Cryptotanshinone (CPT), which is an important active ingredient of herbs, has shown great value for research. In particular, CPT exerts antitumor effects on various types of cancer; however, there are relatively few studies of CPT on gastric cancer cells. The objectives of this study were to investigate how CPT affects apoptosis in 5-fluorouracil-resistant SGC-7901 gastric cancer cells (SGC-7901/5-FU cells) and the molecular mechanism underlying its action. In this study, SGC-7901/5-FU cells were treated with 5-fluorouracil (5-FU) and CPT and the viability of the cells was assessed by CCK8 assay. Additionally, cellular apoptosis rates were evaluated using immunofluorescence and flow cytometry. Related gene expression was evaluated using Quantitative Real-time PCR methods and Western Blot, respectively. CPT inhibited SGC-7901/5-FU cell growth. The immunofluorescence results showed that CPT caused nuclear shrinkage in the cells. Quantitative Real-time PCR and Western Blot results also showed CPT decreased the expression of Mcl-1, Bcl-xl and Bcl-2 levels, and increased the expression of Bax. We demonstrated that CPT can inhibit the growth of SGC-7901/5-FU cells, and the mechanism may be related to the inhibition of the JAK2/STAT3 pathway. Additionally, CPT increased the inhibitory effect of 5-fluorouracil on SGC-7901/5-FU cells, an effect that correlated with changes in cellular resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Gastric cancer (GC) is a common malignancy worldwide. Globally, the survival rate in patients with stomach cancer has increased as a result of advancements in medical technology; however, whether this increased survival has had beneficial effects worldwide remains controversial [1]. Most gastric cancer patients miss the best time for treatment by the time they are diagnosed because the early symptoms of gastric cancer (discomfort such as acid reflux, belching or slight epigastric pain) are very similar to those of gastric ulcer and gastritis. Behavioral, dietary, metabolic and environmental factors all increase the incidence of gastric cancer [2]. Chemotherapy is the primary treatment and in some cases the only treatment for people with advanced-stage gastric cancer who are unable to receive surgical treatment. However, there are numerous issues with the present use of clinical medications, the most serious of which is the emergence of drug resistance. 5-fluorouracil is a drug commonly used in clinical chemotherapy and has developed resistance problems following its widespread use. Resistance to chemotherapy drugs is an essential element that leads to lower quality of life for patients. For this reason, the discovery and exploration of new chemotherapeutic agents or drug combinations that can be used to treat gastric cancer are important directions for ongoing medical research.
The JAK2/STAT3 signaling pathway acts as the vital chain of intracellular signaling. It regulates several biological processes of cellular activity, including cell proliferation and apoptosis. Scholars are deeply interested in JAK2/STAT3 because of its function in cell growth. Sustained activation of this pathway is inextricably linked with tumorigenesis and drug resistance and is observed in many solid tumors [3]. When blood samples from tumor patients are collected for testing, the results usually show high JAK2 expression;, JAK2 could be a promising developmental target for cancer therapy [4]. JAK2 often performs its biological functions in a phosphorylated form. When JAK2 is phosphorylated, downstream signaling pathways are activated. STAT3 is activated by phosphorylated JAK2 and translocates to the nucleus, leading to the transcription of proliferation-related genes in tumor cells [5]. According to reports, the activation of STAT3 in gastric cancer tissues is usually accompanied by changes in the production of cytokines such as IL-6. Various studies have demonstrated that the proliferation of cancer cells is regulated by JAK2 and STAT3—for example, STAT3 is phosphorylated and regulates the viability of cells in myeloproliferative neoplasms (MPNs) [6]. Hence, we have placed greater emphasis on the role played by the JAK2/STAT3 signaling pathway in GC. Blocking it may influence GC development.
For the past few years, many studies have focused on herbs in the hope of finding more effective natural agents with anticancer properties. Cryptotanshinone (CPT) is a diterpenoid quinone compound that is extracted from the rhizome of Salvia miltiorrhiza of Labiatae. It has been found to exhibit a variety of biological properties, such as antitumor, antioxidant, and antibacterial abilities, and it does not cause apparent cytotoxicity [7]. Apoptosis is an active, genetically determined, automatic end-of-life process, and it is also known as programmed cell death. The Bcl2 protein family is a group of proteins that play a role in the regulation of induced mitochondrial apoptosis and are major regulators of apoptotic signaling. In particular, changes in the activities of both Bcl-2 and Bax affect cell growth. Previous studies have shown that CPT can induce apoptosis in breast cancer [8]. Recent studies have suggested that CPT also inhibits the proliferation of gastric cancer cells to a certain extent, but the specific mechanism remains unclear. There have been reports that CPT is a potent inhibitor of STAT3 and can inhibit STAT3 activation [9], suggesting an interrelationship between CPT and the JAK2/STAT3 signaling pathway.
To date, there have been few studies on the relationship between CPT and GC, and the molecular processes are unclear. Therefore, this article notes that CPT is involved in the inhibition of GC cell growth by removing the JAK2/STAT3 pathway.
EXPERIMENTAL
Drugs
Cryptotanshinone (purity ≥ 98%, CPT) and 5-fluorouracil (purity ≥ 98%, 5-FU), which were purchased from Shanghai Yuanye Biotechnology Co., Ltd, were used for subsequent experiments. Both 5-FU and CPT were dissolved in dimethyl sulfoxide (DMSO) to form the stock solution. The stock liquor concentration was set to 50 mmol/L and diluted 1000-fold before being used in cell experiments. The primary antibodies that were used in western blotting were from Abcam, and the second antibodies were from ZSGB-BIO.
Cell culture
We are grateful to Fudan University for providing the 5-fluorouracil-resistant gastric cancer cells (SGC-7901/5-FU) for this experiment. The cells were cultured with RPMI-1640 (HyClone) in a 37°C cell incubator containing 5% CO2. CO2 is used to maintain the acid-base balance of the culture medium. When 10% fetal bovine serum (FBS, BI) and penicillin/streptomycin (100 U/mL, 100 μg/mL, Beyotime) were added, the medium was more favorable for cell growth. After the cells had grown to a density of ~80%, they were passaged at 1:3. After two passages, the cells grew stably and were used in succeeding experiments.
CCK8 Assay
The experiment was performed based on the protocol from the Cell Counting Kit-8 Reagent Kit (CCK8, BIOSS). Briefly, cells from the logarithmic growth phase are suspended, made into a cell suspension and counted. Cells were spread out in the center of a 96-well plate at a density of 1×105 per well. The marginal wells were filled with sterile PBS. The cells were incubated in 5% CO2 overnight at 37°C. The medium was then discarded and cells were cultured in fresh RPMI 1640 medium supplemented with different concentrations of CPT (0, 20, 40, 60, 80, 100 μM) or 5-fluorouracil (0, 6.25, 12.5, 25, 50, 100 μM). The experiment was repeated three times for each drug concentration. The fresh medium served as a negative control group. After different incubation times (12, 24, 48, and 72 h), 10 μl of CCK8 was added to each well and incubation was continued for 4 h. The absorbance of each well was measured at an OD of 450 nm using enzyme markers. Based on the assay absorbance values, standard curves were made, half inhibitory concentrations of CPT and 5-FU against SGC-7901/5-FU cells were estimated, and subsequent experiments were performed.
Immunofluorescence Staining
Cells were digested with trypsin suspended in a complete medium, and then seeded in 12-well plates to which glass slides had been added. The cells were treated with 5-FU (25 μM), CPT (60 μM), or 5-FU (25 μM) combined with CPT (60 μM), and fresh medium was used as a control. SGC7901/5-FU cells were fixed in 4% paraformaldehyde after 24 h, the fixative was removed by washing with PBS precooled at 4°C in advance, and then stained with 4′-6-diamidino-2-phenylindole (DAPI). DAPI (P0131) was purchased from Beyotime (China). The coloring solution was added dropwise onto the center of the slides. The stained cell slides stood for 10 minutes at room temperature protected from light. The cell glass slides were then observed and photographed with an OLYMPUS BX51 microscope (Germany). By microscopic observation, karyopyknosis and dense staining could be observed in some of the cells.
Flow Cytometric Assay
A total of 1×106 SGC-7901/5-FU cells were inoculated in a 6-well plate and 2 ml of fresh medium was added to each well. After cell adherence, the medium was discarded. The medium was changed to an RPMI 1640 medium supplemented in 5-FU (25 μM), CPT (60 μM), or the combination of CPT (60 μM) with 5-FU (25 μM), and fresh RPMI 1640 medium was used as a control. Three replicates were set up for each experimental group. The collected cells were washed twice with PBS that had been precooled at 4°C. Cells were trypsinized with EDTA-free Trypsin solution (C0205) that was obtained from Beyotime (China). PBS was used to gently blow the cells twice along the wall of the centrifuge tube. The cells were then transferred to a new centrifuge tube and 500 μl of binding buffer was added to suspend the cells. The cells were mixed with 5 μl annexin V-FITC, and 5 μl of propidium iodide and reacted for a quarter of an hour under light-proof conditions. Apoptosis was analyzed with an FC500 flow cytometry analyzer (USA) and the apoptosis rate was calculated using FlowJo analysis software.
Quantitative Real-time PCR Assay (qPCR)
After treating the cells for 24 h as previously described, total cellular RNA was extracted according to the instructions for use. TRIzol reagent was purchased from Sangon Biotech. Reverse transcription of RNA into cDNA was achieved following instructions of the reverse transcription kit (Servicebio, China). qPCR was performed using SYBR Green qPCR Master Mix (Servicebio, China). The reaction conditions were as follows: the first step was predenaturation at 95°C for 30 sec, the second step was denaturation at 95°C for 10 sec and annealing at 60°C for 30 sec, the second step consisted of 40 cycles. The melting curve was then plotted according to the instrument settings and the mRNA expression levels were quantified using the American ABI STEP ONE PCR instrument. The primer sequences (5′ to 3′) were as follows: JAK2 forward, TGCAGTCTGCCTTCTACACA; JAK2 reverse, TGACCACTGAATTCCACCGT; STAT3 forward, GTGGGAAGAATCACGCCTTC; STAT3 reverse, AGATCCTGCACTCTCTTCCG; Bcl-2 forward, GCGGCCTCTGTTTGATTTCT; Bcl-2 reverse, TCACTTGTGGCCCAGATAGG; Bcl-XL forward, CCCAGGGACAGCATATCAGA; Bcl-XL reverse, GAAGAGTGAGCCCAGCAGAA; Mcl-1 forward, CCAAGAAAGCTGCATCGAACCAT; Mcl-1 reverse, CAGCACATTCCTGATGCCACCT, Bax forward, TCAGGATGCGTCCAAGAAG; Bax reverse, TGTGTCCACGGCGGCAATCATC; Survivin forward, TTTGTCTTGAAAGTGGCACC; Survivin reverse, TCTTCCTCCCTCACTTCTCA; MDR1 forward, GCTGTCAAGGAAGCCAATGCCT; MDR1 reverse, TGCAATGGCGATCCTCTGCTTC; GAPDH forward, TTCCACCCATGGCAAATTCC; and GAPDH reverse, ATCTCGCTCCTGGAAGATGG. GAPDH was used as the internal reference.
Western blot analysis
After treating the cells for 24 h as previously described, total protein was extracted with the SDS lysis buffer (P0013G, Beyotime, China). Protease inhibitors and phosphatase inhibitors purchased from Beyotime Biotechnology Co., Ltd. were added to lysates to prevent protein degradation. After washing the cells twice with PBS, the configured lysis solution was added and the cells were left to stand for ten minutes to allow sufficient lysis. Then, the lysed cells were transferred to a new 1.5 ml centrifuge tube. The cells were centrifuged using a high-speed centrifuge at 4°C at 12,000 rpm. The supernatant obtained via centrifugation was the protein sample used for the experiments. Protein concentration detection was performed using the Enhanced BCA Protein Detection Kit (P0010S, Beyotime, China). After quantification, the proteins were denatured by boiling with a protein loading buffer.
Approximately 20 μg of total protein was separated by 8%-12% SDA-PAGE and transferred onto a nitrocellulose membrane (EMD Millipore, USA). After transfer, the PVDF membrane was marked to allow observation of the protein location. PVDF
membranes were soaked in 5% skimmed milk at room temperature for 1 h. At the end of blocking, the membranes were washed three times with TBST and incubated with the primary antibodies for 10 – 12 h at 4°C to try to get the strips to bind as well as possible to the antibodies. Then, the secondary antibodies were incubated for 1 h at ambient temperature after washing it with TBST. The protein bands were detected, scanned and imaged via an enhanced chemiluminescence system (ECL, BLT GelView 6000Plus, China), and the band intensities were analyzed using Image J. The primary antibodies and concentrations we chose were as follows: anti-JAK2 (1:5000, ab108596), anti-STAT3 (1:1000, ab68153), anti-Bcl-2 (1:1000, ab196495), anti-Bcl-XL (1:1000, ab32370), anti-Mcl-1 (1:2000, ab32087), anti-Bax (1:500, ab32503), anti-Survivin (1:1000, ab134170), anti-p-JAK2 (1:4000, ab195055), anti-p-STAT3 (1:1000, ab76315), anti-P-gp (1:5000, ab168337) and anti-GAPDH (1:10000, ab181602). The secondary antibody we used was goat anti-rabbit IgG (H+L)-HRP (1:10000, ZB-2301).
Statistical analysis
All data analysis and mapping were performed using Prism 9 software. All data represent three or more independent experiments and are expressed as the mean ± SD. Levene’s method was used to test the homogeneity of variance of each group. If the variances were homogeneous, one-way analysis of variance and t-tests were used to compare the means of the samples. If the variances were not consistent, a nonparametric test was used.
RESULTS
CPT Inhibits the Proliferation of SGC-7901/5-FU Cells
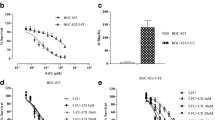
SGC-7901/5-FU cells were cultured with 6.25, 12.5, 25, 50, or 100 μM 5-FU for 12, 24, 48, or 72 h at 37°C. Furthermore, resistant SGC-7901 cells were cultured with 20, 40, 60, 80, and 100 μM CPT for the same periods. The viability of cells treated with each drug was determined by CCK8 assay. The experimental results showed that 5-FU and CPT could inhibit the growth of SGC-7901/5-FU cells (Fig. 1A, B). Cell viability decreased over time. After 24 h, drugs exerted the most significant inhibitory effect on cell viability. When the 5-FU concentration was 26.94 μM (Fig. 1C), the viability of cells was reduced to 50%. This is at least 5 times higher than the normal dosing concentration of SGC-7901 cells; therefore, 25 μM, which is close to the IC50, was selected as the concentration of 5-FU to be used in subsequent experiments. Using the same approach, a concentration of CPT of approximately 63.45 μM resulted in a 50% survival rate for SGC-7901/5-FU cells (Fig 1D); thus, 60 μM was determined to be the model CPT concentration. After measuring the model concentrations of CPT and 5-FU, the two drugs were administered to the SGC-7901/5-FU cells.
The cell viability of SGC-7901/5-FU cells was detected by CCK-8 assay. (A) Cell viability of SGC-7901/5-FU cells after treatment with 5-FU. (B) Cell viability of SGC-7901/5-FU cells after treatment with CPT. (C) Effect of different concentrations of 5-FU on the cell viability of SGC-7901/5-FU cells for 24 h. (D) Effect of different concentrations of CPT on the cell viability of SGC-7901/5-FU cells for 24 h.
CPT Causes the Apoptosis in SGC-7901/5-FU Cells
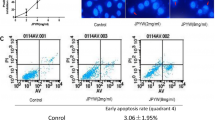
Immunofluorescence experiments and flow cytometry were used to further determine whether CPT can induce apoptosis. In the 5-FU group, cells were processed with 25 μM 5-FU, whereas in the CPT group, cells were processed with 60 μM CPT and in the 5-FU+ CPT group, cells were processed with 25 μM 5-FU and 60 μM CPT. Culture medium was used as a control and groups of cells were incubated for 24 h. Immunofluorescence experiments showed an increase in the number of apoptotic cells in all other groups compared to the control group, particularly the CPT and 5-FU + CPT groups (Fig. 2A). The number of apoptotic cells in the 5-FU+CPT group was significantly increased compared with the 5-FU group. The flow cytometry experimental results showed that CPT increased the partial necrosis and early apoptosis of SGC-7901/5-FU cells (Fig. 2B). We selected apoptotic cells for statistical analyses. The results showed that CPT significantly increased apoptosis (Fig. 2C). Apoptosis was increased in the combined group compared to the 5-FU group (P < 0.01). These results suggest that SGC-7901/5-FU cell apoptosis could be induced by CPT. Moreover, the treatment efficiency of 5-FU was improved.
Effect of CPT on apoptosis of SGC-7901/5-FU cells. (A) Apoptotic cell morphology was detected by DAPI. (B) Apoptosis was detected by Annexin V-FITC/PI kit. (C) Statistical chart of the number of apoptotic SGC-7901/5-FU cells. **p < 0.01, compared with the control group. ****p < 0.0001, compared with the control group. ###p < 0.001, compared with the 5-FU group.
To check the results further, we used qPCR to measure the expression of apoptosis-related genes (Mcl-1, Bcl-XL, Bcl-2, Bax and Survivin) in SGC-7901/5-FU cells. The drug treatment groups included a 5-FU group (25 μM), a CPT group (60 μM), and a combination group (25 μM 5-FU + 60 μM CPT), and the control group was treated with culture media. The results suggest that CPT inhibited the mRNA expression of Mcl-1, Bcl-XL, Bcl-2 and Survivin (Fig. 3). Other apoptosis-related proteins (Bcl-XL, Mcl-1 and Survivin) also showed a trend of decreased expression (Fig. 4). Notably, we observed a more significant decrease in anti-apoptotic protein expression after the combination treatment compared to 5-FU alone (P < 0.05). The increase in apoptotic protein expression was also more pronounced (P < 0.05). Moreover, CPT enhanced the mRNA expression of Bax. In the western blot assay, the findings demonstrated that following a 24-hour treatment of CPT therapy, levels of the proapoptotic protein Bax increased. Additionally, the levels of the antiapoptotic protein Bcl-2 in the CPT group were lower than those in the control group. This is almost consistent with the qPCR results, and we have reason to believe that CPT can cause apoptosis and enhance the effect of 5-FU.
The expression of Bcl-2, Bcl-XL, Mcl-1, Bax and Survivin mRNA in SGC-7901/5-FU cells. *p < 0.05, compared with the control group. **p < 0.01, compared with the control group. ***p < 0.001, compared with the control group. #p < 0.05, compared with the 5-FU group. ##p < 0.01, compared with the 5-FU group. ###p < 0.001, compared with the 5-FU group.
The expression of Bcl-2, Bcl-XL, Mcl-1, Bax and Survivin protein in SGC-7901/5-FU cells. *p < 0.05, compared with the control group. **p < 0.01, compared with the control group. ***p < 0.001, compared with the control group. #p < 0.05, compared with the 5-FU group. ##p < 0.01, compared with the 5-FU group.
CPT Inhibits JAK2/STAT3 Pathway-related Signaling Expression
The qPCR analysis found that the mRNA expression of JAK2 and STAT3 was decreased in the CPT group compared to the control group (P < 0.05) (Fig. 5). There was a trend of decreased expression in the combined group compared to the 5-FU group whereby the difference was significant (P < 0.05). The protein expression of JAK2 and STAT3 in the CPT group and the combination group was not substantially different from that in the control group, according to the western blotting findings (P > 0.05) (Fig. 6). However, p-JAK2 and p-STAT3 protein expression was downregulated (P < 0.05); therefore, we demonstrated that CPT inhibits the activation of the JAK2/STAT3 pathway. The inhibitory effect of CPT on cell growth is associated with increased apoptosis via the inhibition of JAK2 and STAT3.
CPT inhibits the expression of the JAK2/STAT3 signaling pathway-related factors. The expression of JAK2 and STAT3 mRNA in SGC-7901/5-FU cells. *p < 0.05, compared with the control group. **p < 0.01, compared with the control group. ***p < 0.001, compared with the control group. ##p < 0.01, compared with the 5-FU group.
CPT inhibits the expression of the JAK2/STAT3 signaling pathway-related factors. The expression of JAK2 (p-JAK2) and STAT3 (p-STAT3) protein in SGC-7901/5-FU cells. *p < 0.05, compared with the control group. **p < 0.01, compared with the control group. #p < 0.05, compared with the 5-FU group. ##p < 0.01, compared with the 5-FU group.
CPT Reduces the Transcription of MDR1 and the Expression of P-gp
We used qPCR and WB to analyze the drug resistance of the cells. The experiments showed that drug resistance gene MDR1 expression was higher in the control and 5-FU groups (Fig. 7 A). This confirmed the presence of cellular resistance to 5-FU. In contrast, MDR1 expression was lower in the CPT and CPT+5-FU groups. MDR1 expression was considerably increased in the CPT group compared to the control group, with a statistically significant difference. This suggests that the expression of drug-resistant genes in the cells was reduced by the intervention of CPT. WB results were consistent with the qPCR results. P-glycoprotein is a drug resistance protein regulated by MDR1. The P-gp levels were significantly lower in the CPT group than in the control group (Fig. 7 B, C). These results were consistent with the trends of the expression of JAK2 (including p-JAK2), STAT3 (including p-STAT3) and other related proteins. In addition, P-gp levels were reduced in the combination group compared with the 5-FU group (P < 0.05). The protein levels of JAK2 (including p-JAK2), STAT3 (including p-STAT3), Mcl-1, Bcl-2, Bcl-XL and Survivin in the combination group were low, which was consistent with the trend in the P-gp levels. Therefore, we demonstrated that CPT could reduce the expression of P-gp and enhance the effect of 5-FU. Based on the experimental results, it is reasonable to hypothesize that the underlying mechanism is related to the JAK2/STAT3 signaling pathway.
DISCUSSION
For the past few years, herbal medicine has attracted the attention of various researchers in their ongoing quest to find effective herbal treatments for cancer. Gastric cancer was inhibited by a variety of active components from Salvia miltiorrhiza—for example, tanshinone I, tanshinone IIA, and isocryptotanshinone all have been known to exert inhibitory effects on stomach cancer [10, 11]. In addition, the active components of Salvia miltiorrhiza enhance the efficacy of chemotherapy drugs. Tanshinone IIA enhances doxorubicin’s antitumor impact on drug-resistant on drug-resistant gastric cancer cells [12]. CPT is another active ingredient of Salvia miltiorrhiza that exerts effective action regarding various cancers such as prostate, breast and stomach cancers [13]. The tumor-suppressive effect of CPT has been demonstrated in animal models [14]. The precise action of CPT on stomach cancer cells, however, remains unknown. In our research, we evaluated how well CPT works against tumors in SGC-7901/5-FU cells. CPT exerted good antitumor effects on GC by reducing cellular growth and promoting apoptosis. Similarly, CPT enhanced the therapeutic effect of 5-FU on cancer. Further experiments showed that CPT inhibited the JAK2/STAT3 signaling pathway, regulating the manifestation of related key elements, thus promoting the apoptosis of tumor cells and enhancing drug efficacy. In addition, there was a link between CPT and cellular drug resistance.
Apoptosis is a type of orderly cell death that is genetically regulated, and it allows organisms to better adapt to and survive in their environment and maintain internal stability. Apoptosis is characterized by activating, expressing, and regulating several genes and is associated with several signaling pathways. Bcl-2 protein family members have been identified as important apoptotic regulators [15]; these proteins are characterized by their Bcl-2 homology (BH) structural domain and their function in controlling apoptosis [16]. Bcl-2, Bcl-XL and Mcl-1 all belong to the Bcl-2 antiapoptotic subfamily. Bcl-2 and Mcl-1 have long been considered to be direct targets of STAT3. Bax, an apoptotic gene, not only acts as an antagonist of Bcl-2 but also has some proapoptotic effects. The BH1 and BH2 homologous domains of the Bax protein form a hydrophobic groove that binds to its BH3 helix fragment to form a Bax/Bax homodimer. The BH3 helix is exposed on the surface of Bax, which is an apoptotic protein that binds to the surface structure of the pro-apoptotic protein Bcl-2 to produce a Bcl-2/Bax heterodimer. This protein regulates apoptosis by modulating mitochondrial membrane permeability. Both Bcl-2 and Bax proteins exhibit relatively constant levels of expression normally [17]. Bax overexpression results in a large rise in the amount of Bax/Bax homodimers and makes cells more susceptible to death signals, which triggers apoptosis. Whereas when Bcl-2 expression is high, large numbers of the Bax/Bax dimers dissociate, producing more stable Bcl-2/Bax heterodimers that counteract apoptosis-inducing effects and prolong cell survival [18]. When proteins that prevent apoptosis such as Bcl-2, Bcl-XL, and Mcl-1 are saturated or deficient, Bax can become activated and cause a massive release of apoptotic molecules [13]. In the current studies, we looked at how CPT affected cell growth, and we found that it effectively inhibited cell proliferation and induced apoptosis. We first performed cell proliferation assays and observed an inhibitory effect of CPT on SGC-7901/5-FU cells using fluorescence microscopy. Drug treatment resulted in nuclei that were crinkled as well as the formation of apoptotic bodies. Flow cytometry results also showed that the cells underwent early apoptosis.
We looked further into the molecular processes through which the drug promotes apoptosis. A pathway called JAK2/STAT3 is an important transcriptional process of signaling that regulates gene expression and is essential for cell growth and development. JAK2/STAT3 signaling induces cell division, proliferation and antiapoptotic mechanisms [19]. JAK2 and STAT3 are important factors in this pathway, especially when JAK2 is phosphorylated by STAT3 [20]. STAT3 activation controls cell proliferation and death by acting as a transcription factor. There have been reports of STAT3 hyperactivation in multiple kinds of tumors [21]. According to a meta-analysis, high p-STAT3 expression has been linked to poor prognoses in GC patients [22]. The binding of growth factors to tyrosine kinase cell surface receptors allows activated JAK2 kinase function, leading to its phosphorylation. After that, P-STAT3 creates a dimer, where it is phosphorylated by JAK2. Then, P-STAT3 forms a dimer and rapidly enters the nucleus, regulating downstream the expression of related genes [23]. Some researchers believe that the development of many cancers, such as colon and breast cancers, can be inhibited by regulating the JAK2/STAT3 pathway [24, 25]. Studies have indicated that the growth of gastric tumors can be inhibited by downregulating the JAK2/STAT3 pathway [26]. In vitro, studies have shown that disruption of JAK2/STAT3 and ERK signaling causes the death of MGC803 GC cells [27]. Some researchers have suggested that CPT inhibits the JAK2/STAT3 signaling pathway, reduces tumor growth and decreases JAK2/STAT3 phosphorylation in breast cancer [28]. By disrupting the JAK2/STAT3 signaling pathway and inducing apoptosis, CPT reduces the development of Hepa1-6 in hepatocellular carcinoma cells [29]. The biological process underlying the deterrent and pro-apoptotic properties of CPT in several tumor cell lines was confirmed to involve JAK2/STAT3. Previous experimental data suggest that CPT can inhibit gastric tumor growth by inhibiting the JAK2/STAT3 pathway [14]. Then, we performed western blotting assays, which showed a decrease in JAK2 and STAT3 and a decrease in their phosphorylated forms (p-JAK2 and p-STAT3, respectively). These results are also consistent with previous reports in the literature. Similarly, the genetic assay corroborated the results of our method by showing decreased JAK2 and STAT3 gene expression. In our research, we found that CPT inhibits the activity of JAK2 and STAT3.
According to the literature, CPT alleviates the apoptosis induced by hypoxia, and its effects are associated with the inhibition of Bcl-2 [30, 31]. Resveratrol has been shown to modulate the JAK2/STAT3 pathway and thereby reduce apoptosis, an effect associated not only with a reduction in the pro-apoptotic protein Bax but also with an increase in the antiapoptotic protein Bcl-2 [32]. CPT induces apoptosis in cholangiocarcinoma cells by blocking the JAK2/STAT3 signal transduction pathway and changing the expression of the Bcl-2/Bax family members [33]. From this, Bax and Bcl-2 proteins are closely associated with apoptosis. CPT has a regulatory effect on their regulation promoting the process of apoptosis; therefore, it is considered to have anticancer potential [34]. We detected the protein expression levels of Bax and Bcl-2 in SGC-7901/5-FU cells. CPT effectively up-regulated the expression level of Bax while down-regulating the expression levels of Bcl-2. At the same time, we calculated the results of Bcl-2/Bax, which were reduced by the addition of CPT. This study suggests that CPT likely exerts its antitumor effects by reducing the Bcl-2/Bax ratio. This is the same view as previously reported in the literature. Under the intervention of CPT, other apoptosis-related proteins such as Mcl-1 and Bcl-XL also showed a downward trend. It was further proven that the apoptosis of SGC-7901/5-FU cells was promoted by CPT.
The reduced effectiveness of chemotherapeutic agents is mainly associated with the development of cellular resistance. P-glycoprotein (P-gp) is a useful indicator to assess cellular resistance. P-gp on the cell membrane can be involved in apoptosis directly or indirectly through drug efflux, leading to the development of multidrug resistance. The literature shows that P-gp downregulation enhances the efficacy of oxaliplatin and inhibits multidrug resistance [35]. The phenomenon of gefitinib resistance in EGFR mutant lung cancer could be reversed by CPT [36]. Clinically, interferon regulatory factor-1 (IRF-1) improves drug-resistant symptoms in GC by downregulating P-gp [36]. A body of evidence demonstrates that downregulation of P-gp can be effective in addressing drug resistance. In our study, we found that 5-FU+CPT exerted a stronger effect on cells than 5-FU; thus, we examined cellular resistance before and after drug administration. Both MDR1 level and P-gp protein expression level were decreased after drug administration. In contrast, the reduction in resistance to the drugs was considerably greater in the cells in the combination treatment group than in the 5-FU group. This suggests that CPT enhanced the therapeutic efficacy of 5-FU by reducing cell resistance; however, whether there is a correlation between reduced drug resistance and the JAK2/STAT3 pathway remains to be further investigated.
CONCLUSIONS
In summary, the findings of this study prove that CPT can inhibit the growth of SGC-7901/5-FU cells, which is mediated by the inhibition of the JAK2/STAT3 signaling pathway. Furthermore, cryptotanshinone can downregulate the expression of antiapoptotic genes and reduce cellular drug resistance. CPT enhances the effect of 5-FU by promoting apoptosis and reducing cell resistance to 5-FU. All these results suggest that CPT has potential therapeutic value for gastric cancer. Our findings related to the molecular mechanisms and signaling pathways mediated by cryptotanshinone also provide new insights for improving the curative effect of chemotherapeutic agents in gastric carcinoma. Subsequent studies by our group will focus on the interaction of the JAK2/STAT3 signaling pathway and P-gp in the effect of chemotherapy on gastric cancer.
Acknowledgments
This work was supported by the General Project of Anhui Natural Science Foundation (2008085MH266), the Clinical Scientific Research Project of the First Affiliated Hospital of Anhui University of Chinese Medicine (2020yfyzc02), Anhui Province University Outstanding Young Talents Support Program Project (gxyq2021184), the Clinical Scientific Research Project of Anhui University of Chinese Medicine (2021yfylc04), and Anhui Province University Outstanding Young Talents Support Program Project (gxyq2019035).
Conflict of Interest Statement
The authors declare that there is no conflict to ffinterest.
References
M. Sekiguchi, I. Oda, T. Matsuda, and Y. Saito, Digestion, 103(1), 22 – 28 (2022).
C. Xia, X. Dong, H. Li, et al., Chin. Med. J. (Engl.), 135(5), 584 – 590 (2022).
T. Mengie Ayele, Z. Tilahun Muche, A. Behaile Teklemariam, et al., J. Inflamm. Res., 15, 1349 – 1364 (2022).
S. Keeratichamroen, K. Lirdprapamongkol, S. Thongnest, et al., Oncol. Rep., 47(1), Art. No. 6 (2022).
M. Xu, L. Ren, J. Fan, et al., Life Sci., 290, Art. No. 120266 (2022).
M. S. Bader and S. C. Meyer, Pharmaceuticals (Basel), 15(2), Art. No. 160 (2022).
E. J. Kim, S. Y. Kim, S. M. Kim, and M. Lee, Toxicol. Appl. Pharmacol., 330, 84 – 92 (2017).
J. Zhang, L. Shang, W. Jiang, and W. Wu, Bioengineered., 13(3), 7904 – 7918 (2022).
Y. Zhang, W. Lu, X. Zhang, et al., Pharmacol. Res., 147, Art. No. 104307 (2019).
J. Liu, F. Wang, P. Sheng, et al., J. Ethnopharmacol., 272, Art. No. 113923 (2021).
Z. M. Chen, L. Huang, M. M. Li, et al., Sci. Rep., 8(1), Art. No. 9307 (2018).
Z. Xu, L. Chen, Z. Xiao, et al., Phytomedicine, 51, 58 – 67 (2018).
C. Liu, H. N. Sun, Y. H. Luo, et al., Oncotarget., 8(70), 115398 – 115412 (2017).
W. Wu, Y. Cao, L. Cheng, et al., Nat. Prod. Commun., 17(10), 1 – 9 (2022).
C. Denis, J. Sopková-de Oliveira Santos, R. Bureau, and A. S. Voisin-Chiret, J. Med. Chem., 63(3), 928 – 943 (2020).
C. F. A. Warren, M. W. Wong-Brown, and N. A. Bowden, Cell Death Dis., 10(3), Art. No. 177 (2019).
R. Singh, A. Letai, and K. Sarosiek, Nat. Rev. Mol. Cell. Biol., 20(3), 175 – 193 (2019).
Y. J. Lan, Y. T. Wang, C. L. Hung, and Y. W. Chiang, Biochim. Biophys. Acta. Gen. Subj., 1864(4), Art. No. 129541 (2020).
B. Lei, L. Qian, Y. Zhang, et al., J. Cancer, 11(23), 6768 – 6781 (2020).
S. Y. Park, C. J. Lee, J. H. Choi, et al., J. Exp. Clin. Cancer Res., 38(1), Art. No. 399 (2019).
H. Lee, A. J. Jeong, and S. K. Ye, BMB Rep., 52(7), 415 – 423 (2019).
K. Yuan, J. Ye, Z. Liu, et al., J. Exp. Clin. Cancer Res., 39(1), Art. No. 9 (2020).
X. Fan, H. Fu, N. Xie, et al., Aging (Albany NY), 13(19), 22830 – 22842 (2021).
Z. X. Jia, Z. Zhang, Z. Li, et al., Eur. Rev. Med. Pharmacol. Sci., 25(5), 2331 – 2343 (2021).
C. Qiu, T. Zhang, X. Zhu, et al., Reprod. Sci., 26(6), 829 – 838 (2019).
W. Liu, J. Li, D. Zhang, et al., Oncol. Lett., 20(6), Art. No. 318 (2020).
X. Wang, C. Dai, Y. Yin, et al., J. Zhejiang Univ. Sci. B, 22(6), 492 – 503 (2021).
S. Noori, M. Nourbakhsh, H. Imani, et al., BMC Complement Med. Ther., 22(1), Art. No. 145 (2022).
Z. Han, S. Liu, H. Lin, et al., Cancer Immunol. Immunother., 68(7), 1073 – 1085 (2019).
Y. Gu,W. Liu, G. Liu, et al., Mol. Med. Rep., 24(4), Art. No. 739 (2021).
S. A. Kim, O. H. Kang, D. Y. Kwon, Int. J. Mol. Sci., 19(9), Art. No. 2739 (2018).
Y. Hou, K. Wang, W. Wan, et al., Genes Dis., 5(3), 245 – 255 (2018).
F. Ke, Z. Wang, X. Song, et al., Drug Des. Devel. Ther., 11, 1753 – 1766 (2017).
S. Irtegun Kandemir, H. S. Fidan, I. Yener, et al., J. Food Biochem., 46(9), Art. No. e14226 (2022).
E. Xu, H. Zhu, F. Wang, et al., Curr. Mol. Med., 21(10), 922 – 930 (2021).
P. Cai, G. Sheng, S. Jiang, et al., Front. Pharmacol., 13, Art. No. 837055 (2022).
J. Yuan, Z. Yin, L. Tan, et al., Cancer Lett., 457, 28 – 39 (2019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, Y., Wang, L., Cheng, L. et al. Cryptotanshinone Inhibits the Proliferation of 5-Fluorouracil-Resistant Gastric Cancer SGC-7901/5-FU Cells Via the JAK2/STAT3 Pathway. Pharm Chem J 58, 187–196 (2024). https://doi.org/10.1007/s11094-024-03133-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03133-x