’ cultivars (Chen et al. 2016; Huang et al. 2021). Hence, the genes involved in AsA accumulation among jujube germplasm resources remains unclear.
In this study, we systematically investigated the AsA content as well as the expression profiles of genes involved in AsA metabolism and compared the fruit development of ‘Yuanxingxiaosuanzao’ (wild jujube accession) and ‘Luojiangtiaoyuanzao’ (cultivated jujube cultivar). The results could provide a theoretical basis for further studies into the quality and molecular regulation of AsA in jujube fruit.
2 Materials and methods
2.1 Materials
We collected Ziziphus acidojujuba cv. Yuanxingxiaosuanzao (SZ) and Ziziphus jujuba Mill. cv. Luojiangtiaoyuanzao (LZ) in the village of Luojiang County, Sichuan Province, China (31°26′34″ N, 104°42′18″ E). The growth conditions were hilly areas with purple soil, and belong to the subtropical humid monsoon climate, with an annual average temperature of 16.5 °C, average precipitation of 910 mm, a frost-free period of 278 d, and average sunshine duration of 1260 h.
Nine trees of seven-year-old of LZ and SZ with similar growth and field management were selected and randomly divided into three groups of three trees, respectively. At least 20 fruits were randomly collected as a biological replicate, and three replicates were prepared. Fruits at five developmental stages, i.e., young [10 days after anthesis (DAA)], enlarging (30 DAA), white mature (50 DAA), beginning-red (70 DAA), and half-red (90 DAA) were harvested, labeled as stages I, II, III, IV, and V, respectively. All fruit samples were cut into small pieces, immediately frozen in liquid nitrogen, and stored at − 80 °C.
2.2 Soluble sugar and titratable acid content analysis
The soluble sugar content was determined by anthrone colorimetry (Weng et al. 2013) and the titratable acid (TA) content was determined by acid–base titration (Chutichudet et al. 2008).
2.3 AsA content analysis
The AsA content determination method was essentially the same as that used for chestnut rose (Huang et al. 2014) by high-performance liquid chromatography (HPLC). Frozen tissue (0.5 g) was added to 0.2% metaphosphoric acid (5 mL) and ground using a precooled mortar. The resulting homogenate was centrifuged at 12,000 rpm for 15 min (4 °C), and the supernatant was diluted to 10 mL with 0.2% metaphosphoric acid and filtered through a 0.45 μm nylon filter for determination. The AsA content was determined using a HPLC system (Agilent 1260) with a photodiode array detector, and a reversed-phase C18 column (4.6 mm × 250 mm, 5 μm). The mobile phase was composed of 15% methanol and 85% metaphosphoric acid aqueous solution at pH 2.5, using an injection volume of 10 µL, a flow rate of 0.5 mL·min−1, and a column temperature of 35 °C. AsA quantification was performed at 243 nm.
2.4 RNA extraction, cDNA synthesis, and gene expression analysis using real-time PCR
Total RNA was extracted using the modified cetyl trimethylammonium bromide (CTAB) method (Chang et al. 1993). First-strand cDNA was synthesized using a PrimeScript First Strand cDNA Synthesis Kit (Takara, Japan). The genes involved in AsA metabolism were identified from the report of Zhang et al. (2016). Quantitative real-time PCR (qRT-PCR) was performed using a SYBR Premix Ex Taq Kit on a Bio-Rad CFX96 instrument. ZjH3 was the reference gene and primers were designed according to Zhang et al. (2016) and Chen et al. (2016) (Supplementary Table S1). The PCR protocol comprised initial heating at 95 °C for 3 min, followed by 39 cycles at 95 °C for 5 s, 55 °C for 30 s, and 72 °C for 10 s. Primer specificity was determined by qRT-PCR and melting-curve analysis. The 25 µL reaction system contained 2 × SYBR Premix ExTaq (12.5 µL), 10 µmol·L−1 primers (0.5 µL of each), H2O (10.5 µL), and diluted cDNA (1 µL). Gene expression levels were calculated using the formula 2−ΔΔCT.
2.5 Statistical analysis
Statistical analyses were conducted using the SPSS 20.0 statistical software (IBM, Chicago, IL, USA). Data were analyzed by one-way analysis of variance, with significant differences (Duncan′s multiple range test) assessed at the 5% confidence level. Pearson correlation analysis was carried out between AsA content and sugar and TA contents during fruit development.
3 Results
3.1 Fruit growth analysis
SZ and LZ had similar harvest seasons, with the fruits harvested in batches and sorted into five developmental stages by size and color (Fig. 2). During fruit development, the growth curves of the two jujube species were in the shape of a single ‘S’ variation, which showed that the expansion of fruit cells and volume mainly occurred at stages II and III (Fig. 2B and 2C). From stage I to II, both fruit peels were green, with the fruit peels of SZ and LZ becoming white at stages III and IV, respectively. Both fruit peels became half-red at stage V. The results showed that LZ fruit developed faster than SZ fruit in the early stage of fruit development.
Fruit developmental stages of two jujube species. A Whole fruit separated into five stages. B Dynamics of growth and development of LZ fruit. C Dynamics of growth and development of SZ fruit. Values are means of three replicates ± SD and different letters indicate significant differences (Duncan′s multiple range test, p < 0.05)
3.2 Changes in soluble sugar and TA contents during fruit development
The contents of soluble sugar and TA were assessed during fruit development (Fig. 3A and 3B). The soluble sugar content had the same ‘rise-fall-rise’ trend as in the development of both SZ and LZ fruits, with low soluble sugar contents during early fruit development that continuously increased after stage III. The TA content was high at stage I, but decreased at stage II and then increased as the SZ and LZ fruits ripened. At stage V, the soluble sugar contents were 19.64% and 16.19%, while the TA contents were 0.36% and 0.95% in LZ and SZ fruits, respectively. Notably, SZ fruit had significantly lower soluble sugar content, but significantly higher TA content compared with LZ fruit.
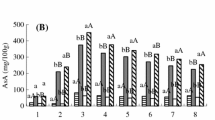
Changes of AsA, soluble sugar and TA contents during fruit development of two jujube species. A Changes of soluble sugar content during fruit development. B Changes of TA content during fruit development. C Changes of AsA content during fruit development. “L” indicates ‘Luojiangtiaoyuanzao’ (LZ) and “S” indicates ‘Yuanxingxiaosuanzao’ (SZ). Values are means of three replicates ± SD and different letters indicate significant differences (Duncan′s multiple range test, p < 0.05)
3.3 Changes in AsA content during fruit development
The AsA accumulation in LZ and SZ fruits was similar during fruit development (Fig. 3C). The AsA concentration in both LZ and SZ fruits was low at the young fruit stage, peaked at stage III, and then declined with fruit ripening. Notably, the AsA content in SZ was higher than that in LZ during fruit development. At stage V, the AsA contents of SZ and LZ fruits were 391.27 and 254.80 mg/100 g−1 FW, respectively.
3.4 Correlation of AsA content with soluble sugar content and TA content during fruit development
The correlation analysis showed that the positive correlation between AsA content and soluble sugar content was not significant in the two jujube species (Table 1). The AsA content was negatively correlated with TA content, but positively correlated with the sugar to acid ratio during LZ fruit development. Furthermore, there was no obvious correlation between AsA content and TA content and the sugar to acid ratio in SZ fruit.
3.5 Expression profiles of genes involved in AsA biosynthesis during fruit development
We examined the expression patterns of seven AsA biosynthetic genes during fruit development of LZ and SZ (Fig. 4A). The expression patterns of GME1, GMP1, GalDH, and GalLDH were similar in LZ and SZ fruits. The expression levels of GME1 and GMP1 were upregulated and then downregulated, peaking at stage III, and the expression levels of GME1 and GMP1 were 1.5-fold higher in SZ than in LZ at stage III. Furthermore, GalDH and GalLDH maintained high expression levels at stages III and V, with higher expression levels observed in LZ. GGP transcripts accumulated rapidly during early fruit development until stage III in both SZ and LZ fruits, and the GGP expression level at stage III was 6.9-fold higher in SZ fruit than in LZ fruit. GPP maintained the highest expression level at stage V in the fruits of SZ and LZ. For MIOX1, the highest expression level was observed at stage III in SZ fruit, but an increase in expression was observed throughout LZ fruit development. GME1, GMP1, GGP, GPP, and MIOX1 had high expression levels, in agreement with the change in AsA content, which indicates these genes might be the key limiting factors involved in AsA biosynthesis in jujube.
Relative expression levels of genes involved in AsA biosynthesis and recycling during fruit development. A Relative expression levels of genes involved in AsA biosynthesis. B Relative expression levels of genes involved in AsA recycling. Values are means of three replicates ± SD and different letters indicate significant differences (Duncan′s multiple range test, p < 0.05)
3.6 Expression profiles of genes involved in AsA degradation and recycling during fruit development
We also analyzed the expression profiles of AsA degradation and recycling genes in the jujube fruits (Fig. 4B). As SZ fruit ripened, the expression of AO1, AO2, AO3, and AO4 significantly increased and then decreased, while the expression of AO5 showed no significant change. In contrast, the expression of AO1, AO3, and AO4 showed little change in LZ fruit, while the expression level of AO5 was low at the early stages and then rose sharply with fruit ripening. The expression of APX3 increased continuously during LZ fruit development, while APX1, APX2, and APX4 showed a rise–fall–rise expression pattern similar to that in SZ. Regarding AsA recycling genes, the expression of MDHAR1 and MDHAR2 showed no significant change during LZ fruit development, but significantly increased during SZ fruit development at stages III and V. For DHAR, LZ and SZ showed similar trends, with two peaks observed at stages III and V, but a higher expression level was maintained in LZ fruit.
4 Discussion
4.1 The difference of AsA content in fruits of two jujube species is determined by multiple factors
AsA accumulation can vary between species or cultivars, as observed in oranges (Martí et al. 2009), blueberries (Liu et al. 2015) and tomatoes (Mellidou et al. 2012b). In the present study, the AsA content in SZ fruit was significantly higher than that in LZ fruit. As the growing environment and harvesting times of LZ and SZ were similar, the differences in AsA content were speculated to be mainly genetically determined.
Sugar is the precursor for AsA and could regulate the expression of enzymes involved in AsA metabolism as a signaling molecule (Sawake et al. 2015). In this study, soluble sugar and AsA contents were not necessarily related (Table 1), which was similar to previous results (Massot et al. 2010; Yang et al. 2013). Although the total soluble solids contents were clearly higher in ‘Newhall’ than in ‘Dream’, the AsA contents in pulps of the two cultivars were similar (Yang et al. 2011). Furthermore, the AsA content showed a very significant negative and positive correlation with the TA content and sugar to acid ratio during LZ fruit development, respectively (Table 1). Together, these differences in AsA content in LZ and SZ appeared not to be linked to primary metabolism of sugars during maturation, while low acid concentration may be considered an advantage for AsA accumulation in LZ fruit. Similar results have been reported in apple and tomato (Fang et al. 2017; Mellidou et al. 2012b). The high concentration of AsA in plants improves the resistance to extreme environments, which is the main reason why the AsA content of wild jujube accessions is higher than that of cultivated jujube cultivars (Mellidou et al. 2021). The difference in AsA content of jujube fruits between two genotypes was the indirect result of human selection of large, low-acid and high-sugar fruits (Zhang et al. 2016).
The pattern of AsA accumulation in different species varies. For instance, the total AsA content remained unchanged or increased slightly in strawberry (Agius et al. 2005) and oranges (Yang et al. 2011), decreased in apple (Li et al. 2011) and sweet cherry (Liang et al. 2017), and increased in grape (Cruz-Rus et al. 2010) and chestnut rose (Huang et al. 2014) during fruit development. In this study, AsA content increased gradually at the early stage of fruit development, peaked at the white mature stage, and then decreased gradually with fruit ripening, which implied that the final content in jujube fruit was subject to developmental regulation. Our results were similar to the findings for kiwifruit (Li et al. 2010) and blueberry (Liu et al. 2015).
4.2 AsA biosynthesis plays crucial roles in AsA accumulation in jujube species
The L-galactose pathway is generally regarded as the main route of AsA biosynthesis (Fenech et al. 2019). GMP converts D-mannose-1-P into GDP-D-mannose (Badejo et al. 2009), and GME converts GDP-D-mannose into GDP-L-galactose (Huang et al. 2014; Liu et al. 2015). GGP catalyzes GDP-L-galactose to release L-galactose-1-P (Bulley et al. 2012; Mellidou et al. 2012a). In this study, the expression patterns of GMP1, GME1 and GGP showed the best correlation with the change in AsA content in both jujube species during fruit development. MIOX is the key gene involved in the myo-inositol pathway (Fenech et al. 2019). We found that MIOX1 was differentially expressed during LZ and SZ fruit development, indicating that the inositol pathway plays diverse roles in AsA accumulation in various jujube species.
More transcripts of GME1, GMP1, GGP, GPP, and MIOX1 were detected in SZ than in LZ before stage IV, particularly at stage III, which was in agreement with the higher AsA content in SZ. These results indicated that the L-galactose and myo-inositol pathways play predominant roles during SZ fruit development, and the higher expression of GME1, GMP1, GGP, GPP, and MIOX1 promoted higher AsA concentrations in SZ fruit. The higher transcript levels of GME, GGP, GalDH and GalLDH in orange was the main reason for its higher ascorbate content than in Satsuma mandarin (Alós et al. 2014). Liu et al. (2015) demonstrated that higher expression levels of GME, GGP, and GalDH were associated with higher AsA content in ‘Bluecrop’ compared with ‘Berkeley’. These results suggested that induction of the genes involved in AsA biosynthesis could be vital for the differences in AsA content between genotypes during jujube fruit development.
4.3 The degradation and regeneration of AsA play crucial roles in AsA accumulation in jujube species
AsA consumption and recycling was mainly determined by the oxidation of AO and APX, and reduction of MDHAR and DHAR (Fenech et al. 2019). In this study, expression of the genes involved in the degradation and regeneration of AsA varied during jujube fruit development. APX1, APX2, APX3, and AO5 showed the highest expression levels in mature LZ and SZ fruit, which was consistent with the decline of AsA content in another jujube cultivar (Zhang et al. 2016). The significant upregulation of AO and APX might be the main reason for the decreased AsA content in the latter stages of fruit development (Huang et al. 2014; Liu et al. 2015).
In this study, DHAR expression was closely related to the high AsA accumulation at stages III and V of SZ and LZ fruits. In addition, the up-regulation of MDHAR1 at stage III and MDHAR2 at stage V contributed to the AsA accumulation in SZ fruit, but had no significant effect on LZ fruit. These results indicated that MDHAR and DHAR are key genes in AsA regeneration of SZ fruits, while DHAR is a key factor in LS fruits. We speculated that the higher AsA regeneration efficiency was partially responsible for higher AsA accumulation in SZ fruit, especially regarding MDHAR expression. MDHAR and DHAR coregulated AsA content and had a complementary relationship in maintaining the redox state of AsA during the development of kiwifruit (Li et al. 2010), bilberry (Cocetta et al. 2012) and blueberry (Liu et al. 2015). In another study, the change in AsA content correlated well with MDHAR expression and was negatively correlated with DHAR expression in strawberry (Cruz-Rus et al. 2011). These results indicated that the AsA regeneration pathway differs in various species.
5 Conclusion
In this study, we compared AsA concentration and expression profiles of genes involved in AsA biosynthesis, degradation, and regeneration between fruits of LZ and SZ. The pattern of AsA accumulation in the two jujube species was similar, and SZ had a higher level of AsA content during fruit development. AsA content was negatively correlated with TA content. The L-galactose and inositol pathways are the predominant routes for AsA biosynthesis in jujube fruits, with higher expression of synthesis genes (GME1, GMP1, GGP, GPP, and MIOX1) and a recycling gene (MDHAR), possibly responsible for the higher AsA concentration in SZ fruits.
Abbreviations
- AsA:
-
Ascorbic acid
- GME:
-
GDP-D-mannose 3′,5’-epimerase
- GMP:
-
GDP-D-mannose pyrophosphorylase
- GPP:
-
L-galactose-1-P phosphatase
- GGP:
-
GDP-L-galactosephosphorylase
- MIOX:
-
Inositol oxygenase
- MDHAR:
-
Monodehydroascorbate reductase
- GME:
-
GDP-D-mannose-30,50-epimerase
- GalDH:
-
L-galactose dehydrogenase
- GalLDH:
-
L-galactono-1,4-lactone dehydrogenase
- GalUR:
-
D-galacturonate reductase
- AO:
-
Ascorbate oxidase
- APX:
-
Ascorbate peroxidase
- DHAR:
-
Dehydroascorbate reductase
- DHA:
-
Dehydroascorbic acid
- TA:
-
Titratable acid
References
Agius F, Amaya I, Botella MA, Valpuesta V (2005) Functional analysis of homologous and heterologous promoters in strawberry fruits using transient expression. J Exp Bot 56(409):37–46. https://doi.org/10.1093/jxb/eri004
Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci 8:613. https://doi.org/10.3389/fpls.2017.00613
Alós E, Rodrigo MJ, Zacarias L (2014) Differential transcriptional regulation of L-ascorbic acid content in peel and pulp of citrus fruits during development and maturation. Planta 239(5):1113–1128. https://doi.org/10.1007/s00425-014-2044-z
Badejo AA, Fujikawa Y, Esaka M (2009) Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff-Wheeler pathway in acerola (Malpighia glabra). J Plant Physiol 166:652–660. https://doi.org/10.1016/j.jplph.2008.09.004
Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-wang K, Andre C, Brewster D, Karunairetnam S, Allan AC (2012) Enhancing ascorbate in fruits and tubers through over-expression of the L-galactose pathway gene GDP-L-galactose phosphorylase. Plant Biotechnol J 10(4):390–397. https://doi.org/10.1111/j.1467-7652.2011.00668.x
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Chen XJ, Zhou Y, Cong YD, Zhu PS, Xing JY, Cui JX, Xu W, Shi QH, Diao M, Liu HY (2021) Ascorbic acid-induced photosynthetic adaptability of processing tomatoes to salt stress probed by fast OJIP fluorescence rise. Front in Plant Sci 12:594400. https://doi.org/10.3389/fpls.2021.594400
Chen YY, Zhao ZH, Zhao J, Liu MJ (2016) Expression profiles of genes and enzymes related to ascorbic acid metabolism in fruits of Ziziphus jujube Mill ‘Jinsixiaozao.’ Front Agric Sci Eng 3(2):131–136. https://doi.org/10.15302/J-FASE-2016096
Chutichudet P, Chutichudet B, Kaewsit S (2008) An analysis on quality, colour, tissue texture, total soluble solid content, titratable acidity and pH of Santol fruits (Sandoricum koetjape Burm. F.) Merr. Pui Fai cultivar, grown in Northern Thailand. Pak J Biol Sci 11:1348–1353. https://doi.org/10.3923/pjbs.2008.1348.1353
Cocetta G, Karppinen K, Suokas M, Hohtola A, Haggman H, Spinardi A, Mignani I, Jaakola L (2012) Ascorbic acid metabolism during bilberry (Vaccinium myrtillus L.) fruit development. J Plant Physiol 169(11):1059–1065. https://doi.org/10.1016/j.jplph.2012.03.010
Cruz-Rus E, Botella MA, Valpuesta V, Gomez-Jimenez MC (2010) Analysis of genes involved in L-ascorbic acid biosynthesis during growth and ripening of grape berries. J Plant Physiol 167:739–748. https://doi.org/10.1016/j.jplph.2009.12.017
Cruz-Rus E, Iraida A, Sánchez-Sevilla FF, Botella MA, Valpuesta V (2011) Regulation of L-ascorbic acid in strawberry fruits. J Exp Bot 62:4191–4201. https://doi.org/10.1093/jxb/err122
Davey MW, Van Monatgu M, Inzé D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J (2000) Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric 80(7):825–860. https://doi.org/10.1002/(SICI)1097-0010(20000515)80:7%3c825:AID-JSFA598%3e3.0.CO;2-6
Fang T, Zhen Q, Liao L, Owiti A, Zhao L, Korban SS, Han YP (2017) Variation of ascorbic acid concentration in fruits of cultivated and wild apples. Food Chem 225:132–137. https://doi.org/10.1016/j.foodchem.2017.01.014
Fenech M, Amaya I, Valpuesta V, Botella MA (2019) Vitamin C content in fruits: biosynthesis and regulation. Front Plant Sci 9:2006. https://doi.org/10.3389/fpls.2018.02006
Fitzpatrick TB, Basset GJC, Borel P, Carrari F, Della PD, Fraser PD, Hellmann H, Osorio S, Rothan C, Vapluesta V et al (2012) Vitamin deficiencies in humans: can plant science help? Plant Cell 24(2):395–414. https://doi.org/10.1105/tpc.111.093120
Huang J, Chen X, He AB, Ma ZB, Gong TQ, Xu KN, Chen RH (2021) Intergrative morphological physiological, proteomics analyses of jujube fruit development provide insights into fruit quality domestication from wild jujube to cultivated jujube. Front Plant Sci 12:773825. https://doi.org/10.3389/fpls.2021.773825
Huang JX, Heyduck R, Richins RD, Van Leeuwen D, O’connell MA, Yao SR (2017) Jujube cultivar vitamin C profile and nutrient dynamics during maturation. HortScience 52(6):859–867. https://doi.org/10.21273/HORTSCI11945-17
Huang M, Xu Q, Deng XX (2014) L-Ascorbic acid metabolism during fruit development in an ascorbate-rich fruit crop chestnut rose (Rosa roxburghii Tratt). J Plant Physiol 171:1205–1216. https://doi.org/10.1016/j.jplph.2014.03.010
Ishikawa T, Dowdle J, Smirnoff N (2006) Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol Plant 126:343–355. https://doi.org/10.1111/j.1399-3054.2007.00871.x
Li M, Chen X, Wang P, Ma FW (2011) Ascorbic acid accumulation and expression of gene involved in its biosynthesis and recycling in developing apple fruit. Am Soc Hortic Sci 136(4):231–238. https://doi.org/10.21273/JASHS.136.4.231
Li MJ, Ma FW, Liang D, Li J, Wang YL (2010) Ascorbate biosynthesis during early fruit development is the main reason for its accumulation in kiwi. PLoS ONE 5(12):e14281. https://doi.org/10.1371/journal.pone.0014281
Liang D, Zhu TT, Ni ZY, Lin LJ, Tang Y, Wang ZH, Wang X, Wang J, Lv XL, Xia H (2017) Ascorbic acid metabolism during sweet cherry (Prunus avium) fruit development. PLoS ONE 12(2):e0172818. https://doi.org/10.1371/journal.pone.0172818
Liu FH, Wang L, Gu L, Zhao W, Su HY, Cheng XH (2015) Higher transcription levels in ascorbic acid biosynthetic and recycling genes were associated with higher ascorbic acid accumulation in blueberry. Food Chem 188:399–405. https://doi.org/10.1016/j.foodchem.2015.05.036
Liu M, Liu P, Liu G (2013) Advances of research on germplasm resources of Chinese jujube. Acta Hortic 993:15–20. https://doi.org/10.17660/ActaHortic.2013.993.1
Liu MJ, Wang JR, Wang LL, Liu P, Zhao J, Zhao ZH, Yao SR, Stanica F, Liu ZG, Wang LX (2020) The historical and current research progress on jujube–a superfruit for the future. Hortic Res 7:119. https://doi.org/10.1038/s41438-020-00346-5
Liu MJ, Zhao J, Cai QL, Liu GC, Wang JR, Zhao ZH, Liu P, Dai L, Yan GJ, Wang WJ et al (2014) The complex jujube genome provides insights into fruit tree biology. Nat Commun 5:5315. https://doi.org/10.1038/ncomms6315
Magwaza LS, Mditshwa A, Tesfay SZ, Opara UL (2017) An overview of preharvest factors affecting vitamin C content of citrus fruit. Sci Hortic 216:12–21. https://doi.org/10.1016/j.scienta.2016.12.021
Martí N, Mena P, Cánovas JA, Micol V, Saura D (2009) Vitamin C and the role of citrus juices as functional food. Nat Prod Commun 4:677–700. https://doi.org/10.1016/j.icarus.2008.07.019
Massot C, Genard M, Stevens R, Gautier H (2010) Fluctuations in sugar content are not determinant in explaining variations in vitamin C in tomato fruit. Plant Physiol Biochem 48(9):751–757. https://doi.org/10.1016/j.plaphy.2010.06.001
Mellidou I, Koukounaras A, Kostas S, Patelou E, Kanellis AK (2021) Regulation of vitamin C accumulation for improved tomato fruit quality and alleviation of abiotic stress. Genes 12:694. https://doi.org/10.3390/genes12050694
Mellidou I, Chagne D, Liang WA, Keulemans J, Davey MW (2012a) Allelic variation in paralogs of GDP-L-Galactose phosphorylase is a major determinant of vitamin C concentrations in apple fruit. Plant Physiol 160:1613–1629. https://doi.org/10.1104/pp.112.203786
Mellidou I, Keulemans J, Kanellis AK, Davey MW (2012b) Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant Biol 12:239. https://doi.org/10.1186/1471-2229-12-239
Sawake S, Tajima N, Mortimer JC, Lao J, Ishikawa T, Yu XL, Yamanashi Y, Yoshimi Y, Kawai-Yamada M, Dupree P et al (2015) KONJAC1 and 2 are key factors for GDP-mannose generation and affect L-ascorbic acid and glucomannan biosynthesis in arabidopsis. Plant Cell 27(12):3397–3409. https://doi.org/10.1105/tpc.15.00379
Wang Z, Xiao Y, Chen W, Tang K, Zhan L (2010) Increased vitamin c content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Integr Plant Biol 52:400–409. https://doi.org/10.1111/j.1744-7909.2010.00921.x
Weng X, Xing G, Li YX (2013) Study on the determination of total sugar of potato starch by anthrone colorimetric method. Food Res Dev 34(17):86–88. https://doi.org/10.3969/j.issn.1005-6521.2013.17.023 (in Chinese)
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369. https://doi.org/10.1038/30728
Wojdyło A, Carbonell-Barrachina ÁA, Legua P, Hernández F (2016) Phenolic composition, ascorbic acid content, and antioxidant capacity of Spanish jujube (Ziziphus jujube Mill.) fruits. Food Chem 201:307–314. https://doi.org/10.1016/j.foodchem.2016.01.090
Yang XY, Xie JX, Wang FF, Zhong J, Liu YZ, Li GH, Peng SA (2011) Comparison of ascorbate metabolism in fruits of two citrus species with obvious difference in ascorbate content in pulp. J Plant Physiol 168:2196–2205. https://doi.org/10.1016/j.jplph.2011.07.015
Yang XY, Wang FF, Teixeira Da Silva JA, Zhong J, Liu YZ, Peng SA (2013) Branch girdling at fruit green mature stage affects fruit ascorbic acid contents and expression of genes involved in L-galactose pathway in citrus. N Z J Crop Hortic Sci 41:23. https://doi.org/10.1080/01140671.2012.724429
Zhang CM, Huang J, Li XG (2016) Transcriptomic analysis reveals the metabolic mechanism of L-ascorbic acid in Ziziphus jujuba Mill. Front Plant Sci 7:122. https://doi.org/10.3389/fpls.2016.00122
Acknowledgements
This work was funded by the Foundation for Disciplines Construction and Development of Sichuan Agricultural University (No: 035/20211993021); the Key Science and Technology Research Project of Deyang (No: 2018CKJ018); the Special Action Plan Project of Science and Technology Rich People and Strong County of Sichuan Province “Integration and Demonstration of Key Technologies for Industrialized Development of Ziziphus jujuba ‘Zhanshangmizao’ of Santai County” (2012-2014) (No: 06320200).
Author information
Authors and Affiliations
Contributions
YW drafted and edited the manuscript, and assisted GYS in completing the experiment. GYS performed the experiments with YW and analyzed the data. DL proposed the re-search, and facilitated the work. HX collected the data. HFZ edited the manuscript with YW. XL revised the manuscript. QXD was the project investigator and research supervisor. The published version of this manuscript was revised and agreed upon by all authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Communicated by Yong-Bum Kwack.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Song, G., Liang, D. et al. Comparison of ascorbate metabolism in fruits of two jujube species with differences in ascorbic acid content. Hortic. Environ. Biotechnol. 63, 759–767 (2022). https://doi.org/10.1007/s13580-022-00431-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-022-00431-9