Abstract
Objective
The prevalence of nonalcoholic fatty liver disease (NAFLD) with type 2 diabetes mellitus (T2DM) is increasing, which causes greater harm to human health. Cyclocarya paliurus (CP) has antihyperglycemic and antihyperlipidemic effects. Here, we investigated the effects of polysaccharides (CPP) and flavonoids (CPF) from CP on gut microbiota, hepatic steatosis, and metabolic parameters in high-fat diet (HFD)/streptozotocin (STZ)-induced NAFLD rats with T2DM.
Methods
NAFLD/T2DM rats, which were induced by high-fat diet (HFD) for 8 weeks and a low dose of 25 mg/kg STZ, were treated with CPP (8 g/kg/d) or CPF (6 g/kg/d) for 12 weeks. The alterations to gut microbiota, hepatic steatosis, and metabolic parameters were measured.
Results
Treatment of both CPP and CPF could improve liver steatosis, NAFLD activity score (NAS), hyperglycemia, and hyperlipidemia. Importantly, administration with both CPP and CPF led to the significant reversion of increased abundance of the pathogenic bacteria Escherichia-Shigella in NAFLD/T2DM rats; moreover, CPP supplement also dramatically increased the beneficial bacteria Akkermansia abundance, while CPF treatment significantly elevated the abundances of the beneficial bacteria Romboutsia and Weissella.
Conclusion
Both CPP and CPF as prebiotics have the significant therapeutic effects on hepatic steatosis and metabolic abnormalities induced by HFD and STZ in rats at least partially by modulating gut microbiota.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emergence of non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) as a global epidemic is one of the major challenges to human health in the twenty-first century. NAFLD is now recognized as the most prevalent chronic liver disease worldwide, with a prevalence as high as 30% in the general population [1]. NAFLD includes a series of diseases ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), and to advanced cirrhosis and hepatocellular carcinoma [2]. It has been well-recognized that obesity, hyperlipidemia, diabetes mellitus, metabolic syndrome, and insulin resistance (IR) are considered as risk factors of NAFLD [3, 4]. T2DM is a complex metabolic disorder characterized by hyperglycemia, low-grade inflammation, IR, and β-cell failure and mainly affects glucose, lipid, and protein metabolism [5, 6]. It is apparent that NAFLD and T2DM share common risk factors, such as obesity, IR. The prevalence of NAFLD in the population of T2DM is increasing year by year, and has been found to be higher compared with that without T2DM [7, 8]. It should be noted that NAFLD with T2DM causes greater harm to health. People with NAFLD and T2DM are more likely to suffer from cardiovascular disease, chronic kidney disease, and carcinoma [3, 9].
Recent studies have shown that gut microbiota dysbiosis is associated with several non-communicable diseases such as obesity, diabetes, cardiovascular diseases, and NAFLD [10,11,–12]. A direct involvement of gut microbiota in the development of NAFLD is suggested by the finding that NAFLD can be delivered to germ-free mice by fecal microbiota transplantation [13]. Imbalances in the structure of gut microbiota are related to gut barrier dysfunctions and cause insulin resistance and endotoxemia, which may finally lead to obesity and T2DM [14,15,16,17,18]. These results suggest that regulating gut microbiota could treat NAFLD and T2DM. At present, many of the anti-diabetic drugs currently in clinical use, though effectively treating symptoms, have several side effects including hepatic and renal lesions [19]. On the other hand, there is still no effective drugs for the treatment of NAFLD except for lifestyle changes, including healthy diet, weight loss, and exercise [20]. In addition, there is no consensus or therapeutic strategies for the management of NAFLD patients with T2DM.
Cyclocarya paliurus (CP) (Batal.) Iljinsk (family Cyclocaryaceae) is a plant with edible and medicinal value, which is grown in mountainous regions of Southern China. The leaves of CP have long been used as a dietary food and a traditional herbal medicine for the prevention or treatment of diabetes mellitus, hypertension, hyperliposis [21,22,23]. CP leaves contain a variety of biologically chemical components, including polysaccharides, flavonoids, coumarins, amino acids, sterols, and triterpenes [24], in which polysaccharides and flavonoids are recognized as the main bioactive components in CP [22, 25, 26]. It has been reported that polysaccharides and flavonoids from CP possess many bioactivities, such as anti-inflammatory, anti-hyperlipidemic, and anti-diabetic activities [23, 25,26,27,28].
However, to the best of our knowledge, no investigation has been performed to explore the protective effects of polysaccharides (CPP) and flavonoids (CPF) from CP on metabolic abnormalities and liver damage in NAFLD rats with T2DM (NAFLD/T2DM rats) induced by high-fat diet and streptozotocin (STZ). In the present study, we explored the effects of CPP and CPF on gut microbiota, hepatic steatosis, and metabolic parameters in NAFLD/T2DM rats.
Materials and methods
Preparation of CPP and CPF
The extraction of CPP was performed according to the method described previously [29]. Briefly, the air-dried and powdered leaves were soaked with 95% (v/v) ethanol for 12 h, and the mixture was filtered. The residues were dried in air and were boiled with distilled water at 95°C for 2 h (1:20, mg/mL). The above operation was repeated twice. Then, the mixture was filtered, and the filter liquor was retained. The filter liquor was concentrated by rotary evaporation at 60°C and then allowed to put overnight at 80% (v/v) ethanol concentration. The protein in the obtained CPP was removed with Savag method, and the CPP was dried under vacuum at −40°C. The CPP content was 79.6%, which was determined by phenol-sulfuric acid method [30] (Fig. 1).
Preparation of CPF was conducted according to the method described by Cheng et al. [31]. Briefly, CP leaves were pulverized and extracted with distilled water at 95∘C for 40min. After extraction, the extract was centrifuged at 4500×g for 15 min, and the above operation was repeated. The supernatants were combined and concentrated using a rotary evaporator. The residue was dissolved with deionized water, filtered by a 0.45-μm microfiltration membrane, and applied to a column (30×1.6 cm) of AB-8 resin. Finally, the effluent of ethanol solution was collected and concentrated, leading to the CPF extract. The CPF content was 84.3%, which was determined by aluminum chloride method [32] (Fig. 1).
Animal model and experiment design
A total of 60 male Sprague Dawley rats (weight, 180±20 g; age, 6 weeks) were purchased from the medical laboratory animal center of Guangdong (Guangzhou, China). All the rats were acclimatized under a temperature of 24±2°C, a relative humidity of 55±10%, and a 12 h light/dark cycle for 10 days before commencement of the animal experiment. All animal experiments were approved by the experimental animal ethics committee of Jinan University and was performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Jinan University.
After acclimation, these rats were randomly divided into the normal control group (NC, n=10) and the model group with both NAFLD and diabetes (NAFLD/T2DM Model, n=50). NAFLD/T2DM Model rats were induced by high-fat diet (HFD) (containing 34% fat, 2% cholesterol, 26% carbohydrate, 26% protein, and 12% basic feed (w/w)) for 8 weeks, followed by an intraperitoneal injection with streptozotocin (STZ) (25 mg/kg in citrate buffer). The rats with fasting glucose level higher than 11 mmol/L were considered as NAFLD/T2DM Model rats, and the NAFLD/T2DM Model rats with consecutive 10-day hyperglycemia (11 mmol/L or greater) were used for the experiment. Finally, 48 NAFLD/T2DM Model rats met the above experimental standard. In parallel, NC rats, which were fed a standard laboratory diet, were injected with an equal volume of citrate buffer solution.
The 48 NAFLD/T2DM Model rats were randomly divided into four groups: NAFLD/T2DM group (n=12), CPP group (n=12), CPF group (n=12), and Glipizide group (n=12), which were continuously fed with the HFD, and administrated by oral gavage once per day with distilled water, CPP (8 g/kg/d), CPF (6 g/kg/d), and Glipizide (2.5 mg/kg/d) (Glipizide extended release tablets, Glucotrol XL), respectively for the next 12 weeks. The NC group rats were fed with the standard laboratory diet for the next 12 weeks.
Observations on the general condition of the rats
The general condition of the rats was monitored daily, including physical activity, fur condition, water intake, food intake, urine output and survival condition. Body mass and food intake were determined weekly.
Analysis of metabolic parameters
At the end of the experiment, overnight-fasted rats were anesthetized by 1% pentobarbital solution (40mg/kg), and the blood samples were collected from abdominal aortic. The fasting blood glucose (FBG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), interleukin (IL)-6, and tumor necrosis factor (TNF)-α were analyzed using commercial assay kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturers’ instructions. The fasting insulin (FINS) was measured with rat insulin kits (R&D Corporation, USA). The insulin resistant index (HOMA-IR) was also calculated by using the following formula:
Analysis of hepatic pathology
At the end of the study, the rats were sacrificed to determine liver mass and liver mass index by using the following formula:
Then, the liver samples were immersed in 10% formalin neutral buffer solution for 48h, then processed routinely, embedded in paraffin, sectioned to 5 μm thickness and stained with hematoxylin and eosin (H&E). Then, we used Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA) to quantitatively analyze fat in liver [13]. The evaluation standard of NAFLD activity score (NAS) is shown in Table S1 [33, 34].
Gut microbiota analysis
At the end of the experiment, feces of rats were collected in sterilized plastic tubes and stored in a -80 °C until use. Total DNA of the samples was extracted using a HiPure Stool DNA Kits (Guangzhou Meiji biotechnology co. LTD, Guangzhou, China) following manufacturer’s recommendations. The V3-V4 region of bacterial 16S rDNA gene was amplified using primers 341F (5’- CCT ACG GGN GGC WGC AG -3’) and 806R (5’-GGA CTA CHV GGG TAT CTA AT-3’). Sequencing libraries were generated using two-step PCR amplification method. The second round of amplification products was purified using AMPure XP Beads (Beckman Coulter, USA) and quantified using a QuantiFluorTM fluoromete (Promega, USA). At last, the library was sequenced on IlluminaHiSeq 2500 platform (Illumina, USA).
Statistical analysis
Statistical analysis was performed using SPSS 21.0 software (IBM Corp., USA). Quantitative data were analyzed by independent-Samples t Test or one-way analysis of variance (ANOVA) followed by Mann-Whitney U test or Kruskal-Wallis H test. Correlation was evaluated by Pearson correlation co-efficient analysis. A difference with p<.05 was considered significant.
Results
Effects of CPP and CPF on general condition
The rats in the NAFLD/T2DM group showed sluggish action, irritability, polydipsia, bulimia, and polyuria, while those in the NC group did not. The above general condition was significantly improved in the CPP group, CPF group, and Glipizide group rats compared with the NAFLD/T2DM group rats.
A significant decrease in the body mass was observed in the NAFLD/T2DM group compared with the NC group. After treatment of both CPP and CPF, the rats showed a significant increase in the body mass compared with the NAFLD/T2DM group rat (Table 1). A significant increase in the body mass was also found in the Glipizide group compared with the NAFLD/T2DM group.
Effects of CPP and CPF on metabolic parameters
A significant increase in serum liver enzymes (ALT, AST, and GGT), serum lipid profile (TC, TG, LDL-C), glucose metabolism indices (FBG, FINS, and HOMA-IR), serum proinflammatory cytokines (TNF-α and IL-6) was observed in the NAFLD/T2DM group compared with the NC group. After treatment of both CPP and CPF, a remarkable decrease in the above metabolic parameters was found compared with the NAFLD/T2DM group. In addition, Glipizide treatment significantly lowered serum TG, glucose metabolism indices (FBG, FINS, and HOMA-IR), serum proinflammatory cytokines (TNF-α and IL-6), but had no effect on serum liver enzymes (ALT, AST, and GGT), and serum TC and LDL-C compared with the NAFLD/T2DM group (Table 1).
Effects of CPP and CPF on hepatic pathological changes
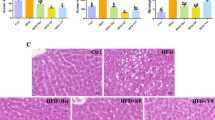
The rats in the NAFLD/T2DM group showed a significant increase in liver mass, liver mass index, liver fat content, and NAS compared with the NC rats, whereas both CPP and CPF intervention significantly decreased the above hepatic pathological parameters. Glipizide treatment also lowered liver mass index, but had no significant influence on liver mass, liver fat content, and NAS compared with the NAFLD/T2DM group (Table 1 and Fig. 2).
Effects of CPP and CPF on gut microbiota composition
The structural changes of gut microbiota are crucial in the pathogenesis of obesity and some other metabolic diseases. To assess the changes of gut microbiota composition after treatment of both CPP and CPF in NAFLD/T2DM rats, 16S rDNA gene sequences from bacterial populations of intestinal contents were analyzed. At the phylum level, the 5 most abundant microbiota in all groups included Firmicutes, Bacteroidetes, Actinobacteria, Verrucomicrobia, and Proteobacteria (Fig. 3A). The relative abundances of Firmicutes (51.1%) and Bacteroidetes (0.3%) decreased significantly, while the relative abundance of Proteobacteria (29.3%) increased significantly in the NAFLD/T2DM group compared with the NC group (Fig. 3B–D). CPP treatment significantly decreased the relative abundances of Firmicutes (24.0%) and Proteobacteria (1.8%) (Fig. 3B and D), and increased the relative abundance of Verrucomicrobia (69.4%) (Fig. 3E), whereas CPF treatment remarkably increased the abundances of Firmicutes (73.4%) and Bacteroidetes (1.9%) compared with the NAFLD/T2DM group (Fig. 3B and C). In addition, the ratio of Firmicutes to Bacteroidetes increased significantly in the NAFLD/T2DM group compared with the NC group, whereas treatment of both CPP and CPF led to the reduction of this ratio but this reduction was not significant compared with the NAFLD/T2DM group (Fig. 3F). In the Glipizide group, the variation of the gut microbiota abundance was similar to the CPF group (Fig. 3B and Table S2).
At the genus level, the composition of the bacteria was substantially changed (Fig. 4A). The relative abundances of Ruminococcaceae_UCG-005 (0.6%), Lactobacillus (4.3%), Weissella (1.0%), Romboutsia (4.1%) decreased significantly (Fig. 4B–E), while the relative abundances of Escherichia-Shigella (22.0%), Collinsella (6.0%), and Blautia (6.6%) increased markedly in the NAFLD/T2DM group compared with the NC group (Fig. 4–H). CPP treatment decreased the relative abundance of Escherichia-Shigella (1.0%) and increased the relative abundance of Akkermansia (45.3%) significantly (Fig. 4F and I), while CPF treatment decreased the relative abundance of Escherichia-Shigella (4.6%) (Fig. 4F) and increased the relative abundances of Romboutsia (10.9%) (Fig. 4E) and Weissella (4.2%) (Fig. 4D) significantly compared with the NAFLD/T2DM group. In the Glipizide group, the relative abundance of Escherichia-Shigella (2.5%) decreased markedly (Fig. 4F) and the relative abundances of Lactobacillus (10.4%) and Romboutsia (6.4%) and Dubosiella (8.1%) (Fig. 4C, E, and J) increased significantly (Table S3).
Correlation between improvements of metabolic indices as well as hepatic pathology and the changes in gut bacterial genera induced by CPP and CPF
We examined whether the improvements of metabolic indices and hepatic pathology were correlated with the alternations of bacterial genera induced by CPP and CPF. Pearson correlation analysis showed that the alternation of Akkermansia level was negatively correlated with the main metabolic and hepatic pathological indices, whereas Escherichia-Shigella level was positively correlated with most of the above results after CPP treatment. Moreover, other CPP-modulated genera, such as Blautia, Lactobacillus, Dubosiella, Collinsell, Romboutsia, Weissella, Aerococcus, and Ruminococcaceae_UCG-005 also contributed to the changes in a few indices of the above results (Fig. 5A). On the other hand, the level of Romboutsia was negatively correlated with most of the metabolic and hepatic pathological parameters, whereas Escherichia-Shigella, Collinsell, Dubosiella, and Aerococcus were positively correlated with the partial above indices after CPF treatment. In addition, Blautia, Lactobacillus, Akkermansia, Weissella, and Ruminococcaceae_UCG-005 enriched by CPF were also negatively associated with some serum liver enzymes and lipid profile (Fig. 5B).
Discussion
In the present study, a rat model of NAFLD/T2DM was induced by HFD for 8 weeks and a low dose of 25 mg/kg STZ, which reveals similar metabolic characteristics and liver damage of NAFLD with T2DM in humans [35, 36]. Treatment with both CPP and CPF significantly improved body mass, liver enzymes (ALT, AST, and GGT), blood lipids (TC, TG, and LDL-C), glucose metabolism parameters (FBG, FINS, and HOMA-IR), proinflammatory cytokines (TNF-α and IL-6), and hepatic pathological parameters (liver mass, liver mass index, liver fat content, and NAS), while Glipizide treatment elevated body mass and only reduced glucose metabolism parameters (FBG, FINS, and HOMA-IR) and proinflammatory cytokines levels (TNF-α and IL-6) in the NAFLD/T2DM rats. These results suggest that the protective effects of both CPP and CPF on NAFLD with T2DM in rat models are superior to Glipizide. Similar to our results, Lin et al. found that chloroform extract of CP markedly decreased the levels of serum liver enzymes (ALT, AST, and ALP), blood lipids (TC and TG) and liver lipids (TC and TG), and serum and liver TNF- α in NAFLD rats [37]. These effects were partially through decreasing serum NEFAs which might lead to a decrease in the amount of liver lipid intake, as well as suppressing hepatic lipid de novo synthesis. CPP and CPF also ameliorated HFD-induced hepatic oxidative stress and inflammation, leading to block the development of NAFLD.
The mechanisms responsible for the protective effect of CP on NAFLD and T2DM have attracted much attention. Accumulating evidence has indicated that gut microbiota dysbiosis is correlated with the pathogenesis of NAFLD, T2DM and obesity [23, 38,39,40], thus speculating that the therapeutic role of CP in metabolic diseases might be partially attributed to the alteration of gut microbes. It has been shown that a higher ratio of Firmicutes to Bacteroidetes is observed in obese individuals than lean individuals [40], and this ratio is significantly reduced after CPF treatment in a high-fat diet-induced obesity mouse model [31].
In the current study, at the phyla level, oral administration of CPP dramatically elevated the abundance of Verrucomicrobia and lowered the abundances of Proteobacteria and Firmicutes in the NAFLD/T2DM rats, and the ratio of Firmicutes to Bacteroidetes also showed a decrease trend after CPP treatment; on the other hand, CPF treatment significantly recovered dysbiosis of Firmicutes and Bacteroidetes and reduced the ratio of Firmicutes to Bacteroidetes (although there was no significant difference in this ratio) in the NAFLD/T2DM rats. At the genus level, oral administration with both CPP and CPF led to the significant reversion of increased abundance of Escherichia-Shigella induced by HFD and STZ in rats; in addition, CPP supplement dramatically increased the abundance of Akkermansia, while CPF treatment led to a significant increase in the abundances of Romboutsia and Weissella. Further pearson linear correlation analysis showed that the significant increase in Akkermansia abundance and the significant decrease in Escherichia-Shigella abundance induced by CPP treatment were associated with the improvements of the main metabolic and hepatic pathological indices; on the other hand, elevated Romboutsia level and reduced Escherichia-Shigella level after CPF treatment were correlated with the alleviation of most abnormal metabolic and hepatic pathological parameters in NAFLD/T2DM rats. Besides, Glipizide treatment led to the increased abundances of Lactobacillus, Romboutsia, and Dubosiella and the decreased abundance of Escherichia-Shigella.
Consistent with our results, the report by Yao et al. indicated that CPP treatment alleviates blood glucose, blood lipid, and HOMA-IR index by increasing the short-chain fatty acids (SCFAs)-producing gut bacteria in rats with T2DM [41]; Li et al. showed that CPP treatment increased the beneficial bacteria genus Ruminococcaceae UCG-005 abundance, which in turn attenuated FBG and HOMA-IR in type 2 diabetic rats [38]; Bai et al. showed that treatment of flavonoids of Quzhou Fructus Aurantii extract significantly reduced obesity, inflammation and liver steatosis by the reduction of Firmicutes to Bacteroidetes ratio, the increase in genera Akkermansia and Alistipes, and the decrease in genera Dubosiella, Faecalibaculum, and Lactobacillus in HFD-induced obesity mouse model [42]; the report by Li et al. revealed that Silybin administration showed protective effects against high-fat diet-induced obesity, insulin resistance, and liver steatosis in mice, which was associated with lowering the Firmicutes to Bacteroidetes ratio and increasing the abundances of SCFA-producing bacteria ( Blautia, Bacteriodes, and Akkermensia) [43].
It has been shown that Escherichia-Shigella, Aerococcus, Collinsella, and Dubosiella may contribute to the development of metabolic diseases such as obesity, NAFLD, and T2DM [42, 44,45,46,47,48,49]. On the other hand, it has been accepted that decreased abundances of some beneficial bacteria in gut, such as Ruminococcaceae_UCG-005, Lactobacillus, Akkermansia, and Blautia, are associated with obesity, NAFLD, and T2DM [42, 43, 50]. These beneficial bacteria can produce SCFAs by metabolizing polysaccharides, and in turn maintain the integrity of the intestinal mucosal barrier [38, 51, 52]. SCFAs have a variety of physiological functions, including shaping the gut environment, influencing the physiology of the colon, being utilized as energy sources by host cells and intestinal microbiota, and participating in different host-signaling mechanisms [53, 54]. Akkermansia can also degrade mucin, thereby protecting the intestinal mucosal barrier and reducing protein deposition [55]. Weissella species, which are Gram-positive coccobacilli, are potential probiotics [56, 57]. The genus Romboutsia, which also are Gram-positive organisms, are in the gut of healthy humans and rats [58,59,60].
Taken together, our findings suggest that both CPP and CPF as prebiotics could partially recover the gut microbiota equilibrium, especially with CPP inhibiting the growth of Escherichia-Shigella and dramatically increasing Akkermansia population, and with CPF restraining the growth of Escherichia-Shigella and enhancing the abundances of Romboutsia and Weissella in NAFLD/T2DM rat model. Such changes in the composition of the gut microbiota in turn improve liver steatosis, hyperglycemia, hyperlipidemia, insulin resistance, and inflammation induced by HFD and STZ in rats.
In conclusion, to the best of our knowledge, this study is the first report that demonstrated administration with both CPP and CPF had the significant therapeutic effects on liver steatosis and metabolic abnormalities induced by HFD and STZ in rats. The mechanism responsible for the effects may be at least partially correlated with modulating gut microbiota composition, as indicated by inhibiting the growth of pathogenic bacteria Escherichia-Shigella and discrepantly expanding the abundances of beneficial bacteria Akkermansia, or Romboutsia and Weissella by CPP and CPF treatment, respectively.
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- T2DM:
-
Type 2 diabetes mellitus
- CP:
-
Cyclocarya paliurus
- CPF:
-
Cyclocarya paliurus polysaccharides
- CPP:
-
Cyclocarya paliurus flavonoids
- HFD:
-
High-fat diet
- STZ:
-
Streptozotocin.
- NAS:
-
NALFD activity score
- NASH:
-
Nonalcoholic steatohepatitis
- IR:
-
Insulin resistance
- FBG:
-
The fasting blood glucose
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- GGT:
-
γ-glutamyl transpeptidase
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- LDL-C:
-
Low-density lipoprotein cholesterol
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- FINS:
-
The fasting insulin
- HOMA-IR:
-
The insulin resistant index
References
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.
Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11(1):1–16.
Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–46.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70(3):531–44.
Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, Lai Z. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine (Baltimore). 2017;96(39):e8179.
Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31.
Loscalzo J. Lipid metabolism by gut microbes and atherosclerosis. Circ Res. 2011;109(2):127–9.
Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58(1):120–7.
Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–94.
Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–103.
Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–23.
Brugman S, Klatter FA, Visser JT, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49(9):2105–8.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget Á, Delmée E, Cousin B́, Sulpice T, Chamontin B, Ferrières J, Tanti JF̧, Gibson GR, Casteilla L, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72.
Cani PD, Bibiloni R, Knauf C, Waget Á, Neyrinck AM, Delzenne NM, Burcelin Ŕ. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81.
Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65(3):385–411.
Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol. 2018;24(30):3361–73.
Kurihara H, Fukami H, Kusumoto A, et al. Hypoglycemic action of Cyclocarya paliurus (Batal.) Iljinskaja in normal and diabetic mice. Biosci Biotechnol Biochem. 2003;67(4):877–80.
Xie JH, Dong CJ, Nie SP, Li F, Wang ZJ, Shen MY, Xie MY. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015;186:97–105.
Ma Y, Jiang C, Yao N, Li Y, Wang Q, Fang S, Shang X, Zhao M, Che C, Ni Y, Zhang J, Yin Z. Antihyperlipidemic effect of Cyclocarya paliurus (Batal.) Iljinskaja extract and inhibition of apolipoprotein B48 overproduction in hyperlipidemic mice. J Ethnopharmacol. 2015;166:286–96.
Wang YR, Cui BS, Han SW, Li S. New dammarane triterpenoid saponins from the leaves of Cyclocarya paliurus. J Asian Nat Prod Res. 2018;20(11):1019–27.
Yang Z, Wang J, Li J, Xiong L, Chen H, Liu X, Wang N, Ouyang K, Wang W. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohydr Polym. 2018;183:11–20.
Xiong L, Ouyang KH, Jiang Y, et al. Chemical composition of Cyclocarya paliurus polysaccharide and inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophage. Int J Biol Macromol. 2018;107(Pt B):1898–907.
Wu Q, Wang M, Simon JE. Determination of isoflavones in red clover and related species by high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J Chromatogr A. 2003;1016(2):195–209.
Zhang X, Duan X, Liu X. The effect of Cyclocarya paliurus polysaccharide (CP) on blood glucose and histomorphology of pancreas in diabetic mice (in Chinese). Acta Med Sin. 2010;23:15–7.
Xie JH, Liu X, Shen MY, Nie SP, Zhang H, Li C, Gong DM, Xie MY. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013;136(3-4):1453–60.
Xie JH, Shen MY, Xie MY, Nie SP, Chen Y, Li C, Huang DF, Wang YX. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydr Polym. 2012;89(1):177–84.
Cheng L, Chen Y, Zhang X, Zheng X, Cao J, Wu Z, Qin W, Cheng K. A metagenomic analysis of the modulatory effect of Cyclocarya paliurus flavonoids on the intestinal microbiome in a high-fat diet-induced obesity mouse model. J Sci Food Agric. 2019;99(8):3967–75.
Feng Z, Wu C, Fang S. Technology optimization of total flavonoids extraction from Cyclocarya paliurus leaves by ultrasonic assistance (in Chinese). T Chin Soc Agric March. 2009;40:130–4.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21.
Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32(1):3–13.
Fan Y, Xiong W, Li J, Hu A, He Z, Zhang J, Zhou G, Yin Q. Mechanism of TangGanJian on nonalcoholic fatty liver disease with type 2 diabetes mellitus. Pharm Biol. 2018;56(1):567–72.
Ma H, Cui F, Dong JJ, You GP, Yang XJ, Lu HD, Huang YL. Therapeutic effects of globular adiponectin in diabetic rats with nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(40):14950–7.
Lin Z, Wu ZF, Jiang CH, Zhang QW, Ouyang S, Che CT, Zhang J, Yin ZQ. The chloroform extract of Cyclocarya paliurus attenuates high-fat diet induced non-alcoholic hepatic steatosis in Sprague Dawley rats. Phytomedicine. 2016;23(12):1475–83.
Li Q, Hu J, Nie Q, et al. Hypoglycemic mechanism of polysaccharide from Cyclocarya paliurus leaves in type 2 diabetic rats by gut microbiota and host metabolism alteration. Sci China Life Sci. 2020;64:117–32.
Liu Y, Qian C, Ding S, Shang X, Yang W, Fang S. Effect of light regime and provenance on leaf characteristics, growth and flavonoid accumulation in Cyclocarya paliurus (Batal) Iljinskaja coppices. Bot Stud. 2016;57(1):28.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3.
Yao Y, Yan L, Chen H, Wu N, Wang W, Wang D. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine. 2020;77:153268.
Bai YF, Wang SW, Wang XX, Weng YY, Fan XY, Sheng H, Zhu XT, Lou LJ, Zhang F. The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutr Diabetes. 2019;9(1):30.
Li X, Wang Y, Xing Y, Xing R, Liu Y, Xu Y. Changes of gut microbiota during silybin-mediated treatment of high-fat diet-induced non-alcoholic fatty liver disease in mice. Hepatol Res. 2020;50(1):5–14.
Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16(4):375–81.
Yin X, Peng J, Zhao L, Yu Y, Zhang X, Liu P, Feng Q, Hu Y, Pang X. Structural changes of gut microbiota in a rat non-alcoholic fatty liver disease model treated with a Chinese herbal formula. Syst Appl Microbiol. 2013;36(3):188–96.
Xiao S, Liu C, Chen M, Zou J, Zhang Z, Cui X, Jiang S, Shang E, Qian D, Duan J. Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl Microbiol Biotechnol. 2020;104(1):303–17.
Chen M, Liao Z, Lu B, Wang M, Lin L, Zhang S, Li Y, Liu D, Liao Q, Xie Z. Huang-Lian-Jie-Du-Decoction Ameliorates Hyperglycemia and Insulin Resistant in Association With Gut Microbiota Modulation. Front Microbiol. 2018;9:2380.
Astbury S, Atallah E, Vijay A, Aithal GP, Grove JI, Valdes AM. Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis. Gut Microbes. 2020;11(3):569–80.
Lambeth SM, Carson T, Lowe J, Ramaraj T, Leff JW, Luo L, Bell CJ, Shah VO. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J Diabetes Obes. 2015;2(3):1–7.
Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200.
Cui HX, Zhang LS, Luo Y, Yuan K, Huang ZY, Guo Y. A Purified Anthraquinone-Glycoside Preparation From Rhubarb Ameliorates Type 2 Diabetes Mellitus by Modulating the Gut Microbiota and Reducing Inflammation. Front Microbiol. 2019;10:1423.
Zhang DY, Zhu L, Liu HN, Tseng YJ, Weng SQ, Liu TT, Dong L, Shen XZ. The protective effect and mechanism of the FXR agonist obeticholic acid via targeting gut microbiota in non-alcoholic fatty liver disease. Drug Des Devel Ther. 2019;13:2249–70.
Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes (Lond). 2015;39(9):1331–8.
Rios-Covian D, Ruas-Madiedo P, Margolles A, et al. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol. 2016;7:185.
Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73(23):7767–70.
Fusco V, Quero GM, Cho GS, Kabisch J, Meske D, Neve H, Bockelmann W, Franz CMAP. The genus Weissella: taxonomy, ecology and biotechnological potential. Front Microbiol. 2015;6:155.
Kot W, Neve H, Heller KJ, Vogensen FK. Bacteriophages of leuconostoc, oenococcus, and weissella. Front Microbiol. 2014;5:186.
Russell JT, Roesch L, Ordberg M, et al. Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome. Nat Commun. 2019;10(1):3621.
Song YL, Liu CX, McTeague M, Summanen P, Finegold SM. Clostridium bartlettii sp. nov., isolated from human faeces. Anaerobe. 2004;10(3):179–84.
Gerritsen J, Fuentes S, Grievink W, van Niftrik L, Tindall BJ, Timmerman HM, Rijkers GT, Smidt H. Characterization of Romboutsia ilealis gen. nov., sp. nov., isolated from the gastro-intestinal tract of a rat, and proposal for the reclassification of five closely related members of the genus Clostridium into the genera Romboutsia gen. nov., Intestinibacter gen. nov., Terrisporobacter gen. nov. and Asaccharospora gen. nov. Int J Syst Evol Microbiol. 2014;64(Pt 5):1600–16.
Acknowledgements
The authors appreciate study investigators and staff who participated in this study.
Funding
This study was supported by the following funding source: XJ. Peng: Health Commission Funding Project of Hunan province, China (No. B2019145); XJ. Peng: Science and Technology Funding Project of Chenzhou, Hunan province, China (No. zdyf201848).
Author information
Authors and Affiliations
Contributions
Lu Zhong, Xiaojuan Peng, Chutian Wu, and Qing Li contributed equally to this paper. Lu Zhong, Xiaojuan Peng, Chutian Wu, and Qing Li helped design the study, analyzed the study data, helped draft the manuscript, made critical revisions of the manuscript, and provided final approval of the version. Lu Zhong, Yanfang Chen, Min Wang, Yuting Li, Kaiyin He, and Ying Shi performed the experiment, analyzed the study data, helped draft the manuscript, and provided final approval of the version. Shaohui Tang designed the study, analyzed study data, drafted the manuscript, made critical revisions of the manuscript, and provided final approval of the version.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were approved by the experimental animal ethics committee of Jinan University and was performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Jinan University (2019021101).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, L., Peng, X., Wu, C. et al. Polysaccharides and flavonoids from cyclocarya paliurus modulate gut microbiota and attenuate hepatic steatosis, hyperglycemia, and hyperlipidemia in nonalcoholic fatty liver disease rats with type 2 diabetes mellitus. Int J Diabetes Dev Ctries 43, 317–327 (2023). https://doi.org/10.1007/s13410-022-01080-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01080-5