Abstract
Ethylene is an important regulator of developmental and stress responses in plants, and 1-aminocyclopropane-1-carboxylic acid synthase (ACS) proteins catalyze one of the rate-limiting steps in endogenous ethylene biosynthesis. Accordingly, the function of ACS family genes has been extensively studied in plants including Arabidopsis and tomato; however, little is known about the systemic function of ACS genes in cucumber (Cucumis sativus L.). Here, we investigated the expression patterns of eight cucumber ACS family genes (CsACSs) in a variety of tissues and sex types, as well as in response to exogenous ethylene application, and their ACS activities. Tissue-specific expression profiling in monoecious and gynoecious cucumber plants revealed that some CsACSs were differentially expressed. In particular, the five genes CsACS1, CsACS1-2, CsACS2, CsACS6, and CsACS11 were highly expressed in the shoot apex regions of gynoecious and hermaphroditic cucumber plants. The expression of most CsACSs was also induced by exogenous ethylene application. Furthermore, three CsACS isoforms (CsACS9, CsACS10, and CsACS12) showed no ACS enzymatic activity, which was associated with the amino acid variations in the conserved active residues of CsACS proteins. However, an in vitro pull-down assay revealed that two enzymatically inactive isoforms (CsACS9 and CsACS10) did not significantly interact with four active isoforms (CsACS1, CsACS1-2, CsACS2, and CsACS6). Taken together, our findings will be valuable for elucidating the relationship between RNA expression, ACS activity, protein–protein interactions between CsACSs and cucumber sex types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The plant hormone ethylene is an important regulator of plant growth and development that plays essential roles in many aspects of the plant life cycle including seed germination, root hair development, root nodulation, flower senescence, abscission, and fruit ripening (Ecker 1995; Johnson and Ecker 1998). In addition, ethylene is also recognized as the primary hormone affecting sex expression in cucumber (Cucumis sativus L.) (Malepszy and Niemirowicz-Szczytt 1991). Physiological studies have shown that a significant correlation exists between ethylene and sex expression in cucumber (Atsmon and Tabbak 1979; Takahashi and Jaffe 1984). The application of ethylene or ethylene-releasing agents promotes femaleness, whereas treatment with agents that inhibit ethylene biosynthesis or action suppresses the development of female organs (Augustine et al. 1973; Yin and Quinn 1995). Consistent with female flower development in response to exogenous ethylene treatment, the shoot apex regions of gynoecious cucumber seedlings endogenously produce 2–3 times more ethylene than the shoot apex regions in monoecious and andromonoecious seedlings (Rudich et al. 1972; Trebitsh et al. 1987; Yamasaki et al. 2001).
Biochemical studies have shown that ethylene is synthesized via a two-step process from S-adenosyl methionine (S-AdoMet, SAM), which is converted to 1-aminocyclopropane-1-carboxylate (ACC) by ACC synthase (ACS) and then oxidized to ethylene by ACC oxidase (ACO) (Yang and Hoffman 1984). The levels of RNA expression of the multigene families encoding ACS and ACO are regulated by a complicated network of developmental and environmental cues (Johnson and Ecker 1998). In addition, nonfunctional ACS isoforms via heterodimeric interactions among ACS proteins may modulate ACS enzymatic activity by a post-translational regulatory mechanism (Tsuchisaka and Theologis 2004). In addition to the control of ACS family gene at a variety of steps, ethylene production is regulated at various metabolic steps, including the synthesis, oxidation, and sequestration of ACC (Wang et al. 2002). Furthermore, recent reports have shown that the pool of available ACC can also affect the rate of ethylene synthesis (Van de Poel and Van Der Straeten 2014).
Recent molecular genetic analyses have demonstrated that the three major genetic loci, a dominant Female (F) locus and the recessive Monoecious (M) and Androecious (A) loci, are involved in the sex determination of cucumber flowers (Kubicki 1969; Trebitsh et al. 1997; Yamasaki et al. 2001). Moreover, genes at two of the loci encode ACSs; the F gene (CsACS1G) results from CsACS1 duplication and controls the development of female flowers (Trebitsh et al. 1997; Mibus and Tatlioglu 2004; Knopf and Trebitsh 2006; Zhang et al. 2015), whereas the M gene (CsACS2) represses stamen development (Boualem et al. 2009). In addition, the A gene (CsACS11) loss-of-function has been reported to result in female to male sexual conversion (androecy) (Boualem et al. 2015), while CsACS1 and CsACS2 are differentially expressed at the initiation of transition to the female phase or upon applying exogenous ethylene (Kamachi et al. 2000; Yamasaki et al. 2001, 2003a).
Among the three major CsACSs (CsACS1, CsACS2, and CsACS11) that affect flower sex type in cucumber, CsACS2 expression patterns and its role in flower development are not correlated. Specifically, although the expression of inactive CsACS2 affects only stamen development and not carpel formation (Boualem et al. 2014), CsACS2 transcripts are mainly restricted to carpel primordia in flowers fated to develop as female and are not observed in male flower primordia (Yamasaki et al. 2003b; Saito et al. 2007). This pattern implies that other CsACS genes also function in cucumber flower development but at different stages or in different tissues. However, even though the cucumber genome contains eight CsACSs (Huang et al. 2009), their role in cucumber sex determination has only been reported for three.
Here, we used in silico analysis to identify the chromosomal locations, gene structures, phylogenetic relationships, and conserved motifs of eight CsACS genes (CsACSs). In addition, we analyzed the spatial expression profiles of these eight CsACSs in different cucumber sex types and the response of CsACSs expression to exogenous ethylene treatment. Furthermore, we examined the enzymatic activities of CsACSs and the protein–protein interactions among some CsACSs in vitro.
2 Materials and methods
2.1 Database searching and sequence analysis
ACS sequence data (Table 1) were collected from cucumber, specifically the Chinese long inbred line, 9930 (ICuGI, http://www.icugi.org/cgi-bin/ICuGI/index.cgi) and the North American inbred line, Gy14 (Phytozome 11, http://www.phytozome.net/cucumber.php#A), and the Arabidopsis (TAIR, https://www.arabidopsis.org) genome databases (Arabidopsis Genome 2000; Huang et al. 2009).
The chromosomal locations of each CsACS were determined using BLASTP to search against the cucumber genome database with default parameters. To determine the molecular weights (MWs) and isoelectric points (pIs) of the CsACS proteins, predicted amino acid sequences of the eight CsACSs were estimated using ExPASy (http://www.cn.expasy.org/tools). Transient signal peptides and their subcellular localization were predicted using TargetP 1.1 (http://www.cbs.dtu.dk/services/TargetP/) (Emanuelsson et al. 2007) and ChloroP 1.1 servers (http://www.cbs.dtu.dk/services/ChloroP/) (Emanuelsson et al. 1999, 2000). To determine the exon–intron gene structures, we compared the predicted coding sequence of each CsACS and AtACS gene with its corresponding genomic sequence derived from the ICuGI and TAIR databases using the gene structure display server (GSDS, http://gsds1.cbi.pku.edu.cn/). Multiple sequence alignments and phylogenetic analysis of eight full-length CsACS and eleven AtACS amino acid sequences were performed using ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/) with default parameters. Because AtACS3 is a pseudogene that resulted from the partial duplication of AtACS1 (Liang et al. 1995; Yamagami et al. 2003), we did not include it in this analysis.
To identify putative cis-acting regulatory elements in CsACS promoter regions, we retrieved ~ 2000-bp nucleotide sequences upstream of the translational start codons (ATG) from the ICuGI database and performed in silico promoter analysis using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
2.2 Plant materials and growing conditions
The monoecious (C. sativus L. ‘WJE F11’), gynoecious (C. sativus L. ‘WNE F8’), and hermaphrodite (C. sativus L. ‘319H’) cucumber plants used in the present study have been described (Boualem et al. 2009; Win et al. 2015). Hermaphrodite seeds were kindly provided by Dr. Rafael Perl-Treves (Bar-Ilan University, Israel), while monoecious and gynoecious seed suppliers were previously described (Win et al. 2015). Plants were cultivated in a growth chamber at 23 °C under long-day (LD) conditions (16 h:8 h light:dark photoperiod) with a light intensity of 120 μmol m−2 s−1.
2.3 Treatment of plant samples for RNA analysis
To examine the expression of the eight CsACSs in the different cucumber cultivars, we collected ~ 5-mm shoot apices from two- or three-leaf-stage seedlings, which is a critical period for cucumber sex determination (Bai et al. 2004). We also collected a variety of tissues (cotyledons, leaves, stems, roots, tendrils, shoot apices, and floral buds) from four- or five-leaf-stage seedlings. To analyze the effect of exogenous ethylene application on the expression of the eight CsACSs, we applied 1.0 mM ethephon (C0143, Sigma, St. Louis, MO, USA) with 0.1% (v/v) Tween-20 to monoecious cucumber plants at the four- or five-leaf stage as previously described (Bai and Xu 2012). Only monoecious plants were examined because the shoot apices of gynoecious cucumber plants had naturally high ethylene production (Yamasaki et al. 2001). The shoot apices were then harvested at 0.5, 1, 3, and 6 h after ethephon application, immediately frozen in liquid nitrogen, and stored at − 80 °C until used for RNA isolation. Five seedlings (each with two biological replicates) were collected per time point.
2.4 RNA expression analysis
For real time-quantitative polymerase chain reaction (RT-qPCR) analysis, total RNAs were extracted from the tissues of hormone-treated plants using the RiboEx Total RNA kit (GeneAll, Seoul, Korea). RNA quality was determined using a Nanodrop ND-2000 spectrophotometer (Nanodrop Technologies, Waltham, MA, USA) and only high-quality RNA samples (A260/A230 > 2.0 and A260/A280 > 1.8) were used for subsequent experiments.
Complementary DNA (cDNA) was synthesized from 5 µg RNA following the protocol for the ReverTra Ace qPCR RT Master Mix kit (Toyobo, Osaka, Japan), and RT-qPCR analysis was conducted in 96-well plates using the THUNDERBIRD SYBR qPCR mix (Toyobo, Osaka, Japan) in a CFX real-time system (Bio-Rad, Hercules, CA, USA). In accordance with established RT-qPCR best practices (Hong et al. 2010; Warzybok and Migocka 2013), CsCACS was included as a stably expressed reference gene and three technical duplicates of the two biological replicates from independently harvested samples were performed. Oligonucleotide sequences used for expression analysis are provided in Table S1. To determine the relative abundance of the transcripts, data were analyzed using the Bio-Rad CFX Manager software (Bio-Rad), and expression values were presented as a heat map or a bar graph.
2.5 Recombinant CsACSs ACS activity in vitro assay
To produce full-length CsACS proteins tagged with histidine (His) in Escherichia coli, these amplified coding sequences were cloned into the pET28a vector (GE Healthcare, Chicago, USA). The resulting recombinant plasmid was sequenced to verify the absence of PCR errors during amplification. Recombinant CsACS proteins from E. coli were purified as previously described (Hwang et al. 2016). For the cloning procedure, products (Exprep Plasmid SV, Expin Combo GP, and Pfu DNA polymerase) provided by GeneAll (Korea) were used. Oligonucleotide sequences used for cloning are listed in Table S1.
To determine CsACSs activities, we measured 5′-methylthioadenosine (MTA) formation using high-performance liquid chromatography (HPLC). We incubated 60 μM S-adenosyl methionine (SAM) in 100 mM potassium phosphate buffer, pH 8.5 in the presence of 100 μM PLP for 20 min at 25 °C after the addition of the purified enzyme with or without heat treatment. The MTA produced by ACS was monitored at 260 nm.
2.6 In vitro pull-down assay
To produce full-length CsACS proteins (CsACS1, CsACS1-2, CsACS2, and CsACS6) in E. coli, amplified CsACS cDNAs were cloned into the pGEX-4T-3 vector (GE Healthcare) and recombinant proteins were purified as previously described (Hwang et al. 2016). Oligonucleotide sequences used for cloning are listed in Table S1.
For the in vitro pull-down assay, glutathione S-transferase (GST) fusion recombinant proteins were mixed with CsACS9-His or CsACS10-His proteins and the mixtures were gently rotated for 2 h at 4 °C. Subsequently, they were washed three times with the washing buffer and eluted with 10 mM reduced glutathione in 100 mM NaCl and 20 mM Tris–HCl (pH 7.2). Finally, the eluted protein samples were analyzed by 12% SDS-PAGE and visualized by western blot analysis. The detailed procedure has been previously reported (Jang et al. 2009).
3 Results
3.1 Identification of ACS genes in cucumber
To identify ACS-encoding genes in cucumber, we searched the 9930 ICuGI (http://www.icugi.org/cgi-bin/ICuGI/index.cgi) and Gy14 Phytozome 11 (http://www.phytozome.net/cucumber.php#A) genome databases. The present study confirmed that eight CsACSs are present in the cucumber genome (Table 1). Because there are 12 ACS genes in Arabidopsis, eight in papaya, 11 in poplar, nine in grapevine, and six in rice, no significant expansion or reduction in ACS gene family members occurred in the cucumber genome (Huang et al. 2009). Based on sequence similarity to Arabidopsis homologs, we designated the five unnamed CsACSs as CsACS1-2 (Csa6M006800), CsACS6 (Csa4M049610), CsACS9 (Csa4M099220), CsACS10 (Csa5M157380), and CsACS12 (Csa3M177920). The range of protein identity between the cucumber and Arabidopsis ACS proteins varied from 57.4% (AtACS10/CsACS10) to 75.3% (AtACS9/CsACS1), and we failed to identify cucumber homologs for AtACS2, AtACS4, AtACS5, and AtACS8 in cucumber.
The CsACSs were mapped to all cucumber chromosomes except chromosome seven (Fig. S1). CsACS2, CsACS11, CsACS12, and CsACS10 were mapped to chromosomes 1, 2, 3, and 5, respectively, whereas CsACS6/CsACS9 and CsACS1/CsACS1-2 were mapped to chromosomes 4 and 6, respectively.
The length of protein sequences encoded by the CsACSs varied from 440 (CsACS11) to 548 amino acids (CsACS10). Meanwhile, the predicted molecular weight (MW) of the CsACSs ranged from 49.72 to 60.37 kDa, and the theoretical isoelectric point (pI) ranged from 5.62 to 8.54 (Table S2). Furthermore, the predicted CsACS subcellular localization using in silico analysis revealed that seven of the eight CsACSs lacked signal peptides (Table S2), suggesting that they are primarily cytoplasmic proteins. This results is consistent with previous findings that apple ACS proteins are cytoplasmic proteins (Yip et al. 1991) and that AtACS7 is localized in the cytoplasm (Huang et al. 2013). However, CsACS12 contained a mitochondrial target peptide in its N-terminus and was therefore, predicted to localize to the mitochondria (Table S2 and Fig. S2). A similar signal peptide sequence was also reported in the N-terminus of AtACS12 (Fig. S2).
3.2 Structure of ACS genes in cucumber
We constructed a phylogenetic tree and performed a gene structure comparison between eight CsACS and 11 AtACS family members. Most CsACSs and AtACSs contained four exons and three introns. CsACS1 and CsACS2 and the corresponding AtACS5 and AtACS7 had three exons (Fig. 1). Interestingly, CsACS1-2 and CsACS6 had five exons, while their homologs in Arabidopsis did not. Detailed analysis of each exon and intron (Table S3) revealed that in CsACSs with, the fourth exon was the longest, followed by the second. Similarly, in CsACSs the third was the longest. In addition, the full-length coding sequences of CsACSs and AtACSs were similar (~ 1400 bp), as were the number and length of exons and introns.
Phylogenetic relationships and structures of cucumber ACS genes. Exons and introns are represented by black boxes and lines, respectively. Asterisks indicate that this gene was previously described shown in Table 1. Detailed sequence information for each exon and intron is provided in Table S2
3.3 Predicted amino acid sequences of ACS genes in cucumber
Because crystallographic analyses and site-directed or random mutagenesis approaches have revealed that some amino acid residues of ACS proteins are essential for enzyme activity, we compared the sequences of CsACS proteins using ClustalW (Tarun et al. 1998; Capitani et al. 1999; Huai et al. 2001). Most CsACS proteins exhibited conserved sequence identity at the ACS active site. However, the sequences of both CsACS10 and CsACS12 included clear amino acid substitutions in the conserved active site and in the four invariant residues of aminotransferases; i.e., Y86S in the CsACS1 protein; D231 N, Y234F, K273E, G283A, and R407G in CsACS10; Y86F and Y234F in CsACS12 (Fig. 2). Furthermore, the CsACS10 and CsACS12 sequences also included changes in the conserved amino acid motifs in boxes 2, 4, 5, 6, and 7 (Fig. 2). These results suggest that the amino acid substitutions in the conserved active residues of CsACS10 and CsACS12 affect ACS activity.
Amino acid sequence alignment of the cucumber ACS proteins. The seven conserved domains in ACS proteins are underlined. Open circles represent amino acid residues that are conserved in at least 75% of all examined ACS proteins (Tarun et al. 1998). Filled circles and squares denote conserved amino acid residues between ACS proteins and subgroup 1 aminotransferases, and the four invariant residues in all aminotransferases. Asterisks and double dots indicate identical and similar amino acid residues, respectively
3.4 Expression of ACS genes in different cucumber sex types
We used RT-qPCR analysis to investigate the spatial expression profiles of CsACSs in a variety of tissues from monoecious and gynoecious cucumber plants. Heat maps indicated that the spatial expression patterns of the eight CsACSs in the two sex types differed slightly (Fig. 3). Based on hierarchical clustering in the monoecious plants, CsACSs were divided into four groups (Fig. 3a). CsACS1 and CsACS6 were strongly expressed in tendrils, but CsACS1 transcripts were not detected in roots. CsACS1-2, CsACS2, and CsACS9 were strongly expressed in roots; CsACS10 and CsACS12 were abundantly expressed in leaves, cotyledons, and tendrils. CsACS11 was highly expressed in shoot apices, stems, and floral buds, but had no expression in roots.
Spatial expression profiles of cucumber ACS genes in cucumber plants with two different flower types. The expression levels of eight cucumber ACS genes in a monoecious and b gynoecious plants were measured with RT-qPCR. We detected both CsACS1 and CsACS1G transcripts in gynoecious cucumber plants using another copy of CsACS1 (CsACS1G). The columns indicate cotyledons (Co), leaves (L), stems (S), roots (R), tendrils (Td), shoot apices (SA), and floral buds (FB). Note that the shoot apices at the four- or five-leaf stage have flower buds that are approximately 15 mm in length. c Expression patterns of eight CsACSs in the shoot apex regions of cucumber. Note that the shoot apex regions at the two- or three-leaf-stage have flower buds approximately 5 mm long. Expression patterns are presented as a heat map and genes sharing similar expression patterns were clustered. The color scale is shown at the right in a: red and green indicate higher and lower expression for each gene, respectively. Mo, Gy, and He represent monoecious, gynoecious, and hermaphroditic cucumber plants, respectively. Two independent biological replicates provided similar results; only one biological replicate is presented. (Color figure online)
Among gynoecious plants, CsACSs were clustered into three groups (Fig. 3B). CsACS2 and CsACS11 were strongly expressed in shoot apices, but no CsACS11 transcripts were detected in cotyledons. CsACS1, CsACS10, and CsACS12 were abundantly expressed in all samples, with especially high expression in tendrils. Lastly, CsACS1-2, CsACS6, and CsACS9 were highly expressed in roots, as well as in cotyledons, tendrils, shoot apices, and floral buds.
Although some CsACSs in both monoecious and gynoecious cucumbers exhibited similar tissue-specific expression (i.e., CsACS1 in tendrils, CsACS11 in shoot apices, and both CsACS1-2 and CsACS9 in roots; Fig. 3), others exhibited sex type-specific expression. Interestingly, the relatively strong expression of some CsACSs (CsACS1-2, CsACS11, and CsACS12) was observed in shoot apices and floral buds of gynoecious cucumber, suggesting that the differential expression patterns were associated with female flower development. Therefore, tissue-specific expression of the eight CsACSs occurred in both sex types, while CsACSs uniquely expressed in the gynoecious cultivar are probably linked to female organ development.
3.5 Expression of ACS genes in shoot apex regions of monoecious, gynoecious, and hermaphrodite cucumbers
To investigate CsACS expression patterns in shoots during the very early stages of flower development, we harvested ~ 5-mm shoot apices with flower buds from cucumber seedlings and performed RT-qPCR. To validate the monoecious, gynoecious, and hermaphrodite sample preparation, we confirmed the preferential expression of CsWIP1 in the shoot apices of both gynoecious and hermaphrodite cucumber plants (Fig. S3) as previously reported (Boualem et al. 2015). According to their expression patterns, the eight CsACSs separated into two groups (Fig. 3c). The first group included CsACS1, CsACS1-2, CsACS2, CsACS6, and CsACS11, all of which were highly expressed in the shoot apices of hermaphrodites only, or in both gynoecious and hermaphrodite plants, suggesting that they function in early stages of cucumber flower development. The increased expression of CsACS1 and CsACS2 in the shoot apices of gynoecious cucumber was consistent with previous findings (Knopf and Trebitsh 2006; Li et al. 2012; Boualem et al. 2015).
The second group included CsACS9, CsACS10, and CsACS12, which were highly expressed in the hermaphrodite cucumber plants (Fig. 3c). In addition, CsACS10 and CsACS12 expression patterns differed across age groups (i.e., two- or three-leaf stage seedlings versus four- or five-leaf stage seedlings). This outcome suggests that CsACS10 and CsACS12 function later in flower development of gynoecious cucumber plants. However, overall results indicate that more CsACSs are preferentially expressed in shoot apex regions of gynoecious or hermaphrodite cucumber plants during flower development.
3.6 Effect of ethylene treatment on ACS gene expression in cucumber
Because exogenous ethylene application has successfully induced CsACS2 expression in cucumber plants (Yamasaki et al. 2000, 2001; Li et al. 2012), we checked CsACS expression in ethylene-treated monoecious lines to examine whether ethylene treatment would have similar effects on other CsACSs expression. RT-qPCR experiments revealed that CsACS transcription in shoot apices peaked dramatically at either 0.5 or 1 h after ethylene treatment (except for CsACS9), followed by a rapid decline, even though the genes all differed in fold-change in transcriptional induction and reduction (Fig. 4). Like other ethylene-inducible genes, CsACS1-2, CsACS2, and CsACS10 contain ethylene-responsive elements in their promoter regions (Table S4), whereas the remaining CsACSs did not. This difference in sequence structure suggests that the inducible expression of CsACSs by ethylene is associated with the occurrence of ethylene-inducible regulatory elements in their promoter sequences.
Ethylene-induced expression patterns of cucumber ACS genes in shoot apex regions of cucumber Monoecious cucumber plants at the four- or five-leaf stages were used in RT-qPCR analysis. Expression levels of zero in each cucumber ACS gene per tissue were set to one. The color scale is shown at the right. The CsCACS gene was used as an internal control
3.7 In vitro enzymatic activities of recombinant CsACS proteins
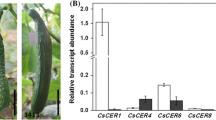
To determine whether the significant changes in the conserved amino acid residues of some CsACS proteins affect their enzymatic activities, we expressed the CsACS proteins as histidine (His) fusion proteins in E. coli and then purified the proteins to assess ACS enzymatic activities in vitro by monitoring 5′-methylthioadenosine (MTA) formation (Fig. 5a). Our enzymatic assays showed that CsACS1-2 and CsACS6 were active, whereas CsACS9, CsACS10, and CsACS12 were not (Fig. 5b). Also, CsACS2 was used as a positive control and had in vitro ACS activity, which was consistent with previous findings (Boualem et al. 2009). Furthermore, the clear differences in the ACS enzyme activities among the three active CsACS proteins were not observed (Fig. 5c). However, denatured CsACS1-2, CsACS2, and CsACS6 were used as negative controls and did not convert S-AdoMet into MTA (Fig. S4). This result indicates that a subset of CsACS proteins have ACS activities in vitro. Taken together with the comparison of deduced amino acid sequences among eight CsACSs (Fig. 2), these results suggest that the amino acid conservation in invariant residues and the conserved motifs of CsACS proteins is important for their ACS activities in vitro.
The enzymatic activities of cucumber ACS proteins in vitro. a High-performance liquid chromatography (HPLC) chromatogram showing the standard peaks of S-adenosyl methionine (S-AdoMet) and 5’-methylthioadenosine (MTA). b HPLC chromatogram showing the peaks of S-AdoMet and MTA in the reaction. Open and filled arrows indicate S-AdoMet and MTA, respectively. c Enzymatic activities of four CsACS proteins. The MTA produced in the reaction are presented. Error bars denote standard error of the mean (SEM) of three biological replicates
3.8 In vitro protein–protein interactions among recombinant CsACS proteins
Eight enzymatically active and one inactive AtACS homodimers interact together to form functional heterodimers (Tsuchisaka and Theologis 2004). In addition, similar spatial expression patterns of CsACSs with or without in vitro ACS activities in some tissues of monoecious and gynoecious cucumbers (Figs. 3, 5) raise the possibility for protein–protein interactions between active and inactive CsACS subunits. For the in vitro pull-down assay, full-length coding sequences of CsACSs were fused with GST or His proteins, and the GST-CsACS1/CsACS1-2/CsACS2/CsACS6 proteins were incubated with the His-CsACS9/CsACS10 proteins purified from E. coli. The interaction signals between the CsACS1-2, CsACS2,CsACS6 proteins and CsACS9 were much weaker than those in the input lane, whereas an interaction between CsACS1 and CsACS9 proteins was not observed (Fig. 6a). Furthermore, the interaction signals of these four proteins with CsACS10 were not detected (Fig. 6b). These data indicate that two enzymatically inactive CsACS proteins do not interact physically with four enzymatically active CsACS proteins to form heterodimers.
Protein–protein interactions between CsACS9 or CsACS10 and four CsACS proteins in vitro GST or GST-tagged CsACS9/10 proteins were incubated with His-tagged CsACS1/1-2/2/6 protein. The eluates were separated in a 12.5% SDS-PAGE gel, transferred to PVDF membranes, and probed with an anti-His antibody. Approximately 10% of the total reaction sample was loaded as an input control. Ponceaus S-stained bands indicated by arrows show the amount and quality of the GST fusion proteins used in this assay
4 Discussion
Ethylene plays an important role in various developmental processes and adaptive stress responses in plants. In addition, the transcriptional regulation of ACSs are involved in converting S-AdoMet to ACC during ethylene biosynthesis, and has been thoroughly studied in a variety of plant species, including Arabidopsis and tomato (Wang et al. 2002). In the present study, we determined the spatial expression profiles of CsACSs among several cucumber sex types and demonstrated that exogenous ethylene application upregulated CsACSs expression. Furthermore, we showed in vitro ACS activity in CsACS proteins.
Several mutagenesis analyses of aminotransferases have reported that mutations of conserved the residues Y86, D231, Y234, K273, and R407 result in the complete or partial loss of ACS enzyme activity (White et al. 1994; Li et al. 1997; Tarun et al. 1998). In addition, crystallographic analyses have shown that amino acid residues in boxes 4 and 7 affect binding of the pyridoxal 5′-phosphate cofactor and the enzyme–substrate S-AdoMet, respectively (Capitani et al. 1999; Huai et al. 2001). In this study, we found that CsACS10 and CsACS12 contained significant changes in the amino acids of conserved residues and domains from both ACS proteins and aminotransferases (Fig. 2). This result suggests that the amino acid substitutions observed in CsACS10 and CsACS12 could affect their enzyme activities. This hypothesis is supported by the observation that missense mutations in conserved cucumber and melon ACS amino acid residues lead to a loss or reduction in ACS activity (Boualem et al. 2008, 2009, 2015). However, we cannot dismiss the possibility that changes in other regions, such as the SAM binding region, could also affect ACS activity because the G33C mutation in CsACS2 resulted in sexual transition (Boualem et al. 2014).
One intriguing observation was that CsACS9 showed no ACS activity in vitro (Fig. 5), although CsACS9 had no significant changes of conserved residues or motifs in ACS proteins (Fig. 2). In this study, we showed that CsACS9 was highly expressed in roots of both monoecious and gynoecious cucumbers (Fig. 3). In addition, some reports have revealed that AtACSs expression is significantly induced by a variety of stimuli (Wang et al. 2002). Based on these results, CsACS9 may be involved in other functions but not female organ development. However, we cannot exclude the possibility that CsACS9 encodes aminotransferase activity, as seen in AtACS10 and AtACS12 proteins (Yamagami et al. 2003).
One very interesting observation was that CsACS proteins had enzymatically active or inactive forms (Fig. 5), suggesting that a subset of inactive CsACS isoforms may function as regulators of ACS activity through heterodimerization with other active CsACS enzymes at the post-translational level. This model is supported by the observation that CsACS10 and CsACS12 without in vitro ACS activity were highly expressed in shoot apices or floral buds of gynoecious cucumbers (Figs. 3, 5), and CsACS12 expression was dramatically induced by ethylene (Fig. 4). Considering that AtACS1 or AtACS3 did not show ACS activity in either bacteria or yeast (Liang et al. 1995) and AtACS proteins physically interact with other AtACS proteins to form functional or nonfunctional heterodimers (Tsuchisaka and Theologis 2004), it is likely that CsACS10 and CsACS12 may function as regulators of ACS activities through the dimerization with other CsACS enzymes at the post-translational level. However, our in vitro pull-down assay did not show a physical interaction between CsACS10 and four enzymatically active proteins (Fig. 6). This result suggests that heterodimerization between functional and nonfunctional CsACS forms may not be a regulatory mechanism in cucumber; however, we cannot dismiss the possibility that in vitro purified CsACS proteins do not mimic in vivo proteins.
Male and female flower buds can be distinguished morphologically, and carpel or stamen primordia are selectively arrested at stages 6 and 7 of cucumber flower development (Bai et al. 2004). CsACS2 is expressed in all flower buds of gynoecious cucumber at different developmental stages (Saito et al. 2007), whereas CsACS11 is only expressed in female flower buds of monoecious cucumber at stages 4 and 8 (Boualem et al. 2015). These results indicate that CsACSs are specifically expressed in female flower buds before and after determination of flower sex types. In the present study, we demonstrated that most CsACS gene expression in the shoot apices of gynoecious and hermaphrodite cucumber was higher at either early (~ stage 8) or late (~ stage 12) stages of floral development (Fig. 3). These results suggest that some CsACSs have important roles in the sexual organ development of flowers; however, the direct link between CsACS expression and sex determination remains unclear. Therefore, in situ hybridization should be used to further investigate the dynamics of CsACS expression in specific developmental stages.
Ethylene has been reported as the primary hormone regulating sex type in typical monoecious cucumber with unisexual flowers (Malepszy and Niemirowicz-Szczytt 1991). Other studies have demonstrated that CsACS2 expression is restricted to the carpel primordia of flowers fated to develop as female (Yamasaki et al. 2003b; Saito et al. 2007) and that ethylene affects CsACS2 expression (Li et al. 2012). Li et al. (2012) proposed that CsACS1G expression is first activated and triggers ethylene production, which subsequently induces CsACS2 expression via positive feedback. Eventually, this mechanism arrests stamen development, thereby leading to the production of female flowers. This is further supported by our findings that ethylene treatment caused a dramatic increase in most CsACS gene expression (Fig. 4), as well as previous studies demonstrating the presence of ethylene-responsive elements in the promoter regions of some CsACSs (Table S4; Huang et al. 2009). These results suggest that a subset of CsACSs may influence cucumber flower development via positive feedback regulation.
In addition to ethylene biosynthesis genes, ethylene signaling genes involved in perception and signal transduction also function in cucumber flower development. Evidence of this involvement includes the application of ethylene-action inhibitors, such as AgNO3, which increases the number of male and bisexual flowers in gynoecious cucumber plants (Yamasaki et al. 2001). Moreover, the expression patterns of ethylene receptor genes like CsETR1 and CsETR2 can overlap with CsACS2 in cucumber flower buds of specific sex types (Yamasaki et al. 2003a). Lastly, overexpression of a deficient ETR1 allele in the stamens of melon inhibits carpel formation (Little et al. 2007). These findings suggest that ethylene perception in male or female organs is important to cucumber flower development. Thus, further investigation of the relationship between the expression profiles of ethylene biosynthesis-related genes and ethylene signaling components would provide a better understanding of sex determination in cucumber plants.
References
Arabidopsis Genome I (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796–815
Atsmon D, Tabbak C (1979) Comparative effects of gibberellin, silver nitrate and aminoethoxyvinyl glycine on sexual tendency and ethylene evolution in cucumber plant. Plant Cell Physiol 20:1547–1555
Augustine JJ, Baker LR, Sell HM (1973) Female flower induction on androecious cucumber, Cucumis sativus L. J Am Soc Hortic Sci 98:197–199
Bai SN, Xu ZH (2012) Bird-nest puzzle: can the study of unisexual flowers such as cucumber solve the problem of plant sex determination? Protoplasma 249(Suppl 2):S119–S123
Bai SL, Peng YB, Cui JX, Gu HT, Xu LY, Li YQ, Xu ZH, Bai SN (2004) Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta 220:230–240
Boualem A, Fergany M, Fernandez R, Troadec C, Martin A, Morin H, Sari MA, Collin F, Flowers JM et al (2008) A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science 321:836–838
Boualem A, Troadec C, Kovalski I, Sari MA, Perl-Treves R, Bendahmane A (2009) A conserved ethylene biosynthesis enzyme leads to andromonoecy in two cucumis species. PLoS ONE 4:e6144
Boualem A, Fleurier S, Troadec C, Audigier P, Kumar AP, Chatterjee M, Alsadon AA, Sadder MT, Wahb-Allah MA et al (2014) Development of a Cucumis sativus TILLinG platform for forward and reverse genetics. PLoS ONE 9:e97963
Boualem A, Troadec C, Camps C, Lemhemdi A, Morin H, Sari MA, Fraenkel-Zagouri R, Kovalski I, Dogimont C et al (2015) A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 350:688–691
Capitani G, Hohenester E, Feng L, Storici P, Kirsch JF, Jansonius JN (1999) Structure of 1-aminocyclopropane-1-carboxylate synthase, a key enzyme in the biosynthesis of the plant hormone ethylene. J Mol Biol 294:745–756
Ecker JR (1995) The ethylene signal transduction pathway in plants. Science 268:667–675
Emanuelsson O, Nielsen H, von Heijne G (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8:978–984
Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300:1005–1016
Emanuelsson O, Brunak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2:953–971
Hong SM, Bahn SC, Lyu A, Jung HS, Ahn JH (2010) Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant Cell Physiol 51:1694–1706
Huai Q, Xia Y, Chen Y, Callahan B, Li N, Ke H (2001) Crystal structures of 1-aminocyclopropane-1-carboxylate (ACC) synthase in complex with aminoethoxyvinylglycine and pyridoxal-5′-phosphate provide new insight into catalytic mechanisms. J Biol Chem 276:38210–38216
Huang S, Li R, Zhang Z, Li L, Gu X, Fan W, Lucas WJ, Wang X, Xie B et al (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41:1275–1281
Huang SJ, Chang CL, Wang PH, Tsai MC, Hsu PH, Chang IF (2013) A type III ACC synthase, ACS7, is involved in root gravitropism in Arabidopsis thaliana. J Exp Bot 64:4343–4360
Hwang YH, Kim SK, Lee KC, Chung YS, Lee JH, Kim JK (2016) Functional conservation of rice OsNF-YB/YC and Arabidopsis AtNF-YB/YC proteins in the regulation of flowering time. Plant Cell Rep 35:857–865
Jang YH, Park HY, Kim SK, Lee JH, Suh MC, Chung YS, Paek KH, Kim JK (2009) Survey of rice proteins interacting with OsFCA and OsFY proteins which are homologous to the Arabidopsis flowering time proteins, FCA and FY. Plant Cell Physiol 50:1479–1492
Johnson PR, Ecker JR (1998) The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet 32:227–254
Kamachi S, Mizusawa H, Matsura S, Sakai S (2000) Expression of two 1-aminocyclopropane-1-carboxylate synthase genes, CS-ACS1 and CS-ACS2, correlated with sex phenotypes in cucumber plants (Cucumis sativus L.). Plant Biotechnol 17:69–74
Knopf RR, Trebitsh T (2006) The female-specific Cs-ACS1G gene of cucumber. A case of gene duplication and recombination between the non-sex-specific 1-aminocyclopropane-1-carboxylate synthase gene and a branched-chain amino acid transaminase gene. Plant Cell Physiol 47:1217–1228
Kubicki B (1969) Investigation of sex determination in cucumber (Cucumis sativus L.). Genet Pol 10:101–121
Li Y, Feng L, Kirsch JF (1997) Kinetic and spectroscopic investigations of wild-type and mutant forms of apple 1-aminocyclopropane-1-carboxylate synthase. Biochemistry 36:15477–15488
Li Z, Wang S, Tao Q, Pan J, Si L, Gong Z, Cai R (2012) A putative positive feedback regulation mechanism in CsACS2 expression suggests a modified model for sex determination in cucumber (Cucumis sativus L.). J Exp Bot 63:4475–4484
Liang X, Oono Y, Shen NF, Kohler C, Li K, Scolnik PA, Theologis A (1995) Characterization of two members (ACS1 and ACS3) of the 1-aminocyclopropane-1-carboxylate synthase gene family of Arabidopsis thaliana. Gene 167:17–24
Little HA, Papadopoulou E, Hammar SA, Grumet R (2007) The influence of ethylene perception on sex expression in melon (Cucumis melo L.) as assessed by expression of the mutant ethylene receptor, At-etr1-1, under the control of constitutive and floral targeted promoters. Sex Plant Reprod 20:123–136
Malepszy S, Niemirowicz-Szczytt K (1991) Sex determination in cucumber (Cucumis sativus) as a model system for molecular biology. Plant Sci 80:39–47
Mathooko FM, Mwaniki MW, Nakatsuka A, Shiomi S, Kubo Y, Inaba A, Nakamura R (1999) Expression characteristics of CS-ACS1, CS-ACS2 and CS-ACS3, three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in cucumber (Cucumis sativus L.) fruit under carbon dioxide stress. Plant Cell Physiol 40:164–172
Mibus H, Tatlioglu T (2004) Molecular characterization and isolation of the F/f gene for femaleness in cucumber (Cucumis sativus L.). Theor Appl Genet 109:1669–1676
Rudich J, Halevy AH, Kedar N (1972) Ethylene evolution from cucumber plants as related to sex expression. Plant Physiol 49:998–999
Saito S, Fujii N, Miyazawa Y, Yamasaki S, Matsuura S, Mizusawa H, Fujita Y, Takahashi H (2007) Correlation between development of female flower buds and expression of the CS-ACS2 gene in cucumber plants. J Exp Bot 58:2897–2907
Takahashi H, Jaffe MJ (1984) Further studies of auxin and ACC induced feminization in the cucumber plant using ethylene inhibitors. Phyton 44:81–86
Tarun AS, Lee JS, Theologis A (1998) Random mutagenesis of 1-aminocyclopropane-1-carboxylate synthase: a key enzyme in ethylene biosynthesis. Proc Natl Acad Sci USA 95:9796–9801
Trebitsh T, Rudich J, Riov J (1987) Auxin, biosynthesis of ethylene and sex expression in cucumber (Cucumis sativus). Plant Growth Regul 5:105–113
Trebitsh T, Staub JE, O’Neill SD (1997) Identification of a 1-aminocyclopropane-1-carboxylic acid synthase gene linked to the female (F) locus that enhances female sex expression in cucumber. Plant Physiol 113:987–995
Tsuchisaka A, Theologis A (2004) Heterodimeric interactions among the 1-amino-cyclopropane-1-carboxylate synthase polypeptides encoded by the Arabidopsis gene family. Proc Natl Acad Sci USA 101:2275–2280
Van de Poel B, Van Der Straeten D (2014) 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: more than just the precursor of ethylene! Front Plant Sci 5:640
Wang KL, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14(Suppl):S131–S151
Warzybok A, Migocka M (2013) Reliable reference genes for normalization of gene expression in cucumber grown under different nitrogen nutrition. PLoS ONE 8:e72887
White MF, Vasquez J, Yang SF, Kirsch JF (1994) Expression of apple 1-aminocyclopropane-1-carboxylate synthase in Escherichia coli: kinetic characterization of wild-type and active-site mutant forms. Proc Natl Acad Sci USA 91:12428–12432
Win KT, Zhang C, Song K, Lee JH, Lee S (2015) Development and characterization of a co-dominant molecular marker via sequence analysis of a genomic region containing the Female (F) locus in cucumber (Cucumis sativus L.). Mol Breed 35:229
Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LE, Theologis A (2003) Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J Biol Chem 278:49102–49112
Yamasaki S, Fujii N, Takahashi H (2000) The ethylene-regulated expression of CS-ETR2 and CS-ERS genes in cucumber plants and their possible involvement with sex expression in flowers. Plant Cell Physiol 41:608–616
Yamasaki S, Fujii N, Matsuura S, Mizusawa H, Takahashi H (2001) The M locus and ethylene-controlled sex determination in andromonoecious cucumber plants. Plant Cell Physiol 42:608–619
Yamasaki S, Fujii N, Takahashi H (2003a) Characterization of ethylene effects on sex determination in cucumber plants. Sex Plant Reprod 16:103–111
Yamasaki S, Fujii N, Takahashi H (2003b) Photoperiodic regulation of CS-ACS2, CS-ACS4 and CS-ERS gene expression contributes to the femaleness of cucumber flowers through diurnal ethylene production under short-day conditions. Plant, Cell Environ 26:537–546
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher-plants. Annu Rev Plant Physiol Plant Mol Biol 35:155–189
Yin T, Quinn JA (1995) Tests of a mechanistic model of one hormone regulating both sexes in Cucumis sativus (Cucurbitaceae). Am J Bot 82:1537–1546
Yip WK, Dong JG, Yang SF (1991) Purification and characterization of 1-aminocyclopropane-1-carboxylate synthase from apple fruits. Plant Physiol 95:251–257
Zhang Z, Mao L, Chen H, Bu F, Li G, Sun J, Li S, Sun H, Jiao C et al (2015) Genome-wide mapping of structural variations reveals a copy number variant that determines reproductive morphology in cucumber. Plant Cell 27:1595–1604
Acknowledgements
We are grateful to Dr. Rafael Perl-Treves (Bar-Ilan University, Israel) for providing the cucumber seeds. The work was supported by the Bio-industry Technology Development Program (111057-5) to SL of iPET (Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry) and research funds for newly appointed professors of Chonbuk National University in 2017 to JHL.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, J.H., Kim, YC., Choi, D. et al. RNA expression, protein activity, and interactions in the ACC synthase gene family in cucumber (Cucumis sativus L.). Hortic. Environ. Biotechnol. 59, 81–91 (2018). https://doi.org/10.1007/s13580-018-0009-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-018-0009-z