Abstract
Introduction
Despite the fact that high-dose radiotherapy is a main therapeutic modality in cancer treatment, recent evidence suggests that it might confer radioresistance. Hyper-radiosensitivity (HRS) is one of the important biological effects of low-dose ionizing radiation (LDIR) in mammalian cell lines. LDIR is considered as a promising assistant method of clinical cancer therapy. The purpose of this study was to evaluate the efficiency of intermittent LDIR followed by a high-dose radiation therapeutic approach compared with the conventional high-dose radiotherapy in the breast cancer MDA-MB-231 cell line.
Materials and methods
MDA-MB-231 cells were divided into four experimental groups—intermittent LDIR group: cells were irradiated for 10 fractions with a dose of 30 mGy at each time (interval 24 h) followed by 2 Gy, single LDIR group: cells have accepted a dose of 300 mGy LDIR and after 24 h a high dose of 2 Gy, high-dose ionizing radiation (HDIR) group: cells were exposed to a single high dose of 2 Gy, and control group.
Results
MTT and flow cytometry assay were used for cell proliferation and apoptosis after 24 h of the last irradiation dose (2 Gy). Also, we examined p21 and cespase3 gene expression by RT-qPCR. We observed that intermittent LDIR significantly increased the killing effect of radiotherapy (viability, 71.95 + 1.25%) (P < 0.01). The apoptosis is proposed to increase up to 32.55 + 0.07% in the intermittent LDIR that was markedly higher than those of other groups (P < 0.01). Caspase3 gene expression in this group was the highest (5.2-fold), 4.26-fold and 1.42-fold in single LDIR and HDIR, respectively. It was observed that the intermittent LDIR potentially decreases p21 expression in comparison with the challenge dose of 2 Gy (0.681-fold).
Conclusion
LDIR may result in HRS through a concurrent increase of apoptosis and a significant decrease in cell viability. The therapeutic effects of this approach should be further investigated in animal models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is globally the second most common cancer after lung cancer. It is estimated that 2,088,849 (11.6%) new cases of breast cancer have occurred in 2018 in the world and the mortality of breast cancer is estimated to be 626,679 (6.6% of global cancer mortality). Therefore, breast cancer is considered a major public health problem worldwide [1, 2]. So far, tremendous efforts have been made to apply numerous modalities of treatment, including surgery, chemotherapy, immunotherapy, and radiotherapy [3]. Conservative breast surgery followed by radiotherapy is an acceptable and standard approach that provides this possibility to preserve the breast in most cases of early-stage breast cancers [4]. In the case of advanced breast cancer, postmastectomy radiation therapy is still widely used as a standard approach [5, 6]. During the last decade, several advanced methods of radiation therapy have been introduced, including accelerated partial radiotherapy and hypofractionated treatment [7,8,9,10]. Due to considerable variations in the size and shape of the breast and/or chest wall, there are important disputed topics in radiotherapy planning. Moreover, a great deal of care has to be taken to save organs at risk (OARs), more seriously the lungs, heart, and contralateral breast (CB) to stave off them from undesirable side effects [11].
Cellular exposure to radiation results in damage to DNA and other cellular structures that triggers a complex cascade of downstream response pathways in both the nucleus and cytoplasm, including the modulation of the cell cycle, DNA repair, reactive oxygen species (ROS) defense, cytokine production, and apoptosis. In certain tumor cell subpopulations, these gene networks can be innately biased toward a radioresistant, prosurvival phenotype, for example, via decreased proliferation or accelerated cell-cycle arrest, more efficient or prolonged DNA repair, or dampened apoptotic signaling [12,13,14]. However, a range of side effects is associated with conventional high-dose radiotherapies [15], such as radioresistance leading to tumor recurrence and consequent poor prognosis [16]. Therefore, enhancing radiosensitivity, overcoming radioresistance, and improving the efficiency of radiotherapy is of great practical importance in breast cancer treatment.
Application of low-dose radiation therapy in breast cancer is one of the well-established submodalities that exhibit some noteworthy advantages like reduced damage to normal tissues, increased safety, and better tolerance by patients. It may, therefore, offer a promising approach in radiotherapy [17].
Low-dose ionizing radiation (LDIR) is defined as doses of less than 0.2 Gy for low–linear energy transfer (LET) and doses less than 0.05 Gy for high-LET radiations, respectively [18]. Low-dose radiotherapy exhibits the advantages of reduced damage, increased safety, and easier acceptance by patients and may, therefore, offer a promising approach in the field of radiotherapy [17].
Unlike in normal cells, LDIR induces different biological effects in cancer cells, such as stimulating cell proliferation in normal cells such as mesenchymal stem cells although this may not occur in solid tumor cells [19, 20]. LDIR also may affect the growth of cancer cells through the activation of certain cell signaling pathways, which does not exist in normal cells [21, 22].
LDIR has been suggested to have several features including hormesis [23, 24], the adaptive effect [25], the bystander effect [26], and HRS [27].
Three independent studies [28,29,30,31,32] demonstrated that many human tumor cell lines show a low-dose HRS (LD-HRS).
Various roles have been defined for LD-HRS by different studies. Of these, p21 and caspase3 are two genes responsible for apoptosis and cell proliferation. In this context, Enns et al. [33] and later Krueger et al. [34] identified a role for apoptosis in HRS. Interestingly, it was demonstrated that the apoptotic response was mediated through the p53-dependent activation of caspase3, which forms part of the signaling cascade downstream of ATM activation. On the other hand, Li et al. [35] suggested that the ATM/p21 pathway directly participated in the LDIR-induced cell proliferation inhibition in p53null-type prostate tumor cells, whereas this mechanism was absent in normal prostate cells.
HRS does not induce cellular repair mechanisms often observed at clinically relevant or higher radiation doses and thus provides a plausible explanation as to why there is no induction of radiation resistance with HRS as measured in vitro [36]. However, the extent of LDIR utilization in clinical cancer therapy is still a debate and more study is needed.
Regarding the previous undeniable roles of the two determinative genes, caspase3 and p21, in the induction of radioresistance or radiosensitivity, caspase3 is known as the molecule responsible for the apoptotic process in various cancers, such as breast cancer. Nonetheless, the prognostic value of caspase3 is still a debate in breast cancer [37].
In this study, we developed a different LDIR model. The aim of this was to evaluate the efficiency of intermittent LDIR followed by the high-dose radiation therapeutic approach compared with the conventional high-dose radiotherapy in the breast cancer MDA-MB-231 cell line.
Materials and methods
Cell line culture
The MDA-MB-231 breast cancer cell line was purchased from the Iranian Biological Resource Center (Tehran, Iran) and was cultured in Dulbecco’s modified Eagle’s medium (Gibco; Thermo Fisher Scientific, Inc., Grand Island, NY, USA) supplemented with 10% FBS (Gibco; GE Healthcare Life Sciences, Chalfont, UK) and 5% penicillin/streptomycin. Cultures were maintained at 37 °C with 5% CO2 in a humidified incubator.
LDIR strategy
The MDA-MB-231 cells were exposed to X-ray using a linear accelerator (LINAC) which produces X photons of 6 Mev at a dose rate of 200 mGy/min (Varian, Golestan Hospital, Ahwaz, Iran).
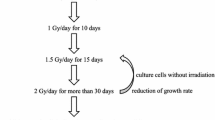
When the cells reached 60% confluency, the culture flasks (T25) were completely filled with the culture medium and placed in a water phantom to compensate for the build-up effect. The SSD was 100 cm, and the field size was 10*10 cm. In order to investigate the intermittent LDIR effect on breast cancer, the MDA-MB-231 cells were divided into four experimental groups (Fig. 1a). In group A, the intermittent group, the cells were irradiated in 10 fractions with a dose of 30 mGy each time. The time interval between two irradiations was 24 h [38,39,40]. After that, the cells were irradiated with 2 Gy high-dose ionizing radiation (HDIR). Group B was cultured simultaneously for the same 10 days and received a dose of 300 mGy and subsequently a high dose of 2 Gy after 24 h. Group C accepted a single high dose of 2 Gy. Group D, the sham-irradiated group, was the control. At each time that the cells of other groups were exposed, the flasks of the control cells were also removed from the incubator and full of culture medium in the same conditions. They were taken to the radiotherapy center, but they did not receive any irradiation (Fig. 1b).
a Chart of irradiation groups. b Irradiation protocol. MDA-MB-231 cells were divided into four experimental groups—intermittent LDIR + HDIR group: cells were irradiated for 10 fractions with a dose of 30 mGy at each time (interval 24 h) followed by 2 Gy, single LDIR + HDIR group: cells have accepted a dose of 300 mGy LDIR and after 24 h a high dose of 2 Gy, HDIR group: cells were exposed with a single high dose of 2 Gy, and control group
MTT assay
The MDA-MB-231 cells were seeded into 96-well plates at a density of 2 × 104 cells/well and a final volume of 150 μL/well. Each experiment was triplicated. Twenty-four hours after the last session of radiation [35, 41, 42], the previous culture medium was removed and new medium (100 μL) was added. Next, 20 μL of MTT reagent (Sigma-Aldrich, Shanghai, China) was added and cells were incubated for 4 h at 37 °C in the dark. The medium was then discarded and cells oscillated in 100 μL/well dimethyl sulfoxide for 30 min. The absorbance was measured at 570 nm using a Fluoroskan Ascent FL Microplate Fluorometer (Thermo Scientific, Sunnyvale, CA, USA). The viability percent of each group was calculated as
Flow cytometry for cell apoptosis
Annexin V-FITC and PI double-staining flow cytometry analyses were employed for the assay of cell apoptosis after irradiation. Cells were trypsinized and collected in centrifuge tubes 24 h after the last radiation; subsequent cells were washed three times with cold PBS and binding buffer, followed by staining with Annexin V-FITC and PI (Annexin V-FITC Apoptosis Detection kit, eBioscience) for apoptosis assay. Briefly, the MDA-MB-231 cells were first resuspended in 100 μL binding buffer. Then, the cells were mixed with 5 μL of Annexin V-FITC and were incubated for 15 min followed by adding 300 μL binding buffer and 5 μL PI (Sigma). After 15-min incubation in PI buffer, the cells were immediately analyzed using a flow cytometer (BD FACSCalibur) with the FlowJo FACS analysis software. The cells in the different portions represent the different cell states as follows: the late-apoptotic cells are present in the upper-right portion, the viable cells are present in the lower-left portion, and the early apoptotic cells are in the lower-right portion.
Reverse transcription-quantitative polymerase chain reaction
For further investigation, the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) technique was used to assay gene expression. The p21, caspase3, and HPRT gene sequences were collected from https://www.ncbi.nlm.nih.gov/. Then, BLAST and Snapgene software were used for primer designing and ordered for synthesis. Cell total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen). Then, a Reverse Transcription Kit (Qiagen) was used to synthesize cDNA. Finally, the SYBER Green PCR Kit was used to run the RT-qPCR. The mRNA expression level of caspase3 and p21 was quantified by normalizing over HPRT as the internal control gene. The sequences of the primers used for RT-qPCR were as follows:
-
p21-fwd, GTTCACAGGTGTTTCTGCGGC
-
p21-rev, CCATTAGCGCATCACAGTCGC
-
Caspase3-fwd, GAGGCGGTTGTAGAAGAGTTT
-
Caspase3-rev, GGCTCGCTAACTCCTCACG
-
HPRT-fwd, TAG CCC TCT GTG TGC TCA AG
-
HPRT-rev, ACT TTT ATG TCC CCT GTT GAC TG
The fold of difference relative to the reference gene (HPRT) was determined by conversion of 2−ΔΔCT. ΔΔCT = (CTobjective gene − CTreference gene) of the experimental group − (CTobjective gene − CTreference gene) of the control group.
Statistical analysis
All experiments were repeated at least three times. Data and statistics are presented as means ± SD. The significance was determined by one-way ANOVA using SPSS 24.0. P < 0.05 was considered to indicate a statistically significant difference (*P < 0.05; **P < 0.01).
Results
In this study, the MDA-MB-231 breast cancer cell line was chosen because it is a highly aggressive, invasive, and poorly differentiated triple-negative breast cancer (TNBC) cell line as it lacks estrogen receptor (ER) and progesterone receptor (PR) expression, as well as HER2 (human epidermal growth factor receptor 2) amplification. TNBC is an aggressive form of breast cancer with limited treatment options [43, 44].
Effects of LDIR on Cell Growth
At first, in order to investigate the effects of LDIR on cell growth, MDA-MB-231 breast cancer cells were pretreated by an intermittent LDIR and single LDIR before HDIR. Cell proliferation activity was evaluated using MTT assay after 24 h of the last irradiation dose (2 Gy). The results of MTT are shown in Fig. 2a.
a The results of cell viability in irradiated groups of breast cancer cells (MDA-MB 231). The survival rate in int. L + H decreased significantly 71.95 + 1.25% unlike in the control group. (*P < 0.05, **P < 0.01 vs. control). Intermittent LDIR promotes the therapeutic effects of radiotherapy. The cell growth was determined by MTT assay. b Comparison of the viability of int. L + H with other groups. The vitality of the int. L + H group has decreased by about 12.55 + 1.2% compared to the HDIR which shows that the efficacy of intermittent low-dose before high-dose irradiation is higher than high-dose irradiation alone. (*P < 0.05, **P < 0.01 vs. int. L + H)
The viability was 71.95 ± 1.25%, 89.66 ± 1.07%, and 84.5 ± 1.04% in intermittent LDIR + HDIR, single LDIR + HDIR, and HDIR compared to the control group, respectively. The viability of cells in the int. L + H group is 17.71% less than that of single L + H, indicating that using intermittent LDIR is more effective. Compared with the HDIR, it has been found that cell survival is 12.55% less in int. L + H, suggesting the use of intermittent low-dose radiation before the HDIR to increase the radiosensitivity of the cells, thereby further reducing cell survival (Fig.2b). Briefly, the intermittent LDIR most significantly inhibited cell growth compared with the HDIR or single LDIR (P < 0.01). These findings supported that intermittent LDIR followed by HDIR induced radiosensitivity in the cells and can increase the therapeutic efficacy through reduced cell survival.
Apoptosis induced by LDIR
After observing the decreased viability of cells in the int. L + H group compared to those of other groups, for further study of the effectiveness of this regimen on cells, cell apoptosis was investigated, using the Annexin V-PI kit. As shown in Fig. 3a, the intermittent LDIR increased cell apoptosis up to 32.55 + 0.07%. The apoptosis was 30.14 + 0.01% in single LDIR, 25.49 + 0.07% in HDIR, and 8.37 + 0.07% in control groups, respectively. The percentage of apoptosis had the highest value in the intermittent LDIR group (Fig. b, c). Moreover, in the single LDIR group, apoptosis was significantly higher in comparison with the HDIR and control groups. Therefore, it seems that the use of low-dose irradiation as an intermittent regimen is more effective on apoptosis than a single low-dose and high-dose irradiation alone.
a Flow cytometry results. Analysis of MDA-MB-231 cell apoptosis by flow cytometry. b The results of flow cytometry. Intermittent L + H promotes the therapeutic effects of radiotherapy. (*P < 0.05, **P < 0.01 compared with the control group). c Comparison of apoptosis of int. L + H with other groups. The apoptosis percentage of the int. L + H group has increased by about 7.06% compared to the HDIR. (*P < 0.05, **P < 0.01 vs. int. L + H)
Effect of intermittent low-dose irradiation on gene expression
Subsequently, to confirm the results, we examined the expression of p21 and caspase3 genes. The results of caspase3 expression have shown 5.2-, 4.26-, and 1.42-fold change in int. L + H, single L + H, and HDIR groups, respectively. RT-qPCR analysis indicated that caspase3 expression in int. L + H compared with the HDIR was most significant and approximately a 3.6-fold increase was observed in int. L + H (P < 0.01) (Fig. 4a). The results of the evaluation of the caspase3 expression, which is associated with apoptosis, are consistent with flow cytometric results. The int. L + H group with the highest percentage of apoptosis has the highest expression of the caspase3 gene (P < 0.01). So the use of low-dose irradiation intermittently is more effective for breast cancer radiotherapy.
a RT-qPCR results. Caspase3 expression, int. L + H most significantly increased the caspase3 expression. (**P < 0.01 vs control group, †P < 0.05 and ††P < 0.01 comparison between the two groups). b RT-qPCR results. P21 expression, intermittent low-dose irradiation most significantly reduced the expression of the p21 gene. (**P < 0.01 vs control group, †P < 0.05 comparison between the two groups)
On the other hand, the consequence of p21 expression indicated that the highest decrease of p21 gene expression was measured in the intermittent LDIR group by a 0.102-fold change than in the other irradiated groups (P < 0.01). Furthermore, the difference between the int. L + H and HDIR groups was significant, and this implies that this method is an effective treatment. The decline in p21 gene expression was not significantly different between single L + H and HDIR (P > 0.05; Fig. 4b).
Discussion
Radiotherapy is a well-established approach in cancer treatment which is carried out either following surgery or alone for inoperable tumors. The main obstacles in radiotherapy include normal tissue toxicity and tumor radioresistance [45, 46]. Intriguingly, experimental studies with low-dose ionizing radiation in various cell lines and animal models have demonstrated the phenomenon of hormesis and adaptive response in normal cells and tissues while not in malignant tumors [47, 48]. In this study, the efficacy of the combination of intermittent LDIR and HDIR was evaluated as a novel strategy in breast cancer treatment. HRS seems to be more induced in pre-treatment by low-dose fractionated radiotherapy followed by conventional radiotherapy than the conventional regimen alone.
Surprisingly, this combined modality of radiotherapy increased dramatically the killing ability of HDIR via a strong induction of apoptosis, concurrently with a significant decline in cell proliferation.
Wang et al. studied the effect of intermittent LDIR on HT29 cells [49]. Their data suggested that the intermittent LDIR noticeably inhibited cell growth in comparison with the HDIR or single LDIR pretreatments. These results are in agreement with our study and they confirm that the effect of radiotherapy has been increased by using intermittent LDIR followed by a high-dose irradiation challenge such as 2 Gy that is a conventional dose in radiotherapy. Also, Schwarz et al. studied the effect of LDIR (0.03–0.1 Gy) either alone or followed by a 2-Gy challenging dose on the survival of the HT29 cell line [50]. They concluded that the dose of 0.05 Gy before 2 Gy reduced the survival of these cells which is consistent with our results. Otherwise, they claimed that a dose of 0.03 Gy (the same dose applied in our study) did not affect the cell survival significantly, which is contrary to our results. This conflict of conclusions may reflect that various cell lines at different doses have individual responses. Jiang reported that LDIR may stimulate the growth of normal cells, whereas the same results were not obtained about leukemia and solid tumor cells in vitro [51]. Marples et al. in a review article reported that mammalian cells exhibit HRS to radiation doses of less than ~ 0.3 Gy when given at acute dose rates. Over the ~ 0.3- to 0.6-Gy dose range, a more radioresistant response per unit dose is evident, as illustrated by the shallower slope of the radiation dose-response curve. The transition toward radiation resistance associated with overcoming HRS is generically described by the term “increased radioresistance” (IRR) [52]. As that was consistent with this article, the proliferation rate of the single LDIR + HDIR (1*0.3 Gy + 1*2 Gy) group was higher than that of the HDIR group in our result, indicating that 0.3 Gy LDIR in this group has induced IRR.
MDA-MB-231 cells include a p53 mutant allele on exon 8 [21]. The ATM/p53/p21 pathway was activated by LDIR in the MDA-MB-231 cells [53].
Despite the search in valid databases, we found a few articles about the effect of LDIR on p21 expression. Wang reported that the intermittent LDIR increases p21 expression and apoptosis [49], and Li’s report showed that p21 expression was reduced after LDIR (750 mGy) without HDIR [53]. In fact, the relationship between the different regimes of LDIR (intermittent and single LDIR) followed by a challenge high-dose irradiation (2 Gy) on apoptosis and cell proliferation is not investigated. The results of these research are not comparable to our observations, because the irradiation regimes, the cell lines, and the main purpose are different from our research. At first, in the intermittent LDIR group, we considered 3 days the interval between LDIR sessions. After 10*30-mGy sessions followed by a 2-Gy HDIR, we observed that the cell proliferation in this group is higher than that of other groups, which may be due to damage repair and radioresistance (data not published). Then, the interval between LDIR sessions was reduced to 24 h (group A) and it was observed that apoptosis and cell proliferation were decreased compared to those of the HDIR group, in spite of downregulation of the p21 gene. We suggest that the p21 gene be considered with such a LDIR therapeutic strategy for other radioresistant cancer cells.
Caspase3 is an important apoptosis-related gene; it is located at 4q35.1 [54]. Caspase3 is the effector of the caspase apoptotic cascade pathway; the mitochondrial apoptotic pathway and the death receptor apoptotic pathway can both activate CASP3, making it the central hub of the whole apoptotic pathway [54,55,56]. In our study, caspase3 expression increased in the intermittent LDIR group compared with HDIR which indicates that int. L + H will cause HRS through a significant increase in the caspase3 expression as a result of increased apoptosis, thereby increasing the therapeutic efficacy of high-dose radiotherapy for breast cancer. Also, Wang reported that LDIR increased the expression of caspase3 in HT29 cells in comparison to HDIR [49]. Enns reported that A549 and T98G cells showed a discernable activation of caspase3 at low doses that induce HRS; meanwhile, this HRS was not observed in MCF7 cells at a similar radiation protocol [33]. Therefore, these results indicate that the effects of LDIR on caspase3 expression in cancer cells are dose and, most importantly, cell line dependent. It is noteworthy that further studies are required to evaluate LDIR effects on caspase3, particularly on its protein level.
There are some limitations to the present study. First, it is also necessary to treat this regimen for normal breast cells to check the hormesis effect. Secondly, more parameters especially protein expression need to be analyzed to indicate the effectiveness of this therapy. Thirdly, to evaluate the therapeutic effects of this method, it should be studied on other cancer cell lines and even in animal and clinical models. Finally, the LDIR requires an investigation to apply as a combination therapy with the other therapeutic modalities such as conventional radiotherapy, chemotherapy, and even immunotherapy as well.
Conclusion
The presented findings suggest that intermittent low-dose ionizing radiation by inducing HRS can increase the effect of high-dose radiotherapy on breast cancer cells. In fact, this study shows that the intermittent LDIR followed by HDIR could be a novel strategy to improve radiotherapy efficiency. However, the other effects of LDIR, such as bystander effects, and adopting response have not been fully investigated.
Limitations
Due to the financial limitations of the project, which belongs to a postgraduate degree (MSc), it was not possible to add additional techniques to this project. So the referee’s insightful suggestions will be considered in future research that will follow this project.
Abbreviations
- HDIR:
-
High-dose ionizing radiation
- HRS:
-
Hyper-radiosensitivity
- LDIR:
-
Low-dose ionizing radiation
- Int. L + H:
-
Intermittent LDIR followed by HDIR
- Single L + H:
-
Single LDIR followed by HDIR
References
Druesne-Pecollo N, Touvier M, Barrandon E, Chan DS, Norat T, Zelek L, Hercberg S, Latino-Martel P (2012) Excess body weight and second primary cancer risk after breast cancer: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat 135(3):647–654
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Ferlay J, Héry C, Autier P, Sankaranarayanan R (2010) Global burden of breast cancer. In: Breast cancer epidemiology. Springer, pp 1–19
van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, van der Schueren E, Helle PA, van Zijl K, Bartelink H (2000) Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 92(14):1143–1150
Overgaard M, Jensen M-B, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC, Rasmussen BB, Blichert-Toft M, Mouridsen HT (1999) Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353(9165):1641–1648. https://doi.org/10.1016/S0140-6736(98)09201-0
Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen M-B, Zedeler K (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 337(14):949–955. https://doi.org/10.1056/nejm199710023371401
Buchholz TA, Somerfield MR, Griggs JJ, El-Eid S, Hammond ME, Lyman GH, Mason G, Newman LA (2014) Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol Off J Am Soc Clin Oncol 32(14):1502–1506. https://doi.org/10.1200/jco.2014.55.1572
Morrow M, Van Zee KJ, Solin LJ, Houssami N, Chavez-MacGregor M, Harris JR, Horton J, Hwang S, Johnson PL, Marinovich ML, Schnitt SJ, Wapnir I, Moran MS (2016) Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Pract Radiat Oncol 6(5):287–295. https://doi.org/10.1016/j.prro.2016.06.011
Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, White J, Harris JR (2017) Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol 7(2):73–79. https://doi.org/10.1016/j.prro.2016.09.007
Recht A, Comen EA, Fine RE, Fleming GF, Hardenbergh PH, Ho AY, Hudis CA, Hwang ES, Kirshner JJ, Morrow M, Salerno KE, Sledge GW, Solin LJ, Spears PA, Whelan TJ, Somerfield MR, Edge SB (2016) Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Pract Radiat Oncol 6(6):e219–e234. https://doi.org/10.1016/j.prro.2016.08.009
Mayo CS, Urie MM, Fitzgerald TJ (2005) Hybrid IMRT plans—concurrently treating conventional and IMRT beams for improved breast irradiation and reduced planning time. Int J Radiat Oncol Biol Phys 61(3):922–932. https://doi.org/10.1016/j.ijrobp.2004.10.033
Gewirtz DA, Hilliker ML, Wilson EN (2009) Promotion of autophagy as a mechanism for radiation sensitization of breast tumor cells. Radiother Oncol 92(3):323–328
Goldstein M, Kastan MB (2015) The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med 66:129–143
Karar J, Maity A (2009) Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol Ther 8(21):1994–2001
Kitahara O, Katagiri T, Tsunoda T, Harima Y, Nakamura Y (2002) Classification of sensitivity or resistance of cervical cancers to ionizing radiation according to expression profiles of 62 genes selected by cDNA microarray analysis. Neoplasia 4(4):295–303
Hu T, Zhou R, Zhao Y, Wu G (2016) Integrin alpha6/Akt/Erk signaling is essential for human breast cancer resistance to radiotherapy. Sci Rep 6:33376. https://doi.org/10.1038/srep33376
Wang X, Liu R, Ma B, Yang KH, Tian J, Jiang L, Bai ZG, Hao XY, Wang J, Li J (2010) High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev (7)
Mettler FA, Sinclair WK, Anspaugh L, Selby PB, Edington C, Webster EW, Harley JH, Wyckoff HO, Ricks RC (1990) The 1986 and 1988 UNSCEAR reports: findings and implications. Health Phys 58(3):241–250
Liang X, So YH, Cui J, XU X, ZHAO Y, CAI L, LI W (2011) The low-dose ionizing radiation stimulates cell proliferation via activation of the MAPK/ERK pathway in rat cultured mesenchymal stem cells. J Radiat Res 52(3):380–386
Jiang H, Li W, Li X, Cai L, Wang G (2008) Low-dose radiation induces adaptive response in normal cells, but not in tumor cells: in vitro and in vivo studies. J Radiat Res 49(3):219–230
Yang G, Li W, Jiang H, Liang X, Zhao Y, Yu D, Zhou L, Wang G, Tian H, Han F (2016) Low-dose radiation may be a novel approach to enhance the effectiveness of cancer therapeutics. Int J Cancer 139(10):2157–2168
Liang X, Gu J, Yu D, Wang G, Zhou L, Zhang X, Zhao Y, Chen X, Zheng S, Liu Q (2016) Low-dose radiation induces cell proliferation in human embryonic lung fibroblasts but not in lung cancer cells: importance of ERK1/2 and AKT signaling pathways. Dose-Response 14(1):1559325815622174
Luckey TD (1982) Physiological benefits from low levels of ionizing radiation. Health Phys 43(6):771–789
Feinendegen L (2005) Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol 78(925):3–7
Olivieri G, Bodycote J, Wolff S (1984) Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 223:594–598
Ballarini F, Biaggi M, Ottolenghi A, Sapora O (2002) Cellular communication and bystander effects: a critical review for modelling low-dose radiation action. Mutat Res Fundam Mol Mech Mutagen 501(1):1–12
Fernet M, Mégnin-Chanet F, Hall J, Favaudon V (2010) Control of the G2/M checkpoints after exposure to low doses of ionising radiation: implications for hyper-radiosensitivity. DNA repair 9(1):48–57
Marples B, Joiner M (1993) The response of Chinese hamster V79 cells to low radiation doses: evidence of enhanced sensitivity of the whole cell population. Radiat Res 133(1):41–51
Joiner M, Lambin P, Malaise E, Robson T, Arrand J, Skov K, Marples B (1996) Hypersensitivity to very-low single radiation doses: its relationship to the adaptive response and induced radioresistance. Mutat Res Fundam Mol Mech Mutagen 358(2):171–183
Wouters B, Skarsgard L (1994) The response of a human tumor cell line to low radiation doses: evidence of enhanced sensitivity. Radiat Res 138(1s):S76–S80
Wouters BG, Sy AM, Skarsgard LD (1996) Low-dose hypersensitivity and increased radioresistance in a panel of human tumor cell lines with different radiosensitivity. Radiat Res 146(4):399–413
Wouters BG, Skarsgard LD (1997) Low-dose radiation sensitivity and induced radioresistance to cell killing in HT-29 cells is distinct from the" adaptive response" and cannot be explained by a subpopulation of sensitive cells. Radiat Res 148(5):435–442
Enns L, Bogen KT, Wizniak J, Murtha AD, Weinfeld M (2004) Low-dose radiation hypersensitivity is associated with p53-dependent Apoptosis11U. S. Department of Energy, University of California Lawrence Livermore National Laboratory contract W-7405-Eng-48 (KT Bogen), National Cancer Institute (Canada) grant 013104 (M. Weinfeld), Alberta Cancer Board Bridge and Pilot grant R-418 (AD Murtha), and US Department of Energy Low-Dose Radiation Research Program (KT Bogen). Mol Cancer Res 2(10):557–566
Krueger SA, Joiner MC, Weinfeld M, Piasentin E, Marples B (2007) Role of apoptosis in low-dose hyper-radiosensitivity. Radiat Res 167(3):260–267. https://doi.org/10.1667/rr0776.1
Li SJ, Liang XY, Li HJ, Yang GZ, Li W, Li Z, Zhou L, Wen X, Yu DH, Cui JW (2018) Low-dose irradiation inhibits proliferation of the p53null type human prostate cancer cells through the ATM/p21 pathway. Int J Mol Med 41(1):548–554. https://doi.org/10.3892/ijmm.2017.3237
Joiner MC, Marples B, Lambin P, Short SC, Turesson I (2001) Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys 49(2):379–389
Yang X, Zhong D-N, Qin H, Wu P-R, Wei K-L, Chen G, He R-Q, Zhong J-C (2017) Caspase-3 over-expression is associated with poor overall survival and clinicopathological parameters in breast cancer: a meta-analysis of 3091 cases. Oncotarget 9(9):8629–8641. https://doi.org/10.18632/oncotarget.23667
Radwan RR, Abdel Fattah SM (2017) Mechanisms involved in the possible nephroprotective effect of rutin and low dose gamma irradiation against cisplatin-induced nephropathy in rats. J Photochem Photobiol B 169:56–62. https://doi.org/10.1016/j.jphotobiol.2017.02.022
Arenas M, Sabater S, Jimenez PL, Rovirosa A, Biete A, Linares V, Belles M, Panes J (2016) Radiotherapy for Graves’ disease. The possible role of low-dose radiotherapy. Rep Pract Oncol Radiother 21(3):213–218. https://doi.org/10.1016/j.rpor.2016.02.001
Gonc U, Cetinkaya M, Atabek M (2016) The effects of low-dose radiotherapy on fresh osteochondral allografts: an experimental study in rabbits. Acta Orthop Traumatol Turc 50(5):572–577. https://doi.org/10.1016/j.aott.2016.08.004
Chen HP, Tung FI, Chen MH, Liu TY (2016) A magnetic vehicle realized tumor cell-targeted radiotherapy using low-dose radiation. J Control Release 226:182–192. https://doi.org/10.1016/j.jconrel.2016.02.025
Yan S, Li X, Jin Q, Yuan J (2016) MicroRNA-145 sensitizes cervical cancer cells to low-dose irradiation by downregulating OCT4 expression. Exp Ther Med 12(5):3130–3136
Liu H, Zang C, Fenner M, Possinger K, Elstner E (2003) PPARγ ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res Treat 79(1):63–74
Chavez KJ, Garimella SV, Lipkowitz S (2010) Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast disease 32(1–2):35–48
Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA (2001) Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mech Ageing Dev 123(1):39–46
Ghazali N, Shaw RJ, Rogers SN, Risk JM (2012) Genomic determinants of normal tissue toxicity after radiotherapy for head and neck malignancy: a systematic review. Oral Oncol 48(11):1090–1100
Suzuki K, Kodama S, Watanabe M (2001) Extremely low-dose ionizing radiation causes activation of mitogen-activated protein kinase pathway and enhances proliferation of normal human diploid cells. Cancer Res 61(14):5396–5401
Himoto T, Nomura T, Tani J, Miyoshi H, Morishita A, Yoneyama H, Haba R, Masugata H, Masaki T (2015) Exacerbation of insulin resistance and hepatic steatosis deriving from zinc deficiency in patients with HCV-related chronic liver disease. Biol Trace Elem Res 163(1–2):81–88
Wang Y, Li Y, Yang L, Yin D (2017) Intermittent low dose irradiation enhances the effectiveness of radio-and chemo-therapy for human colorectal adenocarcinoma cell line HT-29. Oncol Rep 38(1):591–597
Schwarz SB, Schaffer PM, Kulka U, Ertl-Wagner B, Hell R, Schaffer M (2008) The effect of radio-adaptive doses on HT29 and GM637 cells. Radiat Oncol 3(1):12
Jiang H, Xu Y, Li W, Ma K, Cai L, Wang G (2008) Low-dose radiation does not induce proliferation in tumor cells in vitro and in vivo. Radiat Res 170(4):477–487
Marples B, Collis SJ (2008) Low-dose hyper-radiosensitivity: past, present, and future. Int J Radiat Oncol Biol Phys 70(5):1310–1318
Li S-J, Liang X-Y, Li H-J, Li W, Zhou L, Chen H-Q, Ye S-G, Yu D-H, Cui J-W (2017) Low-dose irradiation promotes proliferation of the human breast cancer MDA-MB-231 cells through accumulation of mutant P53. Int J Oncol 50(1):290–296
Yan F, He Q, Hu X, Li W, Wei K, Li L, Zhong Y, Ding X, Xiang S, Zhang J (2013) Direct regulation of caspase3 by the transcription factor AP2alpha is involved in aspirin induced apoptosis in MDAMB453 breast cancer cells. Mol Med Rep 7(3):909–914. https://doi.org/10.3892/mmr.2013.1257
Soung YH, Lee JW, Kim SY, Park WS, Nam SW, Lee JY, Yoo NJ, Lee SH (2004) Somatic mutations of CASP3 gene in human cancers. Hum Genet 115(2):112–115
Chen K, Zhao H, Hu Z, Wang L-E, Zhang W, Sturgis EM, Wei Q (2008) CASP3 polymorphisms and risk of squamous cell carcinoma of the head and neck. Clin Cancer Res 14(19):6343–6349
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Grant No.: U–96124).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Danyaei, A., Khanbabaei, H., Teimoori, A. et al. Effect of intermittent low-dose irradiation on the radiotherapy efficiency for MDA-MB-231 human breast adenocarcinoma cell line. J Radiat Oncol 8, 199–208 (2019). https://doi.org/10.1007/s13566-019-00388-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-019-00388-w