Abstract
Fibronectin type III domain-containing-5 (Fndc5) is a trans-membrane protein which is involved in a variety of cellular events including neural differentiation of mouse embryonic stem cells (mESCs) as its knockdown and overexpression diminishes and facilitates this process, respectively. However, downstream targets of Fndc5 in neurogenesis are still unclear. Neurotrophins including NGF, BDNF, NT-3, and NT-4 are the primary regulators of neuronal survival, growth, differentiation, and repair. These biomolecules exert their actions through binding to two different receptor families, Trk and p75NTR. In this study, considering the fact that neurotrophins and their receptors play crucial roles in neural differentiation of ESCs, we sought to evaluate whether knockdown of Fndc5 decreased neural differentiation of mESCs by affecting the neurotrophins and their receptors expression. Results showed that at neural progenitor stage, the mRNA and protein levels of BDNF, Trk, and p75NTR receptors decreased following the Fndc5 knockdown. In mature neural cells, still, the expression of Trk and p75NTR receptors at mRNA and protein levels and BDNF and NGF expression only at protein levels showed a significant decrease in Fndc5 knockdown cells compared to control groups. Taken together, our results suggest that decreased efficiency of neural differentiation following the reduction of Fndc5 expression could be attributed to decreased levels of NGF and BDNF proteins in addition to their cognate receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibronectin type III domain-containing-5 (Fndc5), initially known as peroxisome protein (PeP), is a trans-membrane protein with 209 amino acid residues which was first identified in 2002 [1,2,3,4,5]. Fndc5 is identified as a crucial factor for neural, cardiac differentiation and a required factor for energy expenditure and lowering the insulin resistance in adipose tissue [6,7,8,9,10].
The importance of Fndc5 in the process of neural differentiation and protection has been shown in recent studies [6, 7, 11]. It is already well known that Fndc5 induces Bdnf expression in brain. Ectopic expression of Fndc5, could also induce Bdnf expression in the hippocampus [12]. However, except for BDNF, the downstream targets of Fndc5 involved in neurogenesis has not yet been determined.
Neurotrophins play critical roles in neurogenesis including growth, survival, differentiation, and repair of neurons in central and peripheral nervous systems. This family includes NGF, NT-3, NT-4, and BDNF in mammals. Brain-derived neurotrophic factor, also known as BDNF, is a member of the neurotrophin family of growth factors, which are found in the brain and the periphery tissues. Two different cell surface receptors mediate the neurotrophins functions. These receptors are the high–affinity Trk (tropomyosin-related kinase) family (TrkA, TrkB, and TrkC receptors) and the low-affinity p75 neurotrophin receptor (p75NTR), a member of tumor necrosis factor receptor family [13,14,15]. Binding of Trk family members to neurotrophins is specific, as TrkA binds NGF, TrkB binds BDNF, as well as NT-4 and TrkC have a unique ligand for NT-3 (TrkA and TrkB bind NT3 with a lower efficiency) [16]. Neurotrophins bind to Trk receptors and activates extracellular signal-regulated kinase (ERK), phosphatidyl inositol-3 (PI3)-kinase, and phospholipase C-γ1 signaling pathways which mediate cell survival and differentiation [17]. In contrast with Trk receptors, p75NTR binds all known NTs with similar affinity [16]. p75NTR facilitates cell survival through activation of Akt and NFκB pathways or result in neuronal death through activation of Jun N-terminal kinase (JNK) signaling cascade, in the absence of Trk receptors [15, 18].
The survival and development of neurons is based on the functional interaction between Trk and p75NTR signals, because these receptors are often co-expressed and can either augment or oppose each other’s actions. p75NTR can enhance Trk receptors binding to cognate neurotrophins, while Trk receptor-mediated signaling may suppress the pro-apoptotic effects of p75NTR [15, 19,20,21]. Therefore, it is not supersizing to see that abundance of neurotrophins and their receptors are well documented in diseases of the nervous system [15].
Neural cells derived from embryonic stem cells (ESCs) have therapeutic potential for the treatment of neurodegenerative disease of CNS [22]. Due to established crucial roles of neurotrophins and their receptors in neural differentiation of stem cells [23,24,25], we investigated the role of Fndc5 on their expression using a stably transduced Fndc5 knockdown cell line. In this project, we sought to evaluate whether Fndc5 knockdown affects the neurotrophins and their respective receptor expression or not.
Materials and methods
mESCs culture and neural differentiation
In this study, we used mouse ESCs (Royan B20 cells) derived from C57BL/6 strain which were obtained from Royan Institute for Stem Cells and Developmental Biology (Tehran, Iran). mESCs were cultured on 0.1% gelatin (Sigma-Aldrich, St. Louis, MO, USA) coated dishes containing serum-free medium consisted of DMEM/F12 (Invitrogen) and neurobasal (Invitrogen) at a 1:1 (v/v) ratio, 5 mg/mL bovine serum albumin (BSA; Sigma-Aldrich), 2 mM L-glutamine (Invitrogen), 0.1 mM non-essential amino acids (Invitrogen), 1% penicillin–streptomycin (v/v) (Invitrogen), 2% B27 supplement (v/v) (Invitrogen), 1% N2 supplement (v/v) (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma-Aldrich), 1000 U/mL leukemia inhibitory factor (LIF; Chemicon, Temecula, CA, USA), 1 µM PD0325901 (Sigma-Aldrich), and 10 µM SB431542 (Sigma-Aldrich), an activin receptor-like kinase inhibitor, as described previously [26] at 37 °C and 5% CO2. Medium was changed daily and cells were passaged every 2 days for at least two times before the beginning of differentiation. mESCs’ differentiation into neurons was performed according to the protocol previously described by Ostadsharif et al., using embryoid body (EB) [27]. For EBs formation, definite number of cells (~ 103) were cultured for 2 days in 20 µl hanging drops in medium-containing knock-out DMEM (Ko-DMEM, Gibco) and 15% embryonic stem cell qualified fetal bovine serum (ES-FBS, GE Healthcare Hyclone, Illinois, CHI, USA) in the absence of LIF. For neural induction, EBs were suspended for 4 days in differentiation medium composed of Ko-DMEM, 10% ES-FBS, and 1 µM RA. Medium was refreshed every 2 days. Subsequently, for differentiation of NPs to mature neurons, EBs were plated on gelatinized 12-well plates (TPP, Zurich, Switzerland) containing neurobasal medium supplemented with 5% ES-FBS, 2 mM L-glutamine, 0.1 mM non-essential amino acids, 1% penicillin/streptomycin, 0.1 mM β-mercaptoethanol, 2% B27, and 1% N2 for an additional 8 days to allow adherence and development of mature neural cells.
Fndc5 knockdown during neural differentiation
In the present study, we used Fndc5 knockdown mESC line which was prepared previously by our laboratory [9]. Nazem et al. designed two shRNA sequences targeting Fndc5 transcripts and one scramble shRNA (shCtrl) and then cloned these sequences in Doxycycline-controlled recombinant vector pLVPT-tTR-KRAB. Subsequently, human embryonic kidney cells were transfected with packaging vectors including recombinant inducible vector containing shFndc5 or shCtrl using lipofectamine in order to produce lentiviral particles. Afterward, mESCs (Royan B20) were coinfected with these viral particles expressing two shRNA or shCtrl as a scramble. Measurement of transduction efficiency, selection of stable cell lines by antibiotic, verification of the presence of shRNA sequence in genomic DNA, confirmation of the Fndc5 expression reduction, and evaluation of stemness features maintenance were performed [9]. In the current study, we treated shFndc5 cell line with 1.5 µg/mL doxycycline (Dox, Clontech) to shFndc5 expression induction and Fndc5 knockdown from day 2 to day 14 during neural progenitors (NP) and differentiated neural cells formation. Also, untransduced (RB20) and scramble cell lines were treated with the same concentration of Dox as controls.
RNA extraction and real-time quantitative PCR
Total RNA was extracted using total RNA isolation (TRI) reagent (Ambion, USA) protocol from mESCs (day 0), EB on day 6 (NPs) and mature neural cells on day 14. To eliminate residual genomic DNA, total RNA was treated with DNaseI (Thermo Fisher Scientific). cDNA synthesis was performed with 1 µg of total RNA and random hexamer primer using revert Aid first strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA). Real-time PCR was carried out with 25 ng of cDNA, 5 µL SYBR premix ExTaqII (TaKaRa, Kusatsu, Japan), and 0.3 µM of each primer in a final volume of 10 µL in triplicate using a Thermal Cycler Rotor-Gene 6000 (Corbett, Australia). The expression levels of target genes were normalized to the level of housekeeping gene, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [28]. All measurements were carried out in triplicate from three separate cultures and data were calculated by the ΔΔCt method [29, 30]. Primer sequences were designed by the Beacon designer software (Version 7.2, USA) and are presented in Table 1.
Protein preparation and Western blot analysis
Cells and EBs (day 6 and day 14) were washed with phosphate-buffered saline (PBS), and then lysed by TRI reagent. Total protein was extracted according to the manufacturer’s protocol (Ambion, USA). Bradford assay was used to evaluate protein concentration (Sigma-Aldrich) [31]. Equal amounts of protein from each sample (30 µg) were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidenedifluoride membrane (PVDF; Biorad, Hercules, CA USA). The membranes were blocked with 10% skim milk (Merck, Darmstadt, Germany) for an overnight, and then incubated at room temperature (RT) for 2 h with the particular primary antibodies: monoclonal anti-microtubule-associated protein 2 (MAP2) (1:2000 v/v; Sigma-Aldrich, M1406), rabbit anti-BDNF antibody (1:1000 v/v; Sigma-Aldrich, AV41970), mouse monoclonal anti-NGF antibody (1:500 v/v; Santa Cruz, SC-365944), mouse monoclonal anti-TrkB (1:25 v/v; Santa Cruz, SC-377218), and rabbit anti-p75 NGF receptor antibody (1:4000 v/v; Abcam, ab8874) and mouse anti-GAPDH antibody (1:5000 v/v; Sigma-Aldrich, A2228) as a loading control. Next step, membranes were washed with Tris-buffered saline (TBST; 50 mM Tris, 150 mM NaCl, 0.05% Tween 20, pH: 7.5) and then incubated at RT for 1 h with an appropriate secondary antibody: horse radish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:5000 v/v; Dako, P0447) or goat anti-rabbit IgG (1:16,000 v/v; Santa Cruz, SC-2301). Subsequently, after washing membranes with TBST, protein bands were visualized using an Amersham enhanced chemiluminescence (ECL) advance western blotting detection kit (GE Healthcare, Dornstadt, Germany). All experiments were performed in triplicate and Image J processing software was used for quantification of blot intensity.
Immunocytochemistry analysis
Suspended EBs, on day 6 were plated onto gelatinized coverslips in 12-well plates and allowed to attach for an additional 8 days. After washing differentiated cells on day 14 with PBS, they were fixed with 4% paraformaldehyde solution at 4 °C for 30 min and then permeabilized by 0.2% Triton-X100 in PBS for 20 min. For staining, cells were incubated overnight at 4 °C with the primary antibodies: anti-mouse antibody against MAP2 (1:200 v/v; Sigma-Aldrich, M1406) and anti-mouse antibody against β tubulin Isotype III (1:500 v/v; Abcam, ab7751). Subsequently, cells were washed with PBS and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG as secondary antibody (1:200 v/v; Millipore, AP124F) for 1 h at 37 °C. BSA blocking buffer with 10 and 5 mg/mL concentration was used for dilution of primary and secondary antibodies, respectively. Finally, cells were treated with 4, 6‐diamidino‐2‐phenylindole (DAPI, 0.1 mg/mL, Sigma-Aldrich, D8417) in PBS for 5 min for nuclear counterstaining. Stained cells on the coverslips were fixed on glass slides and analyzed using a fluorescent microscope (Olympus, Tokyo, Japan). An Olympus DP70 camera was used to capturing images and Image J software was used to analyzing the level of fluorescence [32].
Statistical analysis
All results were reported as the mean value ± standard error of mean (SEM) from at least three independent replicates for each experiment. Statistical analysis was carried out by SPSS version 20. Independent t test was used to calculating statistical differences between the two independent groups and one-way ANOVA followed by Tukey’s post hoc test was used to comparing multiple groups of samples. Statistical significance was defined at the p < 0.05.
Results
Confirmation of Fndc5 knockdown and stemness maintenance in shFndc5 cell line
As mentioned in Materials and methods, in the current study, we used transduced (shCtrl and shFndc5) cell lines created previously by Nazem et al. To verification of Fndc5 knockdown, we treated these cell lines with 1.5 µg/mL doxycycline for 24 h and assessed the expression level of Fndc5. Result showed a significant decrease of Fndc5 transcript level in Dox-treated shFndc5 cell line compared to untreated, scramble, and untransduced cell lines (Fig. 1a).
Confirmation of Fndc5 knockdown and stemness characteristics’ maintenance in shFndc5 cell line. a RT-qPCR analysis of Fndc5 expression in untransduced and transduced (shCtrl and shFndc5) cell lines in the presence and absence of Dox as an inducer of shRNA expression. b RT-qPCR analysis of stemness markers (Nanog, Rex1, and Oct4) expression in transduced (shCtrl and shFndc5) cell lines compared to untransduced cells in the presence and absence of Dox. Values are presented as mean ± SEM for three independent experiments. *p < 0.05
To evaluate the maintenance of stemness properties of transduced (shCtrl and shFndc5) mESCs, the expression levels of pluripotency markers (Nanog, Rex1, and Oct4) were assessed by RT-qPCR. Data indicated no significant differences in transcript levels of three aforementioned markers in these cell lines compared to untransduced cells (Fig. 1b).
Generation and characterization of mESC-derived mature neural cells
To confirm appropriate occurrence of neural differentiation, we evaluated the expression level of stemness and mature neuronal markers during this process. Figure 2a represents the schematic depiction for neural differentiation procedure of mESCs and the morphology of cells at different stages. RT-qPCR data indicated significant downregulation of stemness markers (Nanog and Oct4) and upregulation of mature neuronal markers (Map2 and Tuj-1) during the process of differentiation (Fig. 2b–e). Furthermore, immunofluorescence staining of mESC-derived cells on day 14 showed mature cells with positive expression of mature neuronal markers MAP2 and TUJ1 (Fig. 2f). Therefore, these results confirmed the accuracy of this protocol for neural differentiation and production of mature neural cells.
Characterization of mESC-derived neural cells. a Schematic illustration of mESCs neural differentiation and morphological characterization of these cells during this process. Relative gene expression of the stemness markers (Nanog and Oct4) (b, c) and mature neuronal markers (Map2 and Tuj-1) (d, e) during the neural differentiation of mESCs. f Immunofluorescence staining of mESC-derived neural cells with anti-MAP2 and anti-TUJ1 antibodies. The nuclei were counterstained with DAPI. Scale bar is 100 µm. Values are presented as mean ± SEM for three independent experiments. *p < 0.05
Confirmation of neural progenitor markers’ expression reduction in shFndc5 cell line
In 2013, Hashemi et al. showed that reduction of Fndc5 expression by shFndc5 cell line resulted in a significant decrease in expression levels of NP markers, Sox1, Sox3, Pax6, and Nestin [6]. Therefore, for this purpose, we assessed the transcript levels of Fndc5 and NP markers (Pax6, Nestin, and Sox1) in neural progenitors (day 6) of transduced (shCtrl and shFndc5) cell lines obtained by Nazem et al., and untransduced cell line following Dox treatment compared to untreated groups. Transduced (shCtrl, shFndc5) and untransduced cell lines were differentiated to NPs with 1 µM RA and treated with 1.5 µg/mL Dox from day 2 to day 6 (Fig. 3a). As shown in Fig. 3b–e, in contrast to the untransduced and shCtrl cell lines, mRNA expression levels of Fndc5, Pax6, Nestin, and Sox1 decreased significantly in neural progenitors of Dox-treated shFndc5 cell line compared to untreated control.
Confirmation of neural progenitor marker expression reduction in Fndc5 knockdown cell line. a Schematic illustration of neural progenitors (NPs) formation. Early embryoid bodies (EBs; day 2) of transduced (shCtrl, shFndc5) and untransduced cell lines were suspended for 4 days in the presence of 1 µM RA and 1.5 µg/mL doxycycline (Dox). b RT-qPCR analysis of Fndc5 expression in Dox-treated cell lines (untransduced, shCtrl and shFndc5) compared to untreated cells in neural progenitors (day 6). c–e RT-qPCR analysis of neural progenitor markers, Pax6, Nestin, and Sox1 following induction of Fndc5 knockdown by Dox in shFndc5 cell line compared to untreated control. Represented values are mean of triplicate experiments ± SEM. *p < 0.05
It is important to note that as previously shown by Hashemi et al., [6], Fndc5 knockdown only decreases the rate and efficiency of neural differentiation in mESCs and attenuates this process, but does not inhibit it completely, our results also confirmed their data as Dox-treated shFndc5 cells in day 6 were also neural progenitors, because neural progenitor (Pax6 and Nestin) and stemness (Nanog and Oct4) markers expression, respectively increased and decreased significantly in these cells compared to mESCs like other groups (Supplementary Fig. 1a–d). Furthermore, as Hashemi et al. had shown, the expression of mesodermal (αSMA and αMHC) and endodermal (Sox17 and Foxa2) markers did not increase in Dox-treated shFndc5 cell line compared to untreated control which proved neural differentiation reduction by Fndc5 knockdown was independent of endodermal or mesodermal differentiation (Supplementary Fig. 1e).
Confirmation of mature neuronal markers expression reduction in shFndc5 cell line
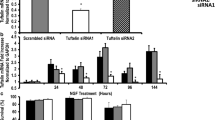
In previous study by Hashemi et al., it has shown that mature neuronal markers (Map2 andTuj-1) and consequently neural differentiation rate of mESCs decreased following Fndc5 expression reduction in shFndc5 cell line compared to control [6]. Therefore, in the present study, to confirm these data, we assessed the transcript levels of Fndc5 and mature neuronal markers in mature neural cells (day 14) of transduced (shCtrl, shFndc5) and untransduced cell lines following Dox treatment compared to untreated groups. Figure 4a depicts the schematic procedure for neural differentiation of mESCs. As shown in Fig. 4, Dox induction reduces Fndc5 RNA level and significantly decreases the transcript levels of Map2 and Tuj-1as mature neuronal markers in shFndc5 cell line compared to untreated control (Fig. 4b–d) which was further verified by western blot analysis (Fig. 4e), and morphological analysis of neural cells derived from mouse embryonic stem cells using phase contrast microscope and immunofluorescence staining against neuronal marker Map2 (Fig. 4f).
Confirmation of mature neuronal markers expression reduction in Fndc5 knockdown cell line. a Illustrated protocol of mESCs neural differentiation. Transduced (shCtrl, shFndc5) and untransduced cell lines were treated with 1.5 µg/mL doxycycline (Dox) during and post-neural progenitor (NPs) formation (day 2–day 14). RT-qPCR analysis of Fndc5 (b) and mature neuronal markers, Map2 and Tuj-1 (c, d) expression in Dox-treated cell lines (untransduced, shCtrl, and shFndc5) compared to untreated cells in mature neurons (day 14). e Western blot analysis for MAP2 in neural cells (day 14) relative to GAPDH in Dox-treated shFndc5 cell line compared to untreated and shCtrl cell lines. f Upper panel: Phase contrast morphology of differentiated mature neural cells (day 14) from shCtrl and shFndc5 cell lines in the presence and absence of Dox. Lower panel: immunofluorescence staining of Map2 in mature neural cells from shCtrl and shFndc5 cell lines in the presence and absence of Dox. The nuclei were counterstained with DAPI. Scale bar is 100 µm. Values are presented as mean ± SEM for three independent experiments. *p < 0.05
As mentioned in the case of progenitor cells, Dox-treated shFndc5 cells in day 14 were also mature neural cells as mature neuronal markers (Map2 and Tuj-1) expression increased significantly in these cells compared to NPs and mESCs like other groups (Supplementary Fig. 2a-b).
Effect of Fndc5 knockdown on expression of neurotrophins and their receptors expression in Fndc5 knockdown neural progenitor cells
To investigate the effect of Fndc5 knockdown on neurotrophins and their receptor expression in neural progenitors (day 6), transduced (shCtrl, shFndc5) and untransduced cell lines were differentiated according to aforementioned protocol and Fndc5 knockdown was performed by Dox induction from day 2 until day 6. As shown in Fig. 5a–d, f–i, Fndc5 knockdown in shFndc5 cell line resulted in reduction of BDNF and all neurotrophin receptors (TrkB, TrkA, TrkC, and p75NTR) at mRNA levels in neural progenitors. Transcript level of other neurotrophins (NGF, NT3, and NT4) did not alter in neural progenitors following Fndc5 knockdown in Dox-treated shFndc5 cell line compared to untreated control. Western blot analysis further confirmed decreased protein levels of BDNF, TrkB, and p75NTR and unchanged protein level of NGF following Fndc5 knockdown in neural progenitors (Fig. 5e, j).
Evaluation the effect of Fndc5 knockdown on neurotrophins and their receptor expression in neural progenitors. RT-qPCR analysis of neurotrophins (a–d) and their receptors (e–h) mRNA expression in Dox-treated cell lines (untransduced, shCtrl and shFndc5) compared to untreated cells in neural progenitors (day 6). Western blot analysis for BDNF and NGF (e) and TrkB and p75NTR (j) in Dox-treated shFndc5 cell line compared to untreated and shCtrl cell lines in neural progenitors (day 6). Data were reported as the mean ± SEM of three independent experiments. *p < 0.05
Effect of Fndc5 knockdown on expression of neurotrophins and their receptor expression in Fndc5 knockdown mature neural cells
To verify the effect of Fndc5 knockdown on neurotrophins and their receptor expression in mature neural cells (day 14), Dox induction was started from day 2 and continued until the end of the process. The expressions of neurotrophins and their receptors were evaluated following Fndc5 knockdown by real-time PCR and western blot analysis. Contrary to our expectation, in mature neural cells, BDNF and NGF mRNA levels increased in Dox-treated shFndc5 cell line compared to untreated control (Fig. 6a, b). Unlike BDNF and NGF, NT3 and NT4 transcript levels were not affected by Fndc5 knockdown in mature neural cells (Fig. 6c, d). Interestingly, western blot analysis revealed a conflicting result with RNA expression analysis. As shown in Fig. 6e, inconsistent with mRNA levels of BDNF and NGF, protein levels of these two neurotrophins decreased following Fndc5 knockdown in mature neural cells, as expected. Furthermore, all neurotrophin receptors (TrkA, TrkB, TrkC, and p75NTR) transcript levels decreased in Dox-treated shFndc5 cell line compared to untreated control (Fig. 6f–i). Western blot analysis further confirmed the reduction of TrkB and p75NTR at protein levels following Fndc5 knockdown in mature neural cells (Fig. 6j).
Evaluation of the effect of Fndc5 knockdown on neurotrophins and their receptor expression in mature neural cells. RT-qPCR analysis of neurotrophins (a–d) and their receptor (f–i) mRNA expression in Dox-treated cell lines (untransduced, shCtrl, and shFndc5) compared to untreated cells in mature neural cells (day 14). Western blot analysis for BDNF and NGF (e) and TrkB and p75NTR (j) in Dox-treated shFndc5 cell line compared to untreated and shCtrl cell lines in mature neural cells (day 14). Data were reported as the mean ± SEM of three independent experiments. *p < 0.05
Discussion
In recent years, transplantation of differentiated neural cells from embryonic stem cells into brain of animal models is an appropriate approach to treat neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases [33, 34]. However, functional recovery of nerve injury after transplant depends on appropriate function of neurotrophic factors by the grafted cells in host which induce survival and regeneration of neurons [34].
Neurotrophins, members of neurotrophic factor family, are the main mediators of survival, development, differentiation, repair, plasticity, and molecular synthesis pathways of neurons in both the central and peripheral nervous systems by signaling through specific receptors [35, 36]. It has been revealed that pathogenesis of significant number of psychiatric and neurodegenerative disorders is associated with alteration in neurotrophins and their receptors expression levels. Supply of neurotrophins as therapeutic agents through neurotrophin-producing cell transplantation or neurotrophin-encoding viral vector delivery is an effective strategy to restore neuronal function in neurodegenerative disorders [37, 38].
It has been reported that beneficial effects of exercise on brain performance were achieved by PGC1α/FNDC5/BDNF pathway [12]. Furthermore, in our laboratory, it has been shown so far that knockdown of Fndc5 decreases neural differentiation rate of mESCs [6] and overexpression of it facilitates this process. In aforementioned study, it was reported that mRNA level of BDNF increased following Fndc5 overexpression and this could be a cause of increased neurogenesis [7]. However, downstream molecular pathways and targets of FNDC5 which influence neuronal health, except BDNF, have not yet been determined.
In this study, considering the crucial roles of neurotrophins and their receptors in neurogenesis and since gene silencing through RNA interference (RNAi) is a general method for analyzing gene function [39, 40], we used a stably transduced Fndc5 knockdown cell line to assess whether decreased level of Fndc5 reduced neural differentiation of mESCs by affecting the expression of neurotrophins and their receptors. Since neurotrophins have important role in neural tissue repair [13,14,15], it would be advantageous to modulate the neurotrophins through Fndc5 for further clinical approaches.
In this paper, we used a shFndc5 expressing mESC line and short hairpin RNA interference (shRNAi) transcript products [9].
Our data were evident that mRNA expression level of neural progenitor markers, Pax6, Nestin, and Sox1, and mature neuronal markers Map2 and Tuj-1 decreased significantly following Fndc5 knockdown in Dox-treated shFndc5 cell line compared to untreated control.
In the present study, among the neurotrophins which were studied, BDNF transcripts were sharply decreased upon downregulation of Fndc5 in NPs. In this circumstance, also the expression of neurotrophin receptors decreased, but NGF, NT3, and NT4 mRNA expression levels unchanged. Western blot analysis further confirmed these data in neural progenitors.
In mature neural cells, following Fndc5 knockdown, the expression of BDNF and NGF at protein level and all neurotrophin receptors at transcript level decreased in Dox-treated shFndc5 cell line compared to untreated control. Western blot analysis for TrkB and p75NTR protein confirmed these data. All neurotrophins mediate their effects via these receptors through activation of three important signaling cascades (ERK, PI3-kinase, and phospholipase C-γ1) and induction of the expression of bHLH transcription factors which are critical for neuronal growth, proliferation, maintenance, and differentiation [41, 42]. Hence, in the current study, the reduction of neurotrophins receptor expression following Fndc5 knockdown results in unfunctional neurotrophins.
It has been revealed that activity-dependent mRNA translation and synthesis of endogenous BDNF is triggered by activation of Trk receptors and regulated by multiple signaling cascades, as inhibition of signaling mediated by Ras, Erk1/2, PI3K, mTOR, or PLC which all control translation through 5′ UTR- dependent mechanisms, inhibit BDNF translation [43].
On the other hand, various studies have already shown that Fndc5/irisin exerts its biological effects through several intracellular signaling pathways. MAPK signaling pathway including Erk1/2 is the major signaling pathway downstream of Fndc5 which is involved in neural and osteoblast differentiation and white adipocytes’ browning [10, 11, 44]. Furthermore, a recent study revealed that recombinant irisin not only induced phosphorylation of ERK in cultured neuron but also stimulates the cAMP/PKA/CREB pathway in human cortical and mouse hippocampal slices [45]. CREB is one of the best-studied factor involved in regulation of BDNF expression. Downregulation of phosphorylated (p)-CREB reduces BDNF level [46]. Furthermore, it has been revealed that the expression of BDNF receptor, trkB, is regulated by the cAMP/CREB pathway in neurons [47].
In conclusion, decreased levels of BDNF and NGF and their receptors in Dox-treated shFndc5 cell line compared to untreated control can be attributed to the decreased activity of signaling cascades such as Erk1/2 and cAMP/CREB following Fndc5 knockdown in these cells.
In the current study, comparing western blot results vs. RT-PCR data, there was a RNA/protein discordant in BDNF and NGF expression when Fndc5 decreased in mature neurons in Dox-treated shFndc5 cell line. Like this discordance in NGF and BDNF RNA/protein levels, observed in our previous study which we used two different media for differentiation of NPs to mature neurons [48]. In the present study, discrepancy between mRNA and protein levels of BDNF and NGF in Dox-treated shFndc5 cell line could presumably due to a compensatory mechanism or the possible miRNAs expression following Fndc5 knockdown which suppress neurotrophins at protein levels [49, 50] or signaling pathways which contribute in neurotrophins translation. For example a study in 2017 revealed that irisin attenuates H2O2-induced apoptosis in cardiomyocyte through upregulation of miR-19b which activates AKT/mTOR signaling pathway [51]. Furthermore, the previous studies reported that miR-19 has functional implications in adult hippocampal neurogenesis as it is enriched in neural progenitor cells (NPCs) and is a key regulator of migration of newborn neurons in the adult brain. In addition, miR-19 affects the maturation of newborn neurons [52, 53]. As mentioned above, inhibition of multiple signaling cascades such as mTOR inhibits BDNF translation. Therefore, if Fndc5 impacts the expression of miR-19b in neural cells like cardiomyocyte, it is possible that Fndc5 knockdown causes downregulation of miR-19b and thereby mTOR signaling activity and BDNF translation.
In the current study, the expressions of NT3 and NT4 did not affect by Fndc5 knockdown. Previous studies revealed that neurotrophins have differences in temporal and spatial expression during the neurogenesis as NT3 is needed for early stages of this process and has important roles in immature neurons. The expression of NT3 is decreased with maturation of CNS neurons [54, 55]. Accordingly, we speculate that the same level of NT3 in Dox-treated shFndc5 cell line compared to untreated control is likely due to the time of gene expression analysis (mature neural cells, day 14). NT3 gene expression analysis before day 14 is required to determine whether Fndc5 knockdown can affect its expression or not.
Moreover, studies revealed that NT4 is widely expressed in non-neuronal tissues as highest level of NT4 has been detected in testis and skeletal muscle and much lower levels in the CNS. NT4 null mice show minimal neurological phenotypes compared with BDNF knock-out mice. Surprisingly, it has been revealed that NT4 is not activity regulated in the brain [56]. Furthermore, data obtained from previous studies suggest that NT4 expression is important in the absence of BDNF [57]. As we showed in our previous study, mRNA expression level of NT4 did not change during neural differentiation of mESCs by RA and it seemed that there was no need to increase NT4 expression, because BDNF upregulated during this process [48]. Accordingly, in the present study, the expression of NT4 has not been affected by Fndc5 knockdown during neural differentiation of mESCs.
In conclusion, these data suggest that the decreased efficiency of neural differentiation following Fndc5 knockdown can be attributed to decreased levels of NGF and BDNF proteins in addition to their cognate receptors. Although, these findings are not sufficient to declare whether neurotrophins and their cognate receptor are direct downstream targets of Fndc5 or not, because molecular mechanisms involved in the control of neural differentiation by Fndc5 are still unclear. Further investigations including overexpression of Fndc5 or treatment with candidate neurotrophins (such as recombinant proteins of NGF and BDNF) to see the recovery of neural differentiation rate in Fndc5 knockdown mouse ES cells will be required to clarify the molecular event of this process.
Abbreviations
- ANOVA:
-
Two-way analysis of variance
- ATP:
-
Adenosine triphosphate
- BDNF:
-
Brain-derived neurotrophic factor
- bHLH:
-
Basic helix–loop–helix
- BSA:
-
Bovine serum albumin
- cAMP:
-
Cyclic adenosine monophosphate
- CNS:
-
Central nervous system
- CREB:
-
CAMP-response element binding protein
- DAPI:
-
4, 6‐Diamidino‐2‐phenylindole
- DMEM/F12:
-
Dulbecco’s modified Eagle’s medium/Hams F12 medium
- Dox:
-
Doxycycline
- EB:
-
Embryoid body
- ECL:
-
Enhanced chemiluminescence
- ERK:
-
Extracellular signal-regulated kinase
- ESC:
-
Embryonic stem cell
- ES-FBS:
-
Embryonic stem cell qualified fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- Fndc5:
-
Fibronectin type III domain-containing-5
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- HRP:
-
Horse radish peroxidase
- JNK:
-
Jun N-terminal kinase
- Ko-DMEM:
-
Knock-out DMEM
- LIF:
-
Leukemia inhibitory factor
- MAP2:
-
Microtubule-associated protein 2
- MAPK:
-
Mitogen-activated protein kinases
- mESC:
-
Mouse embryonic stem cell
- miRNA:
-
MicroRNAs
- mTOR:
-
Mammalian target of rapamycin
- NB:
-
Neurobasal
- NC:
-
Neural cell
- NGF:
-
Nerve growth factor
- NP:
-
Neural progenitor
- NT:
-
Neurotrophin
- NT-3:
-
Neurotrophin-3
- NT-4:
-
Neurotrophin-4
- p75NTR:
-
P75 neurotrophin receptor
- PBS:
-
Phosphate-buffered saline
- PeP:
-
Proxisomal protein
- PI3:
-
Phosphatidylinositol-3-kinase
- PLC:
-
Phospholipase C
- PVDF:
-
Polyvinylidenedifluoride
- RA:
-
Retinoic acid
- RNAi:
-
RNA interference
- ROS:
-
Reactive oxygen species
- RT:
-
Room temperature
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SEM:
-
Standard error of the mean
- shRNA:
-
Small hairpin RNA
- TRI:
-
Total RNA isolation
- Trk:
-
Tropomyosin-related kinase
References
Ferrer-Martínez A, Ruiz-Lozano P, Chien KR. Mouse PeP: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn. 2002;224(2):154–67.
Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297(1–2):79–83.
Ostadsharif M, Ghaedi K, Nasr Esfahani M, Tanhaei S, Karbalaei K, Parivar K, et al. Cytosolic localization of mouse peroxisomal protein/∆ SKI fused with enhanced green fluorescent protein into chinese hamster ovary-K1 and P19 cells. Yakhteh Med J. 2009;11(2):154–9.
Ostadsharif M, Ghaedi K, Nasr-Esfahani MH, Tanhaie S, Karbalaie K, Baharvand H. Sorting analysis of mouse peroxisomal protein by in vitro studies. Iran J Biotechnol. 2010;8(3):186–92.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463.
Hashemi M-S, Ghaedi K, Salamian A, Karbalaie K, Emadi-Baygi M, Tanhaei S, et al. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. 2013;231:296–304.
Forouzanfar M, Rabiee F, Ghaedi K, Beheshti S, Tanhaei S, Shoaraye Nejati A, et al. Fndc5 overexpression facilitated neural differentiation of mouse embryonic stem cells. Cell Biol Int. 2015;39(5):629–37.
Rabiee F, Forouzanfar M, Zadegan FG, Tanhaei S, Ghaedi K, Bashi MM, et al. Induced expression of Fndc5 significantly increased cardiomyocyte differentiation rate of mouse embryonic stem cells. Gene. 2014;551(2):127–37.
Nazem S, Rabiee F, Ghaedi K, Babashah S, Sadeghizadeh M, Nasr-Esfahani MH. Fndc5 knockdown induced suppression of mitochondrial integrity and significantly decreased cardiac differentiation of mouse embryonic stem cells. J Cell Biochem. 2018;119(6):4528–39.
Rabiee F, Lachinani L, Ghaedi S, Nasr-Esfahani MH, Megraw TL, Ghaedi K. New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 2020;10:1–10.
Farahabadi SH, Ghaedi K, Zadegan FG, Karbalaie K, Rabiee F, Nematollahi M, et al. ERK1/2 is a key regulator of Fndc5 and PGC1α expression during neural differentiation of mESCs. Neuroscience. 2015;297:252–61.
Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649–59.
Bartkowska K, Turlejski K, Djavadian RL. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol Exp (Wars). 2010;70(4):454–67.
Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299.
Malva JO, Cunha RA, Oliveira CR, Rego AC. Interaction between neurons and glia in aging and disease. Berlin: Springer; 2007.
Vega JA, García-Suárez O, Hannestad J, Pérez-Pérez M, Germanà A. Neurotrophins and the immune system. J Anat. 2003;203(1):1–19.
Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc B Biol Sci. 2006;361(1473):1545–64.
Schor NF. The p75 neurotrophin receptor in human development and disease. Prog Neurobiol. 2005;77(3):201–14.
Casaccia-Bonnefil P, Gu C, Khursigara G, Chao MV. p75 neurotrophin receptor as a modulator of survival and death decisions. Microsc Res Tech. 1999;45(4–5):217–24.
Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–91.
Dobrowsky RT, Jenkins GM, Hannun YA. Neurotrophins induce sphingomyelin hydrolysis modulation by co-expression of p75NTR with Trk receptors. J Biol Chem. 1995;270(38):22135–42.
Abranches E, Silva M, Pradier L, Schulz H, Hummel O, Henrique D, et al. Neural differentiation of embryonic stem cells in vitro: a road map to neurogenesis in the embryo. PLoS ONE. 2009;4(7):e6286.
Tan B-T, Wang L, Li S, Long Z-Y, Wu Y-M, Liu Y. Retinoic acid induced the differentiation of neural stem cells from embryonic spinal cord into functional neurons in vitro. Int J Clin Exp Pathol. 2015;8(7):8129.
Oliveira SL, Pillat MM, Cheffer A, Lameu C, Schwindt TT, Ulrich H. Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytometry A. 2013;83(1):76–89.
Wang L, Kisaalita WS. Administration of BDNF/ginsenosides combination enhanced synaptic development in human neural stem cells. J Neurosci Methods. 2011;194(2):274–82.
Hassani S-N, Totonchi M, Sharifi-Zarchi A, Mollamohammadi S, Pakzad M, Moradi S, et al. Inhibition of TGFβ signaling promotes ground state pluripotency. Stem Cell Rev Rep. 2014;10(1):16–30.
Ostadsharif M, Ghaedi K, Nasr-Esfahani MH, Mojbafan M, Tanhaie S, Karbalaie K, et al. The expression of peroxisomal protein transcripts increased by retinoic acid during neural differentiation. Differentiation. 2011;81(2):127–32.
Willems E, Mateizel I, Kemp C, Cauffman G, Sermon K, Leyns L. Selection of reference genes in mouse embryos and in differentiating human and mouse ES cells. Int J Dev Biol. 2004;50(7):627–35.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat Protoc. 2008;3(6):1101.
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–22.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54.
Joshi R, Thakuri PS, Buchanan JC, Li J, Tavana H. Microprinted stem cell niches reveal compounding effect of colony size on stromal cells-mediated neural differentiation. Adv Healthc Mater. 2018;7(5):1700832.
Baier PC, Schindehütte J, Thinyane K, Flügge G, Fuchs E, Mansouri A, et al. Behavioral changes in unilaterally 6-hydroxy-dopamine lesioned rats after transplantation of differentiated mouse embryonic stem cells without morphological integration. Stem Cells. 2004;22(3):396–404.
Tripathy D, Haobam R, Nair R, Mohanakumar KP. Engraftment of mouse embryonic stem cells differentiated by default leads to neuroprotection, behaviour revival and astrogliosis in parkinsonian rats. PLoS ONE. 2013;8(9):e72501.
Ramamurthy P. Mouse embryonic stem cells differentiated into neuron-like cells or schwann cell-like cells for the development of strategies to ameliorate deafness. 2012.
Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, et al. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18(6):1111–22.
Razavi S, Nazem G, Mardani M, Esfandiari E, Salehi H, Esfahani SHZ. Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv Biomed Res. 2015;4:53.
Weissmiller AM, Wu C. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl Neurodegen. 2012;1(1):14.
Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci. 2003;100(4):1844–8.
Ziv C, Yarden O. Gene silencing for functional analysis: assessing RNAi as a tool for manipulation of gene expression. Molecular and Cell Biology Methods for Fungi. Berlin: Springer; 2010. p. 77–100.
Liu F, Xuan A, Chen Y, Zhang J, Xu L, Yan Q, et al. Combined effect of nerve growth factor and brain-derived neurotrophic factor on neuronal differentiation of neural stem cells and the potential molecular mechanisms. Mol Med Rep. 2014;10(4):1739–45.
Ito H, Nakajima A, Nomoto H, Furukawa S. Neurotrophins facilitate neuronal differentiation of cultured neural stem cells via induction of mRNA expression of basic helix-loop-helix transcription factors Mash1 and Math1. J Neurosci Res. 2003;71(5):648–58.
Baj G, Pinhero V, Vaghi V, Tongiorgi E. Signaling pathways controlling activity-dependent local translation of BDNF and their localization in dendritic arbors. J Cell Sci. 2016;129(14):2852–64.
Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63(2):514–25.
Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019;25(1):165–75.
Yan X, Liu J, Ye Z, Huang J, He F, Xiao W, et al. CaMKII-mediated CREB phosphorylation is involved in Ca2+-induced BDNF mRNA transcription and neurite outgrowth promoted by electrical stimulation. PLoS ONE. 2016;11(9):e0162784.
Deogracias R, Espliguero G, Iglesias T, Rodrı́guez-Peña A, . Expression of the neurotrophin receptor trkB is regulated by the cAMP/CREB pathway in neurons. Mol Cell Neurosci. 2004;26(3):470–80.
Ebadi R, Kordi-Tamandani DM, Ghaedi K, Nasr-Esfahani MH. Comparison of two different media for maturation rate of neural progenitor cells to neuronal and glial cells emphasizing on expression of neurotrophins and their respective receptors. Mol Biol Rep. 2018;45(6):2377–91.
Shi J. Regulatory networks between neurotrophins and miRNAs in brain diseases and cancers. Acta Pharmacol Sin. 2015;36(2):149–57.
Finley MF, Devata S, Huettner JE. BMP-4 inhibits neural differentiation of murine embryonic stem cells. J Neurobiol. 1999;40(3):271–87.
Peng Q, Wang X, Wu K, Liu K, Wang S, Chen X. Irisin attenuates H2O2-induced apoptosis in cardiomyocytes via microRNA-19b/AKT/mTOR signaling pathway. Int J Clin Exp Pathol. 2017;10(7):7707.
Han J, Kim HJ, Schafer ST, Paquola A, Clemenson GD, Toda T, et al. Functional implications of miR-19 in the migration of newborn neurons in the adult brain. Neuron. 2016;91(1):79–89.
Han J, Gage FH. A role for miR-19 in the migration of adult-born neurons and schizophrenia. Neurogenesis. 2016;3(1):e1251873.
Yaghoobi MM, Mowla SJ. Differential gene expression pattern of neurotrophins and their receptors during neuronal differentiation of rat bone marrow stromal cells. Neurosci Lett. 2006;397(1–2):149–54.
Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, et al. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5(4):501–9.
West A, Pruunsild P, Timmusk T. Neurotrophins: transcription and translation. Neurotrophic factors. Berlin: Springer; 2014. p. 67–100.
Patz S, Wahle P. Neurotrophins induce short-term and long-term changes of cortical neurotrophin expression. Eur J Neurosci. 2004;20(3):701–8.
Acknowledgements
Authors would like to thank University of Sistan and Baluchestan and Royan Institute for the financial support of this project. The authors are thankful to other members of Royan Institute for their excellent technical assistance and advice.
Author information
Authors and Affiliations
Contributions
RE: experimental design, data collection, data analysis, data interpretation, and manuscript writing. FR: data analysis, data interpretation. DMKT: experimental design, financial support, data analysis, data interpretation, and final approval of the manuscript. MHNE: experimental design, financial support, data analysis, data interpretation, and final approval of manuscript. KG: experimental design, data analysis, data interpretation, manuscript writing, and final approval of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors indicate no potential conflicts of interest.
Ethical approval
Approval for this study was obtained from the Institutional Review Board of Royan Institute (Tehran, Iran).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ebadi, R., Rabiee, F., Kordi-Tamandani, D. et al. Fndc5 knockdown significantly decreased the expression of neurotrophins and their respective receptors during neural differentiation of mouse embryonic stem cells. Human Cell 34, 847–861 (2021). https://doi.org/10.1007/s13577-021-00517-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00517-z