Abstract
Recent studies hint that Ginsenoside is involved in cancer prevention and treatment. In this study, we investigated the effect of Ginsenoside Rh2 on drug resistance in human colorectal carcinoma (CRC) cells and its mechanism. The resistance reversion effect of Ginsenoside Rh2 in CRC cells was analyzed using CCK-8 assay. After treating with Ginsenoside Rh2, the cell cycle distribution and cellular apoptosis were analyzed by flow cytometry, cell migration was determined by transwell migration assay, the expression of drug-resistance genes and proteins were evaluated using quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot, respectively. Ginsenoside Rh2 could enhance the cytotoxicity of 5-FU in drug-resistant CRC cells (LoVo/5-FU and HCT-8/5-FU). Treatment with Ginsenoside Rh2 could result in an increase of cell numbers in G0/G1 phase accompanied with a decrease in S-phase, and induced cellular apoptosis in drug-resistant CRC cells. In addition, the migration process and EMT process of drug-resistant CRC cells were suppressed by treatment of Ginsenoside Rh2. Compared to control group, expression of drug-resistance genes, such as MRP1, MDR1, LRP and GST, were negatively correlated to Ginsenoside Rh2. All these results indicated that Ginsenoside Rh2 could effectively reverse drug resistance in human colorectal carcinoma cell and its mechanism involved the prevention of cellular proliferation and migration, the promotion of cellular apoptosis and the alteration of drug-resistance genes, which suggested that Ginsenoside Rh2 may act as a promising candidate for drug resistance in human colorectal carcinoma chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal carcinoma (CRC) is a common malignant tumor and the third leading cancer-related mortality cause around the world [1]. Owing to considerable improvements in systemic therapy, the mortality rate of colorectal cancer has been reduced, still surgery and chemotherapeutic strategies are CRC patients’ two major options. As for chemotherapeutic strategies, 5-fluorouracil (5-FU) is one of the most widely used chemotherapeutic agents for patients [2]. However, frequently occurring drug resistance resulted in treatment failure in CRC [3]. The acquisition of drug resistance could be associated with many mechanisms, including increased drug efflux, alterations in the drug target and drug inactivation, modification of cell cycle checkpoints and defective apoptotic pathways [4,5,6,7,8]. Therefore, novel agents of high efficiency and low toxicity are needed for overcoming 5-FU resistance and increasing patients’ survival rate.
Ginseng is a commonly used Chinese herbal medicine which is considered to be linked with significantly decreased risk of a variety of cancers [9]. Researches proved that ginsenosides is one of the main ingredients with anti-tumor activity in Ginseng [10,11,12]. Ginsenoside Rh2 has been found with a strong ability to inhibit glioma cell proliferation [13] and induced cellular apoptosis in both hepatoma [14] and leukemia Reh cells [15]. In addition, Han’s study suggested that Rh2 has an anti-cancer activity in CRC cells [16]. However, studies related to the resistance reversion effect and mechanism of Ginsenoside Rh2 in human colorectal carcinoma cells were limited.
In the present study, we systematically investigated the reversal effect of Ginsenoside Rh2 on drug resistance in human colorectal carcinoma (CRC) cell line HCT-8/5-FU and LoVo/5-FU. Moreover, the mechanisms that may be involved, such as cellular proliferation, apoptosis, migration and some drug-resistance genes were also studied.
Materials and methods
Reagents and antibodies
Rh2 was purchased from Sigma-Aldrich and the powder was dissolved in dimethylsulfoxide(DMSO). The chemical structure of Rh2 is shown in Fig. 1A. Antibodies of Bcl-2, caspase-3, IκB-α, phospho-IκB-α (S32), E-cadherin, N-cadherin, Vimentin, MMP9, GAPDH were purchased from Cell Signaling Technology (Beverly, MA). Antibodies of MRP1, MDR1, LRP and GST were purchased from Santa Cruz Biotech (Santa Cruz, CA). Antibodies of CyclinD1, CDK2, Rb and phospho-Rb (S807/811) were purchased from Signalway Antibody (SAB, USA). Cell Counting Assay Kit-8 (CCK-8) was purchased from Guangzhou Ladder Biotech (Guangzhou, China). Fetal bovine serum (FBS) and RPMI-1640 medium was purchased from Gibco (Gibco, USA).
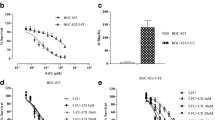
The reversal effect of Rh2 on 5-Fu resistance in HCT/5-FU and LoVo/5-FU cells. a The chemical structure of Rh2. b Cell viability of HCT-8/5-FU cells and its parental HCT-8 cells. c Cell viability of LoVo/5-FU cells and its parental LoVo cells. d Toxic effect of RH2 on HCT-8/5-FU cells and its parental HCT-8 cells. Bars represent mean ± SD, n = 6. *p < 0.05 vs. 0 μM. e Toxic effect of RH2 on LoVo/5-FU cells and its parental LoVo cells. Bars represent mean ± SD, n = 6. *p < 0.05 vs. 0 μM. f Effect of Rh2 on HCT-8/5-FU cell viability. g Effect of Rh2 on LoVo/5-FU cell viability. h Effect of Rh2 on HCT-8 cell viability. i Effect of Rh2 on LoVo cell viability
Cell culture
Human CRC cells (HCT-8 and LoVo) were cultured at 37 °C in RPMI-1640 medium containing 10% fetal bovine serum (FBS), in a humidified atmosphere of 5% CO2. Human 5-FU-resistant CRC cells (HCT-8/5-FU and LoVo/5-FU) were established and cultured in RMPI-1640 medium supplemented with 6.5 or 15.0 μg/ml 5-FU. The establishment of human 5-FU-resistant CRC cells was briefly described as follows: parent human CRC cells HCT-8 were exposed stepwise to escalating concentrations of 5-FU, ranging from 0.5 to 2 μg/ml for about 7 months; and parent human CRC cells Lovo were exposed stepwise to escalating concentrations of 5-FU, ranging from 1 to 2.5 μg/ml for about 7 months. Then the 5-FU resistance characteristics of HCT-8/5-FU and LoVo/5-FU cells were confirmed by CCK-8 assay.
Cell viability assay
The effect of Rh2 on the viability of CRC cells was measured by CCK-8 assay. Single-cell suspensions were seeded in 96-well plates. After incubation with Rh2 for 24 h, 10 µL CCK-8 reagents were added and incubated for another 2 h; the absorbance at 450 nm was measured using a microplate reader (AMR-100, ThermoRui-OS, Jiangsu).
Multidrug resistance reversal assay
The effect of Rh2 on human 5-FU-resistant CRC cells (HCT-8/5-FU and LoVo/5-FU) was detected with CCK-8 assay. Single-cell suspensions were seeded in 96-well plates. Cells were pretreated with different concentrations of 5-FU for 30 min, then the cells were incubated with varying concentrations of Rh2 for 24 h; 10µL CCK-8 reagents were added and incubated for another 2 h; the absorbance at 450 nm was measured using a microplate reader (AMR-100, ThermoRui-OS, Jiangsu).
Cell cycle analysis
Cell cycle distribution was detected by propidium iodide (PI) staining using flow cytometry (CytoFLEX, Beckman, USA), according to the manufacturer’s instructions. Briefly, HCT-8/5-FU cells or LoVo/5-FU cells were pretreated with 4 or 8 μM 5-FU for 30 min, respectively, then the cells were incubated with varying concentrations of Rh2 for 24 h. All the treated cells were collected and fixed in ice-cold 70% methanol. Following centrifugation, cells were resuspended in ice-cold PBS, and incubated with RNase at 37 °C for 30 min, and then were incubated with propidium iodide (PI) at room temperature for 30 min. Finally, cell cycle distribution was analyzed using flow cytometer (CytoFLEX, Beckman, USA). The percentage of cells containing different multiple DNA was quantified.
Cell apoptotic analysis
Cellular apoptosis was measured by Annexin-V/PI double staining using flow cytometry (CytoFLEX, Beckman, USA), according to the manufacturer’s instructions. Briefly, HCT-8/5-FU cells or LoVo/5-FU cells were pretreated with 4 or 8 μM 5-FU for 30 min, respectively, then the cells were incubated with varying concentrations of Rh2 for 24 h. All the treated cells were harvested and resuspended in binding buffer; then incubated with Annexin-V-FITC for 15 min and PI for another 5 min in the dark at room temperature. Finally, each sample was quantitatively analyzed using flow cytometer (CytoFLEX, Beckman, USA).
Transwell assay
Cellular migration was analyzed by transwell assay according to manufacturer’s instructions (Corning, USA). Briefly, HCT-8/5-FU cells or LoVo/5-FU cells were pretreated with 4 or 8 μM 5-FU for 30 min, respectively, then the cells were incubated with varying concentrations of Rh2 for 24 h. All the treated cells were harvested and 1 × 105 cells cultured in 100 μl RMPI-1640 medium containing 1% FBS were seeded on the upper chamber insert with 8-μm-pore membrane (Corning, Lowell, USA), and 600 μl RMPI-1640 medium containing 5% FBS was added to the lower chamber. After migration for 12 h in a 37 °C, 5% CO2 atmosphere, cells that migrated into the lower chamber were fixed in methanol, and stained with 0.5% crystal violet solution. Cell migration was quantized using microscopy (IX73, Olympus, Japan).
Western blot analysis
Protein expressions and phosphorylation levels were measured by Western blot assay. HCT-8/5-FU cells or LoVo/5-FU cells were pretreated with 4 or 8 μM 5-FU for 30 min, respectively, then the cells were incubated with varying concentrations of Rh2 for 24 h. All the treated cells were lysed in RIPA lysis buffer with protease and phosphatase inhibitor cocktail. Protein concentrations were quantized by BCA protein assay kit. 60 μg proteins were separated by SDS-PAGE on 8% gels and transferred to PVDF membranes followed by blockage with 5% non-fat milk diluted with TBST (Tris–HCl 20 mmol/l, NaCl 150 mmol/l, pH 7.5, 0.1% Tween 20) at room temperature for 60 min. Then PVDF membranes were probed with primary antibody overnight at 4 °C. After washing with TBST for 3 × 5 min, the membranes were incubated with HRP-conjugated secondary antibody at room temperature for 90 min, followed by ECL detection. The density of the bands was measured using the Image J software (1.48 μ, USA), and GAPDH was used as internal control.
qRT-PCR
Total RNA was extracted with Trizol reagent (Invitrogen) and 2 mg RNA was applied for gene-specific RT-PCR using a Onestep RT-PCR Kit (Qiagen) according to manufacturer’s instructions. The PCR primer sequences were as follows:
MRP1 forward 5′-TTGCCGTCTACGTGACCATT-3′ and reverse:5′-AGGCGTTTGAGGGAGACACT-3′;
MDR1 forward 5′-CTTGGCAGCAATTAGAAC-3′ and reverse: 5′-TCAGCAGGA AAGCAGCAC-3′;
LRP forward 5′-TATGTGCCATCTGCCAAAGT-3′ and reverse: 5′-CATGTAGGTGCTTCCAATCA-3′;
GST forward 5′-TTCCTGTGGCATAATGTGAT-3′and reverse: 5′-CTGATTCAAAGGCAAATCTC-3′;
GAPDH forward 5′- CCACCCATGGCAAATTCCATGGCA-3′ and reverse: 5′- TCTAGACGGCAGGTCAGGTCCACC-3′.
PCR reactions were performed on Applied Biosystems 7500 real-time PCR system with the following conditions: 94 °C, 12 min for 1 cycle; then 94 °C, 40 s; 75 °C, 1 min, 59 °C, 40 s and 72 °C, 1 min for 32 cycles. GAPDH mRNA was used as an internal control. And the levels for each gene were counted by standardizing the quantified mRNA amount to the GAPDH mRNA.
Statistical analysis
All data were expressed as the mean ± SD. Comparisons between 2 groups were analyzed using Student’s t test and among three groups by ANOVA test using the least significant difference test (SPSS 17.0). Differences were considered statistically significant at a p value of < 0.05.
Results
Rh2 could reverse drug resistance in CRC cells
To investigate the effect of Rh2 on drug resistance in CRC cells, we first established drug-resistant CRC cells (HCT-8/5-FU and LoVo/5-FU cells). As shown in Fig. 1b, c, compared to the half-maximal inhibitory concentration of 5-FU in the parental HCT-8 (IC50 2.82 ± 0.12 μM) and LoVo (IC50 12.3 ± 1.14 μM) cells, HCT-8/5-FU (IC50 28.8 ± 3.22 μM) and LoVo/5-FU (IC50 62.7 ± 4.24 μM) cells were more resistant against 5-FU. And cytotoxicity test of RH2 (Fig. 1d, e) showed that 0-20 μM RH2 does not have toxic effect on CRC cell growth. Then treatment with Rh2 (20 μM) could remarkably reverse 5-FU resistance in HCT-8/5-FU and LoVo/5-FU cells (Fig. 1f, g), while it does not have significant impact on the growth of HCT-8 and LoVo cells (Fig. 1h, i). Furthermore, the resistance reversion effect of Rh2 on the IC50 of 5-FU in HCT-8/5-FU and LoVo/5-FU cells was calculated, as shown in Table 1; the IC50 of 5-FU in HCT-8/5-FU and LoVo/5-FU cells remarkably decreased to 13.7 ± 0.19 and 34.3 ± 0.26 μM by pretreatment with 20 μM Rh2.
Rh2 induces cell cycle arrest and cell apoptosis in drug-resistant CRC cells
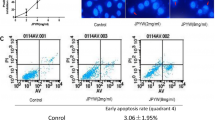
Imbalance between cell proliferation and apoptosis was thought to be related to the progression of cancer and its drug resistance, so we set out to investigate whether RH2 could inhibit the growth of drug-resistant CRC cells. Cell cycle analysis showed that treatment with 5, 10, 20 μM Rh2 could dramatically increase the percentage of cells in the G1-phase and decrease the percentage of cells in the S-phase in both HCT-8/5-FU and LoVo/5-FU cells (Fig. 2a, b). Furthermore, we explored whether pro-apoptosis participated in Rh2-induced anti-proliferative effect. In Fig. 2c, d, Annexin-V-FITC/PI flow cytometry analysis revealed that the number of early apoptotic HCT-8/5-FU and LoVo/5-FU cells induced by 5-FU was evidently higher after treatment with Rh2 at the concentrations of 10 and 20 μM. The data hint that Rh2 sensitized CRC cells to 5-FU by inhibiting proliferation and promoting 5-FU-induced apoptosis.
Rh2 induces cell cycle arrest and cell apoptosis in drug-resistant CRC cells. Drug-resistant CRC cells were pretreated with 4 μM(HCT-8/5-FU cells) or 8 μM(LoVo/5-FU cells) 5-FU for 30 min, then the cells were incubated with varying concentrations of Rh2 for 24 h. Cell cycle distribution of HCT-8/5-FU (a) and LoVo/5-FU (b) were detected by PI staining using flow cytometry. Cell apoptosis of HCT-8/5-FU (c) and LoVo/5-FU (d) were measured by Annexin-V/PI double staining using flow cytometry. Bars represent mean ± SD, n = 6. *p < 0.05 vs. 0 μM
The effects of Rh2 on the expression of cell cycle and apoptosis-related molecules in drug-resistant CRC cells
To investigate the possible mechanism of Rh2 on proliferation, cell cycle and apoptosis, we tested the effect of Rh2 on several cell cycle and apoptosis-related proteins. As shown in Fig. 3, treatment with Rh2 at the concentrations of 10 μM and 20 μM markedly decreased the protein levels of cyclin D1, CDK2, p-Rb and Bcl-2, while increasing the protein levels of cleaved-caspase 3 in both HCT-8/5-FU and LoVo/5-FU cells, which suggested that Rh2 induced cell cycle arrest and apoptosis by regulation of cell cycle and apoptosis-related proteins. In addition, Rh2 up-regulated the expression of p-IκB, suggesting the association of Rh2 with NF-κB activity.
The effects of Rh2 on the expression of cell cycle and apoptosis-related proteins in drug-resistant CRC cells. Drug-resistant CRC cells were pretreated with 4 μM (HCT-8/5-FU cells) or 8 μM (LoVo/5-FU cells) 5-FU for 30 min, then the cells were incubated with varying concentrations of Rh2 for 24 h. The protein expression of cyclin D1, CDK2, p-Rb/Rb, Bcl-2, cleaved-caspase3 and p-IκB-α/IκB-α in HCT-8/5-FU (a) and LoVo/5-FU (b) cells, were determined by Western blot. Bars represent mean ± SD, n = 6. *p < 0.05 vs. 0 μM
Rh2 inhibits the migration and EMT process in drug-resistant CRC cells
To detect whether Rh2 affects the migration and EMT process in drug-resistant CRC cells, transwell migration assay was performed to assess the proportion of cells that migrated to the lower chamber treated with or without Rh2. The results showed that the number of migrated HCT-8/5-FU and LoVo/5-FU cells was markedly decreased after treatment with Rh2 at the concentrations of 10 and 20 μM (Fig. 4a, b), which indicated that Rh2 might inhibit cell migration in HCT-8/5-FU and LoVo/5-FU cells. Furthermore, we examined the effect of Rh2 on the protein expression of EMT-related molecules by Western blot assay. Rh2 at the concentrations of 10 and 20 μM lead to up-regulation of the epithelial marker E-cadherin, and down-regulation of the mesenchymal marker N-cadherin, Vimentin and MMP9 at protein levels in HCT-8/5-FU and LoVo/5-FU cells (Fig. 4c, d). Taken together, our results indicated that Rh2 impaired the migration and EMT process in drug-resistant CRC cells.
The effects of Rh2 on migration and the EMT process in drug-resistant CRC cells. Drug-resistant CRC cells were pretreated with 4 μM (HCT-8/5-FU cells) or 8 μM (LoVo/5-FU cells) 5-FU for 30 min, then the cells were incubated with varying concentrations of Rh2 for 24 h. The migration of HCT-8/5-FU (a) and LoVo/5-FU (b) cells were assessed by transwell assay. The protein expression of EMT-related molecules: E-cadherin, N-cadherin, Vimentin and MMP9 was determined by Western blot in HCT-8/5-FU (c) and LoVo/5-FU (d) cells. Bars represent mean ± SD, n = 6. *p < 0.05 vs. 0 μM
Rh2 attenuates the expression of MRP1, MDR1, LRP and GST in drug-resistant CRC cells
To further investigate the underlying mechanism of Rh2 on drug resistance in CRC cells, we examined the expression of drug-resistance gene MRP1, MDR1, LRP and GST. Results of qRT-PCR and Western blot (Fig. 5a, c, d) showed that HCT-8/5-FU cells had a higher expression of MRP1, MDR1, LRP and GST in both mRNA level and protein level than its parental HCT-8 cells, while treatment with Rh2 partly reverses this high expression of MRP1, MDR1, LRP and GST in HCT-8/5-FU cells. And similar results were observed in LoVo/5-FU and LoVo cells (Fig. 5b, e, f).
The effects of Rh2 on the protein expression of drug-resistant MRP1, MDR1, LRP and GST. Drug-resistant CRC cells were pretreated with 4 μM (HCT-8/5-FU cells) or 8 μM (LoVo/5-FU cells) 5-FU for 30 min, then the cells were incubated with varying concentrations of Rh2 for 24 h. The mRNA expression of MRP1, MDR1, LRP and GST in HCT-8/5-FU (a) and LoVo/5-FU (b) cells, were determined by qRT-PCR. The protein expression of MRP1, MDR1, LRP and GST in HCT-8/5-FU (c, d) and LoVo/5-FU (e, f) cells, was determined by Western blot. Bars represent mean ± SD, n = 6. *p < 0.05 vs. 0 μM; # p < 0.05 vs. HCT-8 or LoVo cells
Discussion
Recently, many groups have confirmed that the use of herbal medicine improved therapeutic effects and reversed drug resistance caused by the long-term chemotherapy. A synergistic effect was observed on the prostate cancer mode treated with Rh2 and paclitaxel or mitoxantrone [17]. Also, there are evidences that suggested that Rh2 might be connected to drug resistance of cancer therapy [18]. In the present study, we confirmed that Rh2 played a protective role in CRC drug resistance. Then we designed a series of experiments to explore its involving mechanism. Consistent with previous reports, low doses of Rh2 inhibited HCT-8/5-FU and LoVo/5-FU cells proliferation and migration, promoted cells apoptosis, and adversely affected 5-FU-resistant tumor therapy.
In sorting out the molecular mechanisms of the anti-drug-resistance activity of Rh2, we need to take into account its known actions on cell abnormal proliferation and apoptosis [19, 20]. In the present study, we found that Rh2 could inhibit proliferation and promote apoptosis of 5-FU-resistant HCT-8 and LoVo cells. As known, cell proliferation always related to cell cycle, and our data also showed that Rh2 inhibited transition of G0/G1 to G2/M, avoiding cell repair for DNA damage induced by 5-FU. To confirm the possible mechanism of Rh2 on regulation of cell cycle and apoptosis, we then investigated the effects of Rh2 on cell cycle and apoptosis-related molecules. The cyclins, CDKs and phosphorylation of Rb have been proved to be pivotal regulators of the G1/S transition of the cell cycle [21, 22]. Bcl-2, an anti-apoptotic protein, and caspase3, a downstream effective protein of apoptotic pathway, are considered to be related to poor response to conventional cancer treatment [23,24,25]. In this paper, we found that treatment with Rh2 at the concentrations of 10 and 20 μM markedly decreased the protein levels of cyclin D1, CDK2, p-Rb and Bcl-2, while increasing the protein levels of cleaved-caspase 3 in both HCT-8/5-FU and LoVo/5-FU cells, which indicted that Rh2 induced cell cycle arrest and apoptosis by regulation of cyclin D1, CDK2, p-Rb, Bcl-2 and caspase 3. In addition, Rh2 up-regulated the expression of p-IκB, suggesting the association of Rh2 with NF-κB activity.
High migration and invasion characteristic is always thought to contribute to the high mortality rate of malignant CRC, so inhibition of cell migration could be vital for controlling drug resistance [26, 27]. Transwell assay showed that Rh2 dramatically inhibited the migration of HCT-8/5-FU and LoVo/5-FU cells. Furthermore, we examined the effect of Rh2 on the protein expression of EMT-related molecules by Western blot assay. Rh2 at the concentrations of 10 and 20 μM led to up-regulation of the epithelial marker E-cadherin, and down-regulation of the mesenchymal marker N-cadherin, Vimentin and MMP9 at protein levels in HCT-8/5-FU and LoVo/5-FU cells. Taken together, our results indicated that Rh2 impaired the migration by restraining EMT process in drug-resistant CRC cells.
Exceptional drug-metabolizing could also be a reason for chemotherapeutic resistance of CRC [28]. Multi-drug resistant protein1 (MRP1) is an important ATP-binding cassette transporter protein, which affects the intracellular drug concentration through the alteration of drug influx or efflux. MRP1 was reported to mediating multidrug resistance in various cancers, such as breast and ovarian cancer [29,30,31]. Over-expression of MDR1 could also increase the level of drug afflux and limit the efficacy of chemotherapy [32, 33]. Lung resistance protein (LRP) was identified as a major vault protein (MVP) implicated in drug resistance of cancer cells in a cell-type-dependent manner by mediating drug efflux from the nucleus to the cytosol [34, 35]. Glutathione-S-transferases (GSTs) are a family of Phase II detoxification enzymes. It could detoxify chemotherapeutic drugs through the GSH-conjugate export pump, thus contributing to drug resistance [36, 37]. Thus, we analyzed the expression of these drug resistance-related proteins in the present study. Results of qRT-PCR and Western blot show that HCT-8/5-FU cells had a higher expression of MRP1, MDR1, LRP and GST in both mRNA level and protein level than its parental HCT-8 cells, while treatment with Rh2 partly reverses this high expression of MRP1, MDR1, LRP and GST in HCT-8/5-FU cells. And the similar results were observed in LoVo/5-FU and LoVo cells.
In conclusion, our study demonstrated that Ginsenoside Rh2 could effectively reverse drug-resistance in human colorectal carcinoma cell and its mechanism involved the prevention of cell proliferation and migration, the promotion of cell apoptosis and the alteration of drug-resistance genes, which suggested that Ginsenoside Rh2 might act as a promising candidate for drug resistance in human colorectal carcinoma chemotherapy.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Seow HF, Yip WK, Fifis T. Advances in targeted and immunobased therapies for colorectal cancer in the genomic era. Onco Targets Ther. 2016;9:1899–920.
Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol. 2014;20:9775–827.
De Mattia E, Cecchin E, Toffoli G. Pharmacogenomics of intrinsic and acquired pharmacoresistance in colorectal cancer: toward targeted personalized therapy. Drug Resist Updat. 2015;20:39–70.
Xiang Z, Kang QJ, Xiang X. Gene and protein expression in the oxaliplatin-resistant HT29/L-OHP human colon cancer cell line. Genet Mol Res. 2015;14:11013–22.
Wu S, Wen F, Li Y, et al. PIK3CA and PIK3CB silencing by RNAi reverse MDR and inhibit tumorigenic properties in human colorectal carcinoma. Tumour Biol. 2016;37:8799–809.
Khaleel SA, Al-Abd AM, Ali AA, et al. Didox and resveratrol sensitize colorectal cancer cells to doxorubicin via activating apoptosis and ameliorating P-glycoprotein activity. Sci Rep. 2016;6:36855.
Sui H, Zhou LH, Zhang YL, et al. Evodiamine Suppresses ABCG2 Mediated Drug Resistance by Inhibiting p50/p65 NF-kappaB Pathway in Colorectal Cancer. J Cell Biochem. 2016;117:1471–81.
Jin X, Che DB, Zhang ZH, et al. Ginseng consumption and risk of cancer: a meta-analysis. J Ginseng Res. 2016;40:269–77.
Xie J, Shao J, Lu Y, et al. Separation of ginseng active ingredients and their roles in cancer metastasis supplementary therapy. Curr Drug Metab. 2013;14:616–23.
Xu FY, Shang WQ, Yu JJ, et al. The antitumor activity study of ginsenosides and metabolites in lung cancer cell. Am J Transl Res. 2016;8:1708–18.
Dong H, Bai LP, Wong VK, et al. The in vitro structure-related anti-cancer activity of ginsenosides and their derivatives. Molecules. 2011;16:10619–30.
Wu N, Wu GC, Hu R, et al. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacol Sin. 2011;32:345–53.
Park HM, Kim SJ, Kim JS, et al. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food Chem Toxicol. 2012;50:2736–41.
Xia T, Wang JC, Xu W, et al. 20S-Ginsenoside Rh2 induces apoptosis in human Leukaemia Reh cells through mitochondrial signaling pathways. Biol Pharm Bull. 2014;37:248–54.
Han S, Jeong AJ, Yang H, et al. Ginsenoside 20(S)-Rh2 exerts anti-cancer activity through targeting IL-6-induced JAK2/STAT3 pathway in human colorectal cancer cells. J Ethnopharmacol. 2016;194:83–90.
Xie X, Eberding A, Madera C, et al. Rh2 synergistically enhances paclitaxel or mitoxantrone in prostate cancer models. J Urol. 2006;175:1926–31.
Zhou B, Xiao X, Xu L, et al. A dynamic study on reversal of multidrug resistance by ginsenoside Rh(2) in adriamycin-resistant human breast cancer MCF-7 cells. Talanta. 2012;88:345–51.
Yang J, Yuan D, Xing T, et al. Ginsenoside Rh2 inhibiting HCT116 colon cancer cell proliferation through blocking PDZ-binding kinase/T-LAK cell-originated protein kinase. J Ginseng Res. 2016;40:400–8.
Chen Y, Liu ZH, Xia J, et al. 20(S)-ginsenoside Rh2 inhibits the proliferation and induces the apoptosis of KG-1a cells through the Wnt/beta-catenin signaling pathway. Oncol Rep. 2016;36:137–46.
Thwaites MJ, Cecchini MJ, Passos DT. Interchangeable roles for E2F transcriptional repression by the retinoblastoma protein and p27KIP1-cyclin-dependent kinase regulation in cell cycle control and tumor suppression. Mol Cell Biol. 2017;37:e00561–616.
Wang N, Wei H, Yin D, et al. MicroRNA-195 inhibits proliferation of cervical cancer cells by targeting cyclin D1a. Tumour Biol. 2016;37:4711–20.
Xiong L, Tang Y, Liu Z, et al. BCL-2 inhibition impairs mitochondrial function and targets oral tongue squamous cell carcinoma. Springerplus. 2016;5:1626.
Gao J, Yan Q, Liu S, et al. Knockdown of EpCAM enhances the chemosensitivity of breast cancer cells to 5-fluorouracil by downregulating the antiapoptotic factor Bcl-2. PLoS One. 2014;9:e102590.
Vegran F, Boidot R, Oudin C, et al. Overexpression of caspase-3 s splice variant in locally advanced breast carcinoma is associated with poor response to neoadjuvant chemotherapy. Clin Cancer Res. 2006;12:5794–800.
Ueda M, Iguchi T, Nambara S, et al. Overexpression of transcription termination factor 1 is associated with a poor prognosis in patients with colorectal cancer. Ann Surg Oncol. 2015;22(Suppl 3):S1490–8.
Gao HX, Yan L, Li C, et al. miR-200c regulates crizotinib-resistant ALK-positive lung cancer cells by reversing epithelial-mesenchymal transition via targeting ZEB1. Mol Med Rep. 2016;14:4135–43.
Schwab R, Micsik T, Szokoloczi O, et al. Functional evaluation of multidrug resistance transporter activity in surgical samples of solid tumors. Assay Drug Dev Technol. 2007;5:541–50.
Schmitt SM, Stefan K, Wiese M. Pyrrolopyrimidine derivatives and purine analogs as novel activators of Multidrug Resistance-associated Protein 1 (MRP1, ABCC1). Biochim Biophys Acta. 2017;1859:69–79.
Torres A, Vargas Y, Uribe D, et al. Adenosine A3 receptor elicits chemoresistance mediated by multiple resistance-associated protein-1 in human glioblastoma stem-like cells. Oncotarget. 2016;7:67373–86.
Gao M, Miao L, Liu M, et al. miR-145 sensitizes breast cancer to doxorubicin by targeting multidrug resistance-associated protein-1. Oncotarget. 2016;7:59714–26.
Samanian S, Mahjoubi F, Mahjoubi B, et al. MDR1 gene polymorphisms: possible association with its expression and clinicopathology characteristics in colorectal cancer patients. Asian Pac J Cancer Prev. 2011;12:3141–5.
Januchowski R, Sterzynska K, Zaorska K, et al. Analysis of MDR genes expression and cross-resistance in eight drug resistant ovarian cancer cell lines. J Ovarian Res. 2016;9:65.
Chen YL, Yang TY, Wu CL, et al. Mechanisms underlying lung resistance-related protein (LRP)-mediated doxorubicin resistance of non-small cell lung cancer cells. Chin J Physiol. 2016;59:331–47.
Zhang W, Zhou H, Yu Y, et al. Combination of gambogic acid with cisplatin enhances the antitumor effects on cisplatin-resistant lung cancer cells by downregulating MRP2 and LRP expression. Onco Targets Ther. 2016;9:3359–68.
Bernig T, Ritz S, Brodt G, et al. Glutathione-S-transferases and chemotherapy resistance of Hodgkin’s lymphoma cell lines. Anticancer Res. 2016;36:3905–15.
Liu S, Liu F, Jia H, et al. A glutathione S-transferase gene associated with antioxidant properties isolated from Apis cerana cerana. Naturwissenschaften. 2016;103:43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Gw., Liu, Yh., Jiang, Gs. et al. The reversal effect of Ginsenoside Rh2 on drug resistance in human colorectal carcinoma cells and its mechanism. Human Cell 31, 189–198 (2018). https://doi.org/10.1007/s13577-017-0189-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-017-0189-3