Abstract
Objective
This work aimed to evaluate the acute and sub-chronic toxicity of the aqueous bark extract of Detarium microcarpum (D. microcarpum), administered orally to rats.
Materials and methods
An acute (2000 mg/kg) and sub-acute (1000 mg/kg) toxicity studies were conducted following OECD guidelines of 425 and 407 respectively. During these studies, general signs of toxicity were observed. The rats used in sub-chronic toxicity were sacrificed, and histological, hematological, and biochemical analyses were done.
Results
No behavioral changes were observed in all rats. The extract did not bring about any deaths after 14 days at 2000 mg/Kg (acute toxicity) and 28 days at 1000 mg/Kg (sub-acute toxicity) days. Although a significant variation was observed in hematocrit, granulocyte, lymphocyte, ALAT, and total cholesterol levels, the extract had no significant effect (p < 0.05) on the other biochemical and hematological parameters evaluated.
Conclusion
The aqueous bark extract of D. microcarpum administered at the repeated dose affected some biochemical and hematological parameters. We can therefore deduce that the extract has lower toxicity (LD50 ˃ 2000 mg/kg)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-income populations in most developing countries generally use traditional medicine for the prevention and/or treatment of various diseases. Nearly 80% of the African population uses traditional medicine for their health needs [1]. The arsenal of therapeutic compounds that make up these plants represents nearly 50% of chemical compounds [2, 3]. These chemical compounds or secondary metabolites are of various classes including polyphenols (phenolic acids, flavonoids…), and alkaloids. In addition to these therapeutic compounds, these plants contain certain compounds (phytates, hydrogen cyanide, heavy metals) that are naturally present and can have undesirable or even harmful physiological effects. In addition, some of the therapeutic compounds present in high-dose plants (alkaloids) can also cause certain toxicity. The latter is believed to result from the interaction between the toxins and/or metabolites of these plants with certain cellular constituents [4, 5]. These interactions cause degenerative changes such as inflammation, and oxidative stress that lead to dysfunction and even cell death due to toxic effects detectable in body fluids [6]. It is therefore necessary to study the toxicity of plants, to have an opinion on the safety of their extracts [7].
Detarium microcarpum also known as small sweet detar or sweet detar, is a woody food plant of the Fabaceae family. The small sweet detar is a plant of height varying between 5 and 10 m found in semi-arid Sub-Saharan Africa, from Senegal to Sudan [8, 9]. D. microcarpum is used in food and feed (Fruit pulp, seed, leaves, and flowers), construction/fuel (Wood), veterinary medicine (Leaves, and roots), and pharmacopeia (Leaves, roots, bark, fruit, and seeds). D. microcarpum consists of various phytochemical compounds, which give it pharmacological properties. These are polyphenols (flavonoids, tannins), saponins, terpenes, coumarins, and alkaloids that are found in the leaves, bark, roots, fruits, and seeds. The quantities of these compounds vary from one part of the plant to another [10, 11]. Decoction and/or maceration of leaves, roots, fruits, and bark; in traditional medicine treat diarrhea, constipation, meningitis, arthritis, tuberculosis, hypertension, and rheumatism [10, 11]. Oibiokpa et al. [10] have demonstrated the antimicrobial properties of the plant. The small scale is also composed of antinutritional agents such as hydrogen cyanide, phytates, oxalate, arsenite, and cadmium, which may give this plant a probable toxic effect. Since the route of administration influences the toxicity of a xenobiotic, the objective of this study was to evaluate the effect of oral administration of the aqueous bark extract of Detarium microcarpum at a single and repeated dose in albino rats of the Wistar strain.
Methods
Chemicals
The chemicals used were of analytical quality and were purchased from Sigma Aldrich Co., 3050 Spruce Street, Saint Louis, MO 63,103 USA.
Plant material
The bark of D. microcarpum was collected in October 2015 in the Far North region of Cameroon and identified at the Herbarium under No 49834. These barks were later dried at room temperature in a dark place and ground to obtain a powder, which was used to prepare the extract.
Extraction
The aqueous extract (AE) of the bark of D. microcarpum was prepared by decoction. Briefly, 500 g of powder was dissolved in 4 L of distilled water. The extraction ratio was 1/8 (w/v). The mixture was homogenized and then brought to a boil for 45 min. The decoction resulting from this boiling was cooled and then filtered in Whatman paper N°1. The filtrate obtained was evaporated in the oven at 60 °C until a powder (extract) was obtained.
Animal and experimentation design
The acute and sub-acute toxicity study was conducted in female and male rats, respectively. A total of fifteen (15) young adult rats weighing between 180 and 200 g, five (05) females and ten (10) males, were used. They were obtained at the animal house of the laboratory of nutrition and nutritional biochemistry and randomly assigned according to sex in cages, 5 per cage, where they had access to water and food ad libitum. Animals were handled according to the European Union on Animal Care (CEE Council 86/609) guideline adopted by the Cameroon Institutional National Ethics Committee. They were acclimatized seven days before the start of each study. The extract was dissolved in distilled water and administered by gavage at a volume of 2 mL/100 g of body weight.
Acute toxicity
Acute toxicity was assessed according to Guideline 425 of OECD [12]. Briefly, the 5 female rats used received a single dose of 2000 mg/kg of body weight of the extract. The extract was first administered to a single rat and observed for 30 min, then successively to the other four rats within a 30-min time interval. Particular attention was paid to rats during the 4 h preceding the administration of the extract. During these 4 h, the animals were fasting. They were then observed individually every 30 min for 24 h and then once a day for 14 days. The weight of the animals was taken at the beginning, on the 7th day, and at the end (14th day) of the experiment.
Sub-chronic toxicity
Sub-chronic toxicity was assessed according to guideline 407 of OECD [13] protocol with some modifications, limit test of 1000 mg/kg of body weight. The ten (10) male rats were divided according to average weight into two (02) groups of five (05) rats each. The first group (normal or control group) received distilled water and the second group (test group) received D. microcarpum extract daily by AE gavage at 1000 mg/kg. The study lasted 28 days during which the rats gained weight at the beginning, every seven days, and the end of the study. On the 29th day and after 12 h of fasting, the rats were sacrificed by cervical dislocation after being anesthetized with ether. Blood was collected separately in EDTA tubes. One part was used to analyze hematological parameters and the other was centrifuged at 3000 rpm for 5 min to obtain the plasma. The plasma obtained was stored at −20 °C for the evaluation of biochemical parameters.
Signs of toxicity and behavioral changes
During these two studies, parameters such as the presence of diarrhea, and eye color were particularly observed, and behavioral changes such as mobility, aggressiveness, noise sensitivity/sound, coat appearance, and respiratory rate as well as the number of possible deaths were noted [14].
Relative weight of organs
After the animals were sacrificed, the heart, liver, kidneys, brain, and lung were isolated via dissection, introduced into a 0.9% saline solution (NaCl 0.9%), part dry, and weighed immediately. The relative organ weight (ROW) was calculated using the formula of Narhari et al. [2] as follows:
Hematological analysis
Red blood cells, white blood cells, hematocrit level, hemoglobin concentration, mean blood cell volume, mean corpuscular hemoglobin content, mean corpuscular hemoglobin concentration, platelet count, mean platelet volume, granulocytes, lymphocytes, and monocytes were determined using an automated hematological analyzer (SFRI, blood cell counter, H18 Light).
Biochemical analysis
Commercial chronolab brand kits were used for the evaluation of renal (creatinine and urea) and hepatic synthesis (total cholesterol and triglycerides) functions. Hepatic cytolysis (alanine aminotransferase and aspartate aminotransferase) and total protein were determined by the methods of Reitman and Franckel [15] and Lowry et al. [16] respectively.
Histopathological examination
The liver, heart, lung, and kidney excised from the experimental rats (normal and test/treated groups) were fixed in 10% formol in labeled bottles and processed for histological analysis. Tissues embedded in paraffin wax were sectioned 5 um thick, stained with hematoxylin and eosin, mounted on glass slides, and examined under a standard light microscope [17].
Statistical analysis
The results of the hematological and biochemical analyses were expressed as an average standard error on the mean. The values were analyzed with SPSS software version 20.0. The Analysis of Variances test was used for the descriptive analysis and a comparison between the normal and test group was performed by the Mann–Whitney U test. A significant difference was noted at p < 0.05.
Results
Acute toxicity
Administration by gavage of aqueous bark extract of D. microcarpum at a single dose of 2000 mg/kg resulted in no deaths in animals used after 14 days. In addition to no significant effect on weight (Table 1), these animals as a whole showed no signs of toxicity and no significant behavioral change (Table 2). As the extract did not show any signs of toxicity, the LD50 is higher than 2000 mg/kg.
Sub-chronic toxicity
Daily administration of D. microcarpum bark extract at 1000 mg/kg resulted in no deaths or behavioral changes in rats receiving the extract compared to the normal group (Table 3).
Effect of the extract on body weight and relative organ weight.
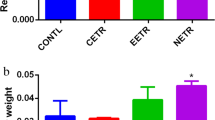
The effect of D. microcarpum bark extract at a dose of 1000 mg/kg administered to animals for 28 days on body weight and relative organ weight (ROW) is presented in Tables 4 and 5, respectively. These results showed that the extract caused a gradual decrease in the weight of rats in the test group from day 7, which was significant (p < 0.05) on day 28 compared to the normal group. Similarly, the ROW of the brain and lungs of the rats receiving the extract was significantly higher than those of the normal group. However, no significant changes (p < 0.05) in liver, heart, and kidney weight were noted when comparing the two groups.
Effect of the extract on hematological parameters.
Table 6 presents the values of all hematological analyses performed on rats. These results showed that at the end of treatment, the extract did not affected (p < 0.05) the levels of red blood cells, monocytes, platelets, hemoglobin, and the mean corpuscular concentration of hemoglobin compared to the normal group. However, an increase in granulocytes and a decrease in lymphocytes and hematocrit were observed in the test group (p < 0.05).
Effect of oral administration of aqueous bark extract of D. microcarpum on biochemical markers associated with organ function.
Plasma values for creatinine, urea, ALAT, ASAT, total cholesterol, triglycerides, and total protein are presented in the table as follows (Table 7). No significant variations (p < 0.05) in creatinine, urea, and triglycerides were observed in the treated group compared to the normal group. However, there was a significantly high ALAT activity and low total cholesterol level in the test group compared to the normal group.
Histopathological assessment
The histopathological analysis of the heart, kidney, and lung of rats treated with D. microcarpum did not show differences compared to rats of the normal group (Fig. 1). But in the liver, histology revealed an expansion of the central vein in rats in the test group compared to those in the normal group. This observation, therefore, shows that the extract caused liver alteration.
Discussion
Toxicity is the expression of adverse effects resulting from the interaction between an administered substance and cells [18]. It, therefore, allows researchers to have a clear idea of the dose of extract or substance that would be safe to administer to animals. The Organisation for Economic Cooperation and Development (OECD) defines acute toxicity as adverse effects that occur at short notice following oral administration of a single or multiple dose substances within 24 h. Sub-chronic toxicity represents adverse effects that occur after repeated administration of a substance [19]. The objective of this work was to study the acute and sub-chronic toxicity of the aqueous bark extract of Detarium microcarpum, administered orally to rats.
The acute toxicity study of aqueous bark extract of D. microcarpum administered at a single dose of 2000 mg/kg resulted in a 1.56% decrease in rat body weight on day 14 compared to the initial weight (Table 1). According to Subramanion et al. [7], a weight loss of more than 10% indicates the adverse effects of a drug or chemical. This shows that oral administration of the bark of D. microcarpum would not influence the growth of rats. No major changes in rat behavior or deaths were observed during the 14 days of the experiment (Table 2). The extract, therefore, appears to be safe for rats and the LD50 would be higher than 2000 mg/kg. Indeed, any substance or compound with an LD50 greater than 1000 mg/kg is considered safe and slightly toxic [14]. This suggests that the extract of D. microcarpum administered at a single dose of 2000 mg/kg body weight is not or only slightly toxic. To confirm this result, analyses of hematological and biochemical parameters after administration of the extract of D. microcarpum in the medium term were performed.
The sub-chronic toxicity study was conducted for 28 days. During this period, the extract of D. microcarpum was administered daily at 1000 mg/kg body weight to rats. Observation of actual behavioral changes and the presence of deaths revealed that the extract did not cause any rat deaths or changes in habits. These results are similar to those obtained in the acute toxicity study, so the extract would be weak or non-toxic. The increase or decrease in body and organ weights is an indicator of a substance's toxicity [12, 13]. During the study, the body weight of animals and the relative weight of organs (heart, liver, kidneys, brain, and lungs) were evaluated. Between the beginning (day 0) and day 14, an insignificant variation (p < 0.05) in body weight was observed in rats in the test group compared to the normal group (Table 4). On day 28, the variation in animal weight observed in the test group (+ 1.19%) was significantly (p < 0.05) lower than that of the normal group (+ 19.48%). Although weight loss was noted, it is important to note that in the test group, the variation in final weight (+ 1.19%) was high compared to the initial day (0%). This result shows that the extract causes poor growth in rats. The relative weight of liver, heart, and kidney organs was not significantly different in the test group with D. microcarpum bark extract compared to the normal group. This weight loss is because the extract prevents the accumulation of fat or has a negative influence on the digestion of fat, and its absorption. However, studies have confirmed the evidence that weight loss is associated with a lack of fat accumulation and physiological adaptation response to plant extracts [20]. This result often underlies a loss of appetite associated with low-calorie intake in animals. A significant increase (p < 0.05) in relative lung and brain weights (ROW) was obtained in the treated/test group compared to the normal group (Table 5). This hypertrophy of these two organs is the result of a possible pro-inflammation activity induced by certain harmful constituents present in the extract. However, the relative weight of the heart, liver, and kidney organs of treated rats was not significantly different (p < 0.05) from that of normal rats. This result is similar to those of Muhammad et al. [14] who evaluated the subchronic toxicity of the ethanolic extract of Pericampylus glaucus administered at 1000 mg/kg. The bone marrow responsible for the synthesis of blood cells is generally the target of toxic compounds [21]. The evaluation of the hematological profile of rats, therefore, showed that the extract caused a significant decrease in the hematocrit level (Table 6). The values obtained in the treated and normal groups of (37.97 ± 1.91) % and (31.27 ± 0.31) %, respectively, are low the reference range (38.90–50.90) % published by Arika et al. [6]. This is proof that this decrease in the hematocrit caused by the extract is however, safe on the blood composition. The extract of D. microcarpum may be non-toxic and would not induce anemia. Leukocytes ensure the body's immunity by protecting it against antigen invasion [6]. They are composed of granulocytes (neutrophils, basophils, and eosinophils) and agranulocytes (lymphocytes and monocytes). In this study, we obtained a non-significant variation (p < 0.05) in total white blood cells. However, the evaluation of specific white blood cells revealed a significant decrease (p < 0.05) of lymphocytes and an increase in granulocytes in the test group compared to the normal group. Although the extract resulted in a decrease in lymphocyte count, the value obtained in the test group was in the reference range (14.10–45.80) % of Arika et al. [6]. The increase in the percentage of granulocytes is explained by the fact that the carbohydrates and anti-nutrients present in the extracts stimulate the production of pro-inflammatory cytokines and advanced glycation products. Indeed, granulocytes and agranulocytes are activated by cytokines, advanced glycation products, oxidative stress, and angiotensin II [22, 23].
Detoxification is carried out by the liver and kidneys [24]. Hence an increase in transaminases (ALAT and ASAT) tells about the inflammation or destruction of the liver while an increase in urea and creatinine refers rather to a malfunction of the nephron [25]. In addition, a slight alteration in ALAT, alkaline phosphatases, ASAT, glucose, urea, and creatinine suggests that sub-chronic administration of extract does not alter hepatocytes and kidneys of rats and their normal metabolism [26]. The extract resulted in a significant increase in ALAT. Similarly, no significant changes (p < 0.05) in urea and creatinine were obtained in either group (Table 7). The extract does not affect kidney function but affects liver function by inducing cytolysis of hepatocytes leading to ALAT loss. Liver synthesis function was assessed by total cholesterol and triglyceride assays. The extract induced a significant decrease in total cholesterol. This result can be due to hepatic cytolysis. Indeed, cytolysis is associated with the loss of membrane integrity and metabolic functions [26]. The significant increase in total plasma protein in the treated group g/mL compared to the normal group results from the loss of brain and lung organ integrity associated with slight liver hypertrophy (Table 7). Indeed, the hypertrophy of an organ can lead to the release of its components (metabolites such as proteins, enzymes, and others) into the extracellular environment, which will lead to abnormally high plasma levels of these components. Histological analysis shows an alteration in the liver of rats in the test group compared to those in the normal group (Fig. 1). This is following the slight hypertrophy of the liver obtained thus increasing plasma levels of total cholesterol metabolites, total proteins, and ALAT activity (Table 7).
Conclusion
This study showed that the aqueous extract of Detarium microcarpum bark administered orally to rats did not result in any deaths, behavioral changes, or significant changes in certain biochemical and hematological parameters in the animals. However, body weight loss and changes in total blood cholesterol, alanine aminotransferase, total protein, granulocytes, and lymphocytes were observed. The bark extract of this plant, therefore, has low toxicity. Doses below 1000 mg/kg may be used for the safe investigation of its therapeutic efficacy.
Abbreviations
- A:
-
Alveol
- AE:
-
Aqueous extract
- ALAT:
-
Alanine amino transferase
- ASAT:
-
Aspartate amino transferase
- CV:
-
Central vein
- G:
-
Glomerular
- LD:
-
Lethal dose
- M:
-
Cardiomyocyte
- NG:
-
Normal group
- OECD:
-
Organization for economic cooperation and development
- ROW:
-
Relative organ weight
- RP:
-
Red pulp
- TG:
-
Test group
- WP:
-
White pulp
References
Antwi-Baffour SS, Bello AI, Adjei DN, Mahmood SA, Ayeh-Kumi PF (2014) The place of traditional medicine in the African society: the science, acceptance, and support. Am J Health Res 2(2):49–54
Narhari D, Durajan G, Sharif H, Sheikh Z (2015) Evaluation of acute and subacute toxicity induced by methanol extract of Terminalia citrina leaves in Sprague Dawley rats. J Acute Dis 4(4):316–321
Tabassum N, Hamdani M (2014) Plants are used to treat skin diseases. Pharmacogn Rev 8:52–60
Fu PP, Xia Q, Lin G, Chou MW (2004) Pyrrolizidine alkaloids–genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab Rev 36:1–55
Madariaga-Mazón A, Hernández-Alvarado RB, Noriega-Colima KO, Osnaya-Hernández A, Martinez-Mayorga K (2019) Toxicity of secondary metabolites. Phys Sci Rev. https://doi.org/10.1515/psr-2018-0116
Arika WM, Nyamai DW, Musila MN, Ngugi MP, Njagi EN (2016) Hematological markers of In Vivo toxicity. J Hematol Thrombo Dis 4(2):1–7
Subramanion L, Zuraini Z, Yeng C, Yee L, Lachimanan Y, Sreenivasan S (2011) Acute oral toxicity of methanolic seed extract of Cassia fistula in Mice. Molecules 16:5268–5282
Aviara NA (2015) Moisture-dependent physical properties of Detarium microcarpum seeds. J Biosyst Eng 40(3):212–223
Gaisberger H, Kindt R, Loo J, Schmidt M, Bognounou F et al (2017) Spatially explicit multi-threat assessment of food tree species in Burkina Faso: a fine-scale approach. PLoS ONE 12(9):e0184457–e0184457
Oibiokpa F, Godwin I, Abubakar N, Kudirat O (2014) Nutritional composition of Detarium microcarpum fruit. AJFS 8(6):342–350
Sani A, Agunu A, Danmalam UH, Ibrahim H (2014) Pharmacognostic studies of the stem bark of Detarium Microcarpum Guill. and Perr. (Fabaceae). Nat Prod Chem Res 1–8
OECD (2008a) Guidelines for testing of chemicals. Test N°425. Acute oral toxicity up and down procedure. Organization for economic co-operation and development publishing. https://doi.org/10.1787/9789264071049-en.
OECD (2008b) Guidelines for testing of chemicals. Test N°407, repeated dose 28-day oral toxicity study in rodents. Organization for Economic Co-operation and Development Publishing. https://doi.org/10.1787/9789264070684-en.
Muhammad K, Mohd S, Pinaki S, Moklesur R, Arindam D, Sreemoy K (2015) Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr in BALB/c mice. J Acute Dis 4(4):309–315
Reitman F (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Lowry O, Rosbrough N, Farr Al, Randall R (1951) Protein determination using Folin Ciocalteu reagent. Biol Chem 193:265–275
Pieme C, Penlap V, Nkegoun B, Taziebou C, Teku E et al (2006) Evaluation of acute and sub-acute extract of leaves of Senna alata (L.) Roxb (Ceasalpiniaceae). Afr J Biotechnol 5(3):283–289
Janet MO, Hadiza LM, Hussaini AM, Musa BB, Abubakar SA (2016) Acute and sub-acute toxicity studies of aqueous and methanol extracts of Nelsonia campestris in rats. J Acute Dis 5:62–70
Gandhare B, Kavimani S, Rajkapoor B (2013) Acute and subacute toxicity study of methanolic extract of Ceiba pentandra (Linn.) Gaertn on Rats. J Sci Res 5(2):315–324
Gepner Y, Shelef I, Schwarzfuchs D, Zelicha H, Tene L et al (2018) Effect of distinct lifestyle interventions on mobilization of fat storage pools. Circulation 137(11):1143–1157
Cartiser N, Bévalot F, Fanton L, Gaillard Y, Guiltton J (2011) State-of-the-art of bone marrow analysis in forensic toxicology: a review. Int J Legal Med 125(2):181–198
Lee P, Peng H, Gelbart T, Beutler E (2004) The IL-6- and lipopolysaccharide-induced transcription of hepcidin in HFE-, transferrin receptor 2-, and beta microglobulin-deficient hepatocytes. Proc Natl Acad Sci USA 101:9263–9265
Shurtz-Swirski R, Sela S, Herskovits A, Shasha S, Shapiro G et al (2004) Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care 24:104–110
Harizal S, Mansor S, Hasman J, Tharakan J, Abdullah J (2010) Acute toxicity study of the standardized methanolic extract of Mitragyna speciose Korth in rodents. J Ethnopharmacol 131:404–409
Mariappan G, Saha B, Sutharson L, Singh A, Garg S et al (2011) Analgesic, anti-inflammatory, anti-pyretic and toxicological evaluation of some newer 3-methyl pyrazolone derivatives. Saudi Pharm J 19(2):115–122
Diallo A, Eklu-Gadegkeku K, Agbonon A, Aklilokou K, Creppy E et al (2010) Acute and sub-chronic (28-day) oral toxicity studies of hydro alcohol leaf extract of Ageratum conyzoids L (Asteraceae). Trop J Pharm Res 9(5):463–467
Acknowledgements
The authors would like to thank the Human Biology Laboratory of the Medical and Medicinal Plants Research Institute (IMPM) of Cameroon for the analysis of hematological parameters and the laboratory of pathological anatomy of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé 1-Cameroon for histopathological examination that facilitated this study.
Author information
Authors and Affiliations
Contributions
JLN was involved in conceptualization, methodology and software. JAYF was involved in investigation and writing—original draft preparation. GRTN performed the investigation. GRTN and MF were involved in reviewing and editing. JEO was involved in supervision.
Corresponding author
Ethics declarations
Conflict of interest
Janvier Aimé Youovop Fotso, Guy Roussel Takuissu Nguemto, Judith Laure Ngondi, Julius Enyong Oben declare that we have no conflict of interest.
Ethical approval
Animals were handled according to the European Union on Animal Care (CEE Council 86/609) guideline adopted by the Cameroon Institutional National Ethics Committee.
Rights and permissions
About this article
Cite this article
Fotso, J.A.Y., Takuissu, G.R.N., Ngondi, J.L. et al. Acute and sub-chronic toxicity studies of aqueous bark extract of Detarium microcarpum guill. and perr in albinos wistar rat. Toxicol. Environ. Health Sci. 14, 269–276 (2022). https://doi.org/10.1007/s13530-022-00139-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-022-00139-4