Abstract
This study reports spore germination, early gametophyte development and change in the reproductive phase of Drynaria fortunei, a medicinal fern, in response to changes in pH and light spectra. Germination of D. fortunei spores occurred on a wide range of pH from 3.7 to 9.7. The highest germination (63.3%) occurred on ½ strength Murashige and Skoog basal medium supplemented with 2% sucrose at pH 7.7 under white light condition. Among the different light spectra tested, red, far-red, blue, and white light resulted in 71.3, 42.3, 52.7, and 71.0% spore germination, respectively. There were no morphological differences among gametophytes grown under white and blue light. Elongated or filamentous but multiseriate gametophytes developed under red light, whereas under far-red light gametophytes grew as uniseriate filaments consisting of mostly elongated cells. Different light spectra influenced development of antheridia and archegonia in the gametophytes. Gametophytes gave rise to new gametophytes and developed antheridia and archegonia after they were transferred to culture flasks. After these gametophytes were transferred to plastic tray cells with potting mix of tree fern trunk fiber mix (TFTF mix) and peatmoss the highest number of sporophytes was found. Sporophytes grown in pots developed rhizomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drynaria fortunei (Kunze) J. Sm (Polypodiaceae), a large epiphytic or petrophilous fern, is a source of traditional Chinese medicine commonly known as “Gu-Sui-Bu”. The plant grows on tree trunks, rocks, and occasionally on brick walls. The species has been widely distributed in Taiwan, China, Vietnam, Thailand, and Laos (Anonymous 1994). The medicine “Gu-Sui-Bu” has been commonly used in the treatment of bone injuries. It has been shown to be effective for the treatment of inflammation, hyperlipemia, and arteriosclerosis (Anonymous 2005). In studies carried out in vitro, “Gu-Sui-Bu” was effective in antiresorptive action in bone cells and prevented osteoporosis (Jeong et al. 2005). Results from a recent study showed that “Gu-Sui-Bu” extract taken orally increased bone density (Wong and Rabie 2006). “Gu-Sui-Bu” has also been shown to possess the therapeutic effects on bone healing (Ma et al. 1996; Lin et al. 2002; Chang et al. 2003; Sun et al. 2004).
Because a finger-thick fleshy rhizome part of the fern is used in “Gu-Sui-Bu” medicine, the plant is rapidly disappearing from natural habitats as a result of overexploitation. Currently, the plant is not under cultivation. Development of in vitro propagation procedure may help in the promotion of its cultivation and compensation of its loss in the wild.
Drynaria fortunei is a typical fern that completes its life cycle in two forms: a diploid sporophyte (most often) and a haploid gametophyte. These forms alternate each other. Gametophyte begins with the germination of spore, followed by a gradual development of prothallus (gametophyte). The phenomenon of spore germination and subsequent growth of gametophyte has been reported to be influenced by several physico-chemical factors, including light. Several studies have been carried out to understand the basic regulatory mechanisms in spore germination and development of gametophyte and sporophyte (Raghavan 1989). In addition to light and gibberellin-like (GA-like) compounds, other factors such as pH of culture medium (Conway 1949), ethylene (Edwards and Miller 1972a, b), CO2 (Edwards 1977), and calcium availability (Wayne and Hepler 1984, 1985) have been shown to influence spore germination and subsequent growth of gametophytes. Results of these studies showed that the optimal conditions for spore germination varied with the fern species. Hence, the study of these factors for spore germination is prerequisite for optimal growth of D. fortunei.
In this study, effects of different factors, including strength of MS basal medium, sucrose concentrations, pH, sugars, spectra of light on germination, development of gametophytes and reproductive organs of D. fortunei spores, were investigated. We also studied the development of sporophytes from these gametophytes under greenhouse conditions.
Materials and Methods
Plant materials. Spores of Drynaria fortunei (Kunze) J. Sm were collected from Hsinchu, northern Taiwan, and stored in dark at 4°C until use. A voucher specimen of the plant (No. CMU-94-DF-01) has been deposited in the China Medical University Herbarium, Taichung, Taiwan.
Culture conditions. Spores were surface-sterilized for 5 min with 0.5% sodium hypochlorite (Clorox®, The Clorox Co., Oakland, CA) solution with two drops of Tween 20®/100 ml (Hayashi Pure Chemical Industries Ltd., Osaka, Japan). After the spores were collected on the sterile filter paper in a funnel, they were rinsed three times with sterile distilled water. Spores in equal density (2,000 spores/0.5 ml sterile water) were inoculated in Petri dishes (90 × 15 mm), each containing 25 ml of 2×, 1×, ½×, ¼× MS (Murashige and Skoog 1962) basal medium to evaluate the effect of MS medium on spore germination.
As the highest spore germination was achieved on ½× MS basal medium, this medium was selected for further experiments. To optimize cultural factors for spore germination, spores were germinated on the medium (1) with the pH of 3.7, 4.7, 5.7, 6.7, 7.7, 8.2, 8.7, 9.2, and 9.7, (2) sucrose at 0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, and 7.0 g l−1, (3) different carbohydrate sources: sucrose, glucose, fructose, and maltose, each at 2% level. Spores were also germinated under different light spectra, including blue light (450 nm), red light (660 nm), far-red light (735 nm), white light (380–780 nm), or complete darkness.
The pH of the medium was adjusted with 1 N NaOH or 1 N HCl. All media were solidified using 0.35% gelrite (Sigma-Aldrich, Inc., St. Louis, MO). Petri dishes were sealed with parafilm and incubated at 25 ± 1°C under the 16 h light and 8 h dark cycle, with the light intensity of 38 μmol m−2 s−1 provided by cool fluorescent tubes. For experiments with different color lights, cultures were incubated in a LED-light plant growth chamber (900FLED, Taiwan Hipoint Corporation, Taiwan) at 25 ± 1°C, with 16-h photoperiod. LEDs are semiconductor diodes that typically emit a single wavelength of light when charged with electricity.
Subculture of gametophytes. For further growth and development, 2-mo.-old gametophytes were subcultured in 500-ml flasks containing 100 ml ½× MS medium supplemented with 2% sucrose, 0.35% gelrite and incubated as previously described.
Ex vitro development of sporophytes. Five-mo.-old gametophytes grown in flasks under white light were transferred to plastic cell tray containing three types of substrata, namely, tree fern trunk fiber mix (TFTF mix), TFTF mix/peatmoss (1:1), and peatmoss. TFTF mix and peatmoss (Klassman Potgrond H, William Sinclair Horticulture Ltd., England) were procured from Yu Kuan, Taichung, Taiwan. The gametophytes (5 g fresh weight) were transferred to each tray with potting mix and kept in a greenhouse. The trays were sprinkler-irrigated daily.
Microphotographs of germinating spores and gametophytes were taken with a microscope (Nikon ECLIPSE E400, Japan) using a digital camera (Nikon Coolpix 4500, Japan). Samples of gametophytes were also frozen in liquid nitrogen and observed using a scanning electron microscope (JEOL-JSM-6330F, Japan).
Statistical analysis. Each treatment consisted of a minimum three replicates and each experiment was repeated three times. Data were statistically analyzed for least significant difference (LSD) using SAS 8.2 statistical software (SAS Institute Inc., Cary, NC 2001).
Results and Discussion
Effects of medium. The spores of D. fortunei germinated within 7 d of sowing. Like many fern species, spore germination in D. fortunei commenced by rupturing of the spore coat and division of the spore cell. However, spores underwent only one asymmetric cell division giving rise to rhizoid and protonemal cell, which continued to divide to form a gametophyte. In the initial experiments, we optimized the basal medium and carbohydrate source for maximum germination of D. fortunei spores. Among different strengths of MS basal medium used, although a higher germination (32.5%) was observed on ¼× MS as compared to ½× MS (27.6%), further growth of gametophytes in the former medium was slow. On the other hand, 1× and 2× medium gave rise to 15.3 and 7.8% germination, respectively. In both cases, gametophyte growth was much lower than those grown on ½× medium. The ½× medium was therefore selected for further study.
Spores of several fern species could be germinated in water, as observed in D. fortunei in this study (data not shown). The gametophyte usually requires a variety of mineral elements found in a balanced nutrient solution for continued growth (Raghavan 1989). As evident from the results of the present study, strength of salts in MS basal medium has a marked effect on not only spore germination but also gametophyte growth. A higher percentage of spore germination but restricted growth of prothalli in ¼× MS medium could be caused by deficiency (suboptimal level) of nutrients, whereas decreased germination and restricted growth on 1× and 2× medium could be caused by the presence of supraoptimal levels of mineral salts in the media. Thus, it appears that gametophytes require balanced mineral salts for its normal growth.
The sucrose concentration in the medium is also important for spore germination. Among the concentrations used (0–7%), the presence of 2% sucrose gave rise to the highest germination (72.3%), whereas the sucrose levels higher than 2% decreased the germination drastically. The lowest germination (20.0%) was observed in the medium with 7% sucrose. With respect to carbohydrate sources in the medium, sucrose resulted in the highest spore germination (74.7%) compared to maltose (59.7%), glucose (23.3%), and fructose (0%). After 4 wk of growth, gametophytes grown in the sucrose medium were cordate compared to spatulate in the maltose and glucose media. However, gametophytes in sucrose developed apical notch after 10 wk, whereas those in maltose became cordate. Gametophyte growth on medium with glucose was drastically inhibited and its shape was irregular without the biplanar form.
There are contrasting reports on the effect of sucrose on spore germination and gametophyte development in several fern species. Sheffield et al. (2001) reported a significant increase in spore germination of four fern species by addition of sucrose in the medium. However, results of other studies showed that sucrose was stimulatory (in terms of cell number) to gametophyte development of Platycerium (Camloh 1993), but it inhibited two-dimension growth of gametophytes of Anemia and Pteridium (Douglas 1994). In several other ferns, the beneficial effects of other sugars such as fructose, maltose, ribose, and xylose on gametophyte development have also been documented (Raghavan 1989).
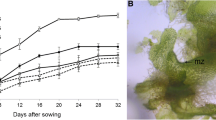
Effects of pH. The medium pH had a significant effect on spore germination in D. fortunei (Fig. 1). The germination frequency increased with an increase in the medium pH, with the highest frequency (63.3%) occurring at pH 7.7 and the lowest frequency (0.8%) at pH 9.7. However, the medium pH higher than 7.7 resulted in decreased germination. In addition to spore germination, pH affected the growth of gametophytes. Development of biplanar gametophytes was observed only at pH 7.7 (Fig. 4 b), but restricted growth occurred at pH levels lower or higher than 7.7 (Fig. 4 a–c). Furthermore, the lowest and the highest pH, i.e., 3.7 and 9.7, inhibited cell divisions of protonema.
The study of pH on spore germination under controlled conditions is an important aspect of germination of fern spores, as this group of plants survive and grow under different soil types and ecological niches. In the previous studies, it was observed that spores of most ferns species germinated at slightly acidic or neutral pH (Raghavan 1989). Tolerance of spores of some fern species, including Notholaena cochisensis and Pellaea limitanea, to pH of 9–10 showed correlation with high alkalinity of the soil in which they grew (Hevly 1963). It was speculated that spore germination was limited to a narrow range of pH on either side of neutrality (Raghavan 1989). However, to our surprise, germination of D. fortunei spores occurred in varying percentages on a wide range of pH from 3.7 to 9.7, although the highest germination frequency occurred at the pH toward alkalinity (pH 7.7).
Observations taken after 14 wk of spore inoculation revealed that media with different pH values had an effect on the number of archegonia formation in gametophytes. The effect was similar to that for spore germination (Fig. 5 a). The highest number of archegonia (27.6 ± 2.7/gametophyte) occurred at pH 7.7, followed by pH 6.7 (20.7 ± 3.8/gametophyte). At pH 4.7, there was only 0.7 ± 0.5 archegonia per gametophyte, whereas no archegonia was observed in media at pH 3.7 and 9.7.
Effects of light spectra. It was observed that spores of D. fortunei germinated only in the light but not in the dark, indicating that light is required for spore germination. Further experiments with different light spectra, i.e., red, far-red, blue, and white light, resulted in 71.3, 42.3, 52.7, and 71.0% germination, respectively (Fig. 2). However, there were striking differences in the development of gametophytes in different light spectra (Fig. 4 d–h). Under the white or blue light, cordate type gametophytes developed after 4 wk of culture, and there was no morphological difference among gametophytes. On the other hand, elongated or filamentous but broad gametophytes developed under red light (Fig. 4 d). In far-red light, gametophytes grew as uniseriate filaments consisting of mostly elongated cells (Fig. 4 f). The morphogenetic index of gametophytes in terms of length/width ratio under different light spectra was 0.9 ± 0.1, 2.6 ± 1.2, 37.4 ± 2.0, and 0.8 ± 0.1 for blue, red, far-red and white lights, respectively (Fig. 3). We also investigated the reversible effects of white and infrared light on the development of gametophytes. Spores germinated in white light for 1 wk, followed by 2 wk in far-red, and another 2 wk in white light resulted in a change in the cell division pattern of protonema (Fig. 4 g). Similarly, spores germinated under far-red light for 1 wk and then shifted to white light for 2 wk, followed by 2 wk in far-red showed a multiseriate, filamentous gametophyte, which indicates cell division under both infrared and white light regimen (Fig. 4 h).
a–h, Development of gametophytes under different pH and light spectra. Gametophytes were grown on the medium at pH 3.7 (bar = 34 μm) (a); pH 7.7 and under white light (Control; bar = 146 μm) (b); pH 9.7 (bar = 34 μm) (c); blue light (bar = 230 μm) (d); red (R) light (bar = 230 μm) (e); far-red (FR) light (bar = 230 μm) (f); gametophytes were grown under white light for 1 wk then shifted to FR for 2 wk and 1 wk in white light (bar = 146 μm) (g); gametophytes were grown under FR for 1 wk, then shifted to white light for 2 wk and FR for 1 wk (bar = 146 μm) (h).
The effect of light spectra on reproductive organ development in gametophytes of D. fortunei was also investigated. Observations taken after 5 mo. of spore inoculation revealed no formation of antheridia and archegonia under far-red light (Table 1). However, under the blue light, 60% gametophytes formed archegonia and 6.7% gave rise to both archegonia and antheridia, but no sex organs were observed in 33.3% gametophytes. Red light appeared to favor antheridia formation, as male organs were observed in 33.3% gametophytes, but 53.3% did not develop reproductive organs. We found a small number of gametophytes (6.7%) developed archegonia alone or both sex organs (bisexual) under red and blue light (Table 1). Under white light, 50% gametophytes developed only archegonia (Fig. 5 a), whereas 3.3% formed only antheridia (Fig. 5 b), but 23.3% gave rise to both antheridia and archegonia (Table 1), whereas 23.3% gametophytes were found to be asexual (showed no sex organs). As elongated and uniseriate gametophytes of D. fortunei developed under far-red light, and growth of these gametophytes was abnormal and did not reach maturity, this may offer an explanation as to why sex organ development is absent in some gametophytes. The diversity in sexual expression in gametophytes has also been reported in Cryptogramma crispa (Pajaron et al. 1999).
Reproductive organs (female—archegonium, and male—antheridium) in D. fortunei. Archegonia in D. fortunei in 14-wk-old gametophyte (a); antheridia in 5 mo. old gametophyte (b). Mass of old and new gametophytes in culture flask, 3 mo. after transfer of gametophytes from Petri dishes (c); sporophytes developed after 14 wk of transfer of gametophytes to plastic cell tray kept in greenhouse (d); sporophytes in a single plastic tray cell (e); sporophytes transferred to a pot kept in greenhouse showing rhizome formation after 5 mo. of potting (arrow indicates rhizome) (f).
Light is one of the most important factors that affect events in the life cycle of a fern. It is a signal known to awaken the dormant fern spore. It has been reported that spore germination involves a light sensitive pigment phytochrome (Raghavan 1992). Phytochrome acts as a germination switch, and wavelength of light flips the switch back and forth between off (the Pr form: P for phytochrome and r for absorbing red light) and on (the Pfr form: fr for absorbing far-red light) in a photoreversible manner. Studies on the sensitive fern Onoclea sensibilis revealed that these effects by phytochrome brought about by altering the intracellular calcium levels in the spore cell (Wayne and Hepler 1984, 1985). Thus, light triggers a cascade of biochemical events in a dormant fern spore and spores incubated under different light spectra show varying germination responses caused by sequential changes in these pigments, thereby making germination a complex event.
Like spores, the sensitivity of protonemata (five- to six-celled stage gametophyte) of ferns to blue, red and far-red light varies greatly. In Lygodium japonicum, gametophytes exhibited planar morphology under red and blue light, but remained filamentous under far-red light (Swami and Raghavan 1980). These light affected growth forms were the result of changed patterns of cell divisions in the protonemata. The pronounced inhibition of elongation growth accompanied by growth in width of the filamentous form provides a firm basis to quantify growth data in terms of length/width ratio, designated as morphogenetic index (Mohr 1956). The morphogenetic index of gametophytes grown under far-red is high, indicating the occurrence of increased cell elongation coupled with low incidence of cell division. Under white, blue, and red light, the index is very low and there is a significant increase in the number of cells formed. Development of gametophytes with normal, planar growth under white light is a result of increased mitotic activity and increase in surface area of the gametophytes.
Transfer of gametophytes from one type of light to another can cause changes in their morphology. The effect of different light spectra on protonemata development in ferns has often been interpreted as a result of differential mitotic activity. Thus, light regimens may act antagonistically by modulating the elongation potential or division potential of cells, as observed in this study (Fig. 4 g, h).
In contrast to the response of D. fortunei in this study, it was observed that spores of Stromatopteris moniliformis (Gleicheniaceae) did not germinate in the light and it took 3 mo. to germinate in the dark on the medium containing minerals and 0.5% glucose (Whittier 1999). In Diphasiastrum sitchense, Whittier (2003) also observed no spore germination in the light even after 11 mo. of culture, whereas 0.5 % spores germinated in the dark.
In general, gametophytes after attaining maturity serves as a center for growth and morphogenesis and development of reproductive organs, e.g., archegonia and antheridia. In most homosporous ferns, development of antheridia precedes archegonia (Raghavan 1989). However, in D. fortunei, development of archegonia was preceded by antheridia. The cause triggering this differentiation in different ferns is not clear, although results of some studies indicated the involvement of endogenous hormones in sexuality induction in fern gametophytes (Raghavan 1989).
Gametophytes transferred to medium in flasks grew further and developed new gametophytes forming a compact mass of old and new gametophytes (Fig. 5 c).
Development of sporophytes ex vitro. The 5-mo.-old gametophytes were transferred to plastic tray cells with potting mix (Fig. 5 d) in a greenhouse and juvenile sporophytes were developed after 14 wk (Fig. 5 d, e). The highest number of sporophytes per tray cell (167.6 ± 5.4) was obtained on peatmoss, followed by TFTF mix and peatmoss (130.6 ± 17.3). TFTF mix alone gave rise to only 73.1 ± 17.1 sporophytes per tray cell (Table 2). These sporophytes grew further and developed rhizome after these were transferred to individual plastic pots with same potting mix (Fig. 5 f).
The present study describes the requirements for in vitro spore germination and gametophyte development of D. fortunei. The results may promote large-scale cultivation of D. fortunei to compensate its depletion in nature.
References
Anonymous. Flora of Taiwan, Vol.1, 2nd edn., Taipei: National Science Council of Taiwan; 1994:484–485.
Anonymous. Pharmacopoeia Commission of People Republic of China (ChPC), Vol. 1. China: Chemical Industry Press; 2005:179–180.
Camloh, M. Spore germination and early gametophyte development of Platycerium bifurcatum. Am. Fern J. 83:124–132; 1993.
Chang, E. J.; Lee, W. J.; Cho, S. H.; Choi, S. W. Proliferative effects of flavan-3 and propelargonidina from rhizomes of Drynaria fortunei on MCF-7 and osteblastic cells. Arch. Pharm. Res. 26:620–630; 2003.
Conway, E. The autecology of bracken [Pteridium aquililinum (L.) Kuhn.]: The germination of spore, and the development of the prothallus and the young sporophyte. Proc. R. Soc. Edinb. 63B:625–643; 1949.
Douglas, G. E. An investigation into the growth, development and ultra structure of fern gametophytes in existing and novel culture systems. PhD dissertation, University of Manchester, U.K; 1994.
Edwards, M. E. Carbon dioxide and ethylene control of spore germination in Onoclea sensibilis L. Plant Physiol. 59:756–758; 1977.
Edwards, M. E.; Miller, J. H. Growth regulation by ethylene in fern gametophytes. II. Inhibition of cell division. Am. J. Bot. 59:450–457; 1972a.
Edwards, M. E.; Miller, J. H. Growth regulation by ethylene in fern gametophytes. III. Inhibition of spore germination. Am. J. Bot. 59:458–465; 1972b.
Hevly, R. H. Adaptations of Cheilanthoid ferns to desert environments. J. Ariz. Acad. Sci. 2:164–175; 1963.
Jeong, J. C.; Lee, J. W.; Yoon, C. H.; Lee, Y. C.; Chung, K. H.; Kim, M. G.; Kim, C. H. Stimulative effects of Drynariae rhizoma extracts on the proliferation and differentiation of osteoblastic MC3T3-E1 cells. J. Ethnopharmacol. 96:489–95; 2005.
Lin, C. Y.; Sun, J. S.; Sheu, S. Y.; Lin, F. H.; Wang, Y. J.; Chen, L. T. The effect of Chinese medicine on bone cell activities. Am. J. Chin. Med. 30:271–285; 2002.
Ma, K. C.; Zhu, T. Y.; Wang, F. X. Stimulatory effects of gu-sui-bu (Drynaria baronii) injection on chick embryo bone primordium calcification in vitro. Am. J. Chin. Med. 24: 77–82; 1996.
Mohr, H. Die Abhangigkeit des protonemawachstums und der protonemapolaritat bei farnen vom licht. Planta 47:127–158; 1956.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15:473–497; 1962.
Pajaron, S.; Pangua, E.; Garcia-Alvarez, L. Sexual expression and genetic diversity in populations of Cryptpgramma crispa (Pteridaceae). Am. J. Bot. 86:964–973; 1999.
Raghavan, V. Developmental biology of fern gametophytes. Cambridge: Cambridge University Press; 1989:7–151.
Raghavan, V. Germination of fern spores. Am. Sci. 80:176–185; 1992.
SAS Institute Inc. SAS/STAT User’s Guide. Version 8.2, vol 2. USA: SAS Institute; 2001:943.
Sheffield, E.; Douglas, G. E.; Hearne, S. J.; Huxham, S.; Wynn, J. M. Enhancement of fern spore germination and gametophyte growth in artificial media. Am. Fern J. 91:179–186; 2001.
Sun, J. S.; Theriault, B. L.; Anderson, G. I. The effect of Gu-Sui-Bu (Drynaria fortunei) on bone cell activity. Am. J. Chin. Med. 32:737–53; 2004.
Swami, P.; Raghavan, V. Control of morphogenesis in the gametophyte of a fern by light and growth hormones. Can. J. Bot. 58:1464–1473; 1980.
Wayne, R.; Hepler, P. K. The role of calcium ions in phytochrome-mediated germination of spores of Onoclea sensibilis L. Planta 160:12–20; 1984.
Wayne, R.; Hepler, P. K. Red light stimulates an increase in intracellular calcium in the spores of Onoclea sensibilis L. Plant Physiol. 77:8–11; 1985.
Whittier, D. P. Spore germination and early gametophyte development in Stromatopteris. Am. Fern J. 89:142–148; 1999.
Whittier, D. P. The gametophyte of Diphasiastrum sitchense. Am. Fern J. 93:20–24; 2003.
Wong, R. W.; Rabie, A. B. Systemic effect of crude extract from rhizome of Drynaria fortunei on bone formation in mice. Phytother. Res. 20:313–315; 2006.
Acknowledgment
The authors gratefully acknowledged a research grant (NSC 95-2317-B-039-002) from the National Science Council, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Guha-Mukherjee
Rights and permissions
About this article
Cite this article
Chang, HC., Agrawal, D.C., Kuo, CL. et al. In vitro culture of Drynaria fortunei, a fern species source of Chinese medicine “Gu-Sui-Bu”. In Vitro Cell.Dev.Biol.-Plant 43, 133–139 (2007). https://doi.org/10.1007/s11627-007-9037-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-007-9037-6