Abstract
Purpose
Diabetic retinopathy is the leading cause of vision loss and impairment in developed countries. Inflammation is considered one of the main reasons for the progression of diabetes complications. We investigated the relationship of monocyte/high-density lipoprotein-cholesterol ratio (MHR) with type 2 diabetes mellitus (T2DM) and diabetic retinopathy (DR).
Methods
A total of 118 T2DM patients, 60 of whom had DR, and 58 age- and sex-matched healthy controls were included in this cross-sectional study. MHR was calculated by blood sampling after a complete ophthalmologic examination on all subjects.
Results
MHR was higher in T2DM patients with DR compared to both the control group and without DR (p=0.018). There was a significant positive correlation between MHR and DR (r=0.256 p=0.004). Additionally, MHR was an independent predictor of DR according to multivariate regression analysis (OR=1.197, p=0.009). DR could be predicted with 92% sensitivity and 84% specificity when MHR was 16.05, whereas DR predicted with 100% sensitivity and 98% specificity when MHR was 23 in ROC curve analysis (AUC: 0.356, 95% CI 0.251–0.460, p = 0.008).
Conclusion
This study showed that patients with T2DM may be more likely to develop DR when they have high MHR values. Based on these results, clinicians can also use MHR as a new laboratory marker to predict DR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a systemic disease characterized by vascular and neuropathic complications and is becoming an increasing problem worldwide [1]. Diabetic retinopathy (DR) is the most common complication of DM and is the primary cause of vision loss and impairment in developed countries [2, 3]. The pathogenesis of DR is complex and inflammation plays an essential role in the development and progression of DR, along with many factors such as hyperglycemia and hypertension [4,5,6,7].
Circulating monocytes migrate to tissues and turn into macrophages and dendritic cells and are involved in antimicrobial defense mechanisms. They also produce inflammatory cytokines and contribute to local and systemic inflammation. They play a role in many diseases with inflammatory mechanisms such as atherosclerosis [8].
High-density lipoprotein cholesterol (HDL-c) prevents atherosclerosis by transporting cholesterol from peripheral tissues to the liver [9]. Also, HDL-c protects endothelial functions, has an antioxidant effect, and modulates inflammation [10].
Until now, it has been shown that the monocyte/HDL-c ratio (MHR) is an important marker of inflammation and oxidative stress in many inflammation-related diseases, especially cardiovascular diseases [11,12,13]. The reason why the MHR shows inflammation strongly is due to the anti-inflammatory and antioxidant effects of HDL-c as well as the pro-inflammatory effects of monocytes. It has been reported that MHR can also be a biomarker for vascular and neuropathic complications of type 2 DM (T2DM) [14, 15]. However, as far as we know, there are not enough studies on MHR and DR.
This study aims to evaluate the relationship between DR and MHR values in T2DM patients.
Methods
Study population
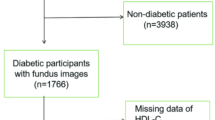
This cross-sectional study was conducted between February 2020 and June 2020 in Ordu University Training and Research Hospital, Internal Medicine, and Ophthalmology Clinic. A total of 118 patients, 60 of whom had DR and diagnosis of T2DM, were included in the study. The control group consisted of 58 healthy individuals who were matched in terms of age and gender. Participants who accepted to participate in the study were questioned in terms of age, gender, hyperlipidemia, smoking, family history, chronic diseases, and medications used.
Smokers, chronic alcoholics, patients with infection, hematological disease, acute or chronic renal failure, chronic liver disease, cardiovascular disease, solid or malignant tumors, and chronic inflammatory diseases were excluded from the study. Our study was approved by the local ethics committee and was conducted in accordance with the ethical principles defined in the Declaration of Helsinki. A written informed consent form was obtained from all participants before the study.
Clinical examination and biochemical analysis
The diagnosis of T2DM was made according to the American Diabetes Association guidelines [16]. After a complete ophthalmological examination was applied to all participants, the diagnosis of retinopathy was made by fundus photographs, fluorescein angiography, and optical coherence tomography. The Diabetic Eye Care Guideline was used for DR diagnostic criteria [17].
Venous blood samples were taken after an overnight fast. All biochemical analyses were studied freshly in our hospital. ARCHITECT c8000 clinical chemistry analyzer (Abbott, IL, USA) was used to analyze creatinine, HDL-c, low-density lipoprotein cholesterol (LDL-c), total cholesterol, triglyceride, and C-reactive protein (CRP) concentrations. Complete blood count was analyzed using the CELL-DYN Ruby automated hematology analyzer (Abbott, IL, USA). Neutrophil/lymphocyte ratio (NLR), monocyte/lymphocyte ratio (MLR), platelet/lymphocyte ratio (PLR), and MHR were calculated and recorded for each participant.
Statistical analysis
In all statistical analyses, SPSS 26.0 Statistical Package Program for Windows (SPSS Inc., Chicago, IL, USA) was used. The Shapiro-Wilk test was used to evaluate the distribution of data. The Kruskal-Wallis test, one-way ANOVA, and Fisher’s exact test were used to compare the groups. The numeric variables as mean± SD and median (minimum-maximum) and the categorical variables as number and percent were expressed. Point biserial was applied to evaluate the correlation between the presence of DR and other parameters. Logistic regression analysis was used to calculate predictors of DR. MHR cutoff value was calculated by receiver operating characteristic (ROC) curve analysis to predict DR. A p-value of <0.05 was accepted as statistically significant.
Results
The study was conducted on 176 subjects, of whom 46 (26.1%) were males and 130 (73.9%) were females. Insulin use, hemoglobin, neutrophil, HbA1c, creatinine, HDL-c, and MHR were statistically different when the groups were compared in terms of age, gender, drug use, hemogram, and biochemical parameters (Table 1). In post hoc analysis, there was a significant difference between group 1 and group 2 in terms of Hba1c, HDL-c. It was found that there were differences in neutrophil, HDL-c, and MHR between group 1 and group 3, and there was a difference between group 2 and group 3 in terms of neutrophil, HbA1c, and creatinine (Table 1).
Considering whether there is a correlation between DR and age, hemogram, biochemical parameters, and MHR, positive correlation with neutrophil, HbA1c, creatinine, NLR, and MHR (r= 0.251 p= 0.005; r= 0.423 p= 0.001; r= 0.256 p= 0.005; r=0.108 p=0.005, r= 0.256 p= 0.004, respectively) and a negative correlation with HDL-c (r = −0.299 p = 0.001) were found (Table 2).
As a result of the regression analysis, HbA1c and MHR were found to be independent predictors of DR (Table 3).
ROC curve analysis for MHR is shown in Figure 1. Accordingly, the area below the ROC value of MHR to distinguish DR was 0.657, and p = 0.003 significance value. The best cutoff value was 10.27, with a sensitivity of 65.2% and a specificity of 58.3%.
Discussion
In the present study, there was no difference in MHR between T2DM patients without DR and healthy subjects; however, MHR was higher in patients with T2DM with DR compared to healthy control subjects and without retinopathy patients. Also, MHR was determined to be an independent predictor of DR. To the best of our knowledge, this study is the first to show the relationship between DR and MHR.
Many epidemiological studies have reported that DM is associated with chronic inflammation and endothelial dysfunction (ED) [18, 19]. Chronic inflammation and ED are two contributing causes of the development and progression of microangiopathic and macroangiopathic complications seen in DM [6]. DR is the most common microangiopathic complication of DM. Increasing evidence indicates that systemic inflammation plays an essential role in the development and progression of DR in the early and later stages of DR by inducing the formation of new blood vessels and macular edema [20], damaging the glial cross and causing neuronal loss [21]. The relationship between DR and blood inflammatory index has been previously demonstrated [22,23,24]. In addition, studies have also found that many inflammatory cytokines such as tumor necrosis factor-α and vascular endothelial growth factor are increased in the systemic circulation in patients with DR [25].
Monocytes and macrophages are cells that play a primary key role in the synthesis and release of pro-inflammatory and pro-oxidant cytokines [26]. Macrophages that differentiate from activated monocytes that secrete cytokines, growth factors, and interleukins adhere to the outer surface of the retinal capillaries and disrupt the blood-retinal barrier in DR. Therefore, the retinal pigment epithelium acts as a gateway for monocytes that directly or indirectly damage the retina [27, 28]. Despite these known contributions of monocytes in DR development, studies have shown that there is no direct relationship between the monocyte count in the blood and DR [24, 29]. Our study supports this evidence for monocyte count and DR.
HDL-c carries cholesterol from peripheral tissues to the liver, prevents the harmful effects of LDL-c, and reduces LDL oxidation [9]. Besides, HDL-c inhibits monocyte activities, prevents the differentiation of monocytes to macrophages, and limits the inflammatory response. It prevents ED by removing cholesterol from lipid-loaded macrophages in atherosclerotic lesions. Thanks to these properties, HDL-c has antiatherosclerotic, antioxidant, anti-inflammatory, and antithrombotic effects [9, 10]. Studies on whether the HDL-c level is a risk factor for the development of DR in DM patients present conflicting results. Some studies state that there is no association between DR and HDL-c [30, 31], while others argue that high HDL-c level is a risk factor for DR [32]. Lyons et al.’s study with 988 people showed that low HDL-c levels are a risk factor for DR [33]. In our study, HDL-c levels were found to be higher in the control group compared to the other two groups, while there was no significant difference between T2DM with DR and without DR groups. These findings suggest that this apparent complexity for the relationship between DR and HDL-c may be influenced by differences in other risk factors such as hypertension and hyperglycemia.
Considering the pro-inflammatory effects of the monocyte-macrophage system and the anti-inflammatory effects of HDL-c, it makes sense to combine these two parameters as an inflammatory marker in a single index (MHR). Previous studies have shown that MHR is associated with many inflammatory diseases, primarily cardiovascular diseases [12,13,14,15]. MHR has also been studied in DM and its complications, and an association of high MHR with peripheral neuropathy, a common complication, has been proven [14]. Also, Karatas et al. [15] and Onalan’s [34] studies have shown a high MHR level relationship with nephropathy, a microvascular complication in DM, and it has been suggested that MHR may be a biomarker for diabetic nephropathy. This high correlation of MHR with DM complications is due to its being a useful marker of both inflammation and vascular ED. In our study, high MHR rates were observed in T2DM patients with DR, but not in others, suggesting that MHR can be used as a marker of inflammation and ED for the development of DR.
The counts of leukocytes including neutrophils, lymphocytes, monocytes, basophils, and eosinophils are used as classical inflammatory markers, especially in cardiovascular diseases. In recent years, the ratios of these parameters (NLR, MLR, PLR, etc.) have been defined as new inflammatory markers and are frequently studied in inflammation-related diseases [35]. Our study has shown that neutrophil count and NLR value are high in DR, and previous studies on this issue also support our findings [22, 36].
This study has some limitations. First of all, this study had a small sample size. DR grading could not be done because sufficient patients could not be reached. Indicating whether there is a difference between non-proliferative and proliferative DR would have made this study more valuable. Secondly, creatinine levels in the DR group were statistically higher, although within clinically normal limits. While we are not sure whether this affected the results, it might have been better for the health of the study to have creatinine levels similar to other groups.
Conclusıon
MHR was not higher in T2DM patients without DR than that in the control group. However, T2DM patients with DR had higher MHR values than the other two groups. There is a positive correlation between MHR and DR. Based on these results, MHR could be a biomarker for DR.
Data availability
All data are included in this paper.
References
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, Lim SC, Tai ES, Mitchell P. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115(11):1869–75.
Varma R, Macias GL, Torres M, Klein R, Peña FY, Azen SP, Los Angeles Latino Eye Study Group. Biologic risk factors associated with diabetic retinopathy: the Los Angeles Latino Eye Study. Ophthalmology. 2007;114(7):1332–40.
El-Asrar AM. Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr J Ophthalmol. 2012;19(1):70–4.
Tomić M, Ljubić S, Kaštelan S, GverovićAntunica A, Jazbec A, Poljičanin T. Inflammation, haemostatic disturbance, and obesity: possible link to pathogenesis of diabetic retinopathy in type 2 diabetes. Mediators Inflamm. 2013;2013:818671.
Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group. Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes–the EURODIAB Prospective Complications Study. Diabetologia. 2005;48(2):370–8.
Klein BE, Knudtson MD, Tsai MY, Klein R. The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy: Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2009;127(9):1175–82.
Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–74.
Assmann G, Gotto AM Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III8-14.
Connelly MA, Shalaurova I, Otvos JD. High-density lipoprotein and inflammation in cardiovascular disease. Transl Res. 2016;173:7–18.
You S, Zhong C, Zheng D, Xu J, Zhang X, Liu H, Zhang Y, Shi J, Huang Z, Cao Y, Liu CF. Monocyte to HDL cholesterol ratio is associated with discharge and 3-month outcome in patients with acute intracerebral hemorrhage. J Neurol Sci. 2017;15(372):157–61.
Karataş MB, Çanga Y, Özcan KS, İpek G, Güngör B, Onuk T, Durmuş G, Öz A, Karaca M, Bolca O. Monocyte to high-density lipoprotein ratio as a new prognostic marker in patients with STEMI undergoing primary percutaneous coronary intervention. Am J Emerg Med. 2016;34(2):240–4.
Kanbay M, Solak Y, Unal HU, Kurt YG, Gok M, Cetinkaya H, Karaman M, Oguz Y, Eyileten T, Vural A, Covic A, Goldsmith D, Turak O, Yilmaz MI. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol. 2014;46(8):1619–25.
Gökçay Canpolat A, Emral R, Keskin Ç, Canlar Ş, Şahin M, Çorapçioğlu D. Association of monocyte-to-high density lipoprotein-cholesterol ratio with peripheral neuropathy in patients with type II diabetes mellitus. Biomark Med. 2019;13(11):907–15.
Karatas A, Turkmen E, Erdem E, Dugeroglu H, Kaya Y. Monocyte to high-density lipoprotein cholesterol ratio in patients with diabetes mellitus and diabetic nephropathy. Biomark Med. 2018;12(9):953–9.
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–16.
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, Maia M, Mathenge W, Moreker S, Muqit MMK, Resnikoff S, Verdaguer J, Zhao P, Ferris F, Aiello LP, Taylor HR. Guidelines on Diabetic Eye Care: the International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1608–22.
Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353(9165):1649–52.
Pitsavos C, Tampourlou M, Panagiotakos DB, Skoumas Y, Chrysohoou C, Nomikos T, Stefanadis C. Association between low-grade systemic inflammation and type 2 diabetes mellitus among men and women from the ATTICA study. Rev Diabet Stud. 2007;4(2):98–104.
Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103.
Lange C, Storkebaum E, de Almodóvar CR, Dewerchin M, Carmeliet P. Vascular endothelial growth factor: a neurovascular target in neurological diseases. Nat Rev Neurol. 2016;12(8):439–54.
Luo WJ, Zhang WF. The relationship of blood cell-associated inflammatory indices and diabetic retinopathy: a meta-analysis and systematic review. Int J Ophthalmol. 2019;12(2):312–23.
Demirtas L, Degirmenci H, Akbas EM, Ozcicek A, Timuroglu A, Gurel A, Ozcicek F. Association of hematological indices with diabetes, impaired glucose regulation and microvascular complications of diabetes. Int J Clin Exp Med. 2015;8(7):11420–7.
Yue S, Zhang J, Wu J, Teng W, Liu L, Chen L. Use of the monocyte-to-lymphocyte ratio to predict diabetic retinopathy. Int J Environ Res Public Health. 2015;12(8):10009–19.
Nalini M, Raghavulu BV, Annapurna A, Avinash P, Chandi V, Swathi N, Wasim. Correlation of various serum biomarkers with the severity of diabetic retinopathy. Diabetes Metab Syndr. 2017;11 Suppl 1:S451–4.
Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80(5):1156–64.
Benhar I, Reemst K, Kalchenko V, Schwartz M. The retinal pigment epithelium as a gateway for monocyte trafficking into the eye. EMBO J. 2016;35(11):1219–35.
Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One. 2014;9(10):e108508.
Fawwad A, Butt AM, Siddiqui IA, Khalid M, Sabir R, Basit A. Neutrophil-to-lymphocyte ratio and microvascular complications in subjects with type 2 diabetes: Pakistan’s perspective. Turk J Med Sci. 2018;48(1):157–61.
Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, Ferris FL 3rd, Knatterud GL. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci. 1998;39(2):233–52.
Benarous R, Sasongko MB, Qureshi S, Fenwick E, Dirani M, Wong TY, Lamoureux EL. Differential association of serum lipids with diabetic retinopathy and diabetic macular edema. Invest Ophthalmol Vis Sci. 2011;52(10):7464–9.
Sasso FC, Pafundi PC, Gelso A, Bono V, Costagliola C, Marfella R, Sardu C, Rinaldi L, Galiero R, Acierno C, de Sio C, Caturano A, Salvatore T, Adinolfi LE, NO BLIND Study Group. High HDL cholesterol: a risk factor for diabetic retinopathy? Findings from NO BLIND study. Diabetes Res Clin Pract. 2019;150:236–44.
Lyons TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT, Klein RL. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45(3):910–8.
Onalan E. The relationship between monocyte to high-density lipoprotein cholesterol ratio and diabetic nephropathy. Pak J Med Sci. 2019;35(4):1081–6.
Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102(6):653–7.
Ulu SM, Dogan M, Ahsen A, Altug A, Demir K, Acartürk G, Inan S. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther. 2013;15(11):942–7.
Acknowledgment
The authors thank all the participants who selflessly collaborated in this research.
Author information
Authors and Affiliations
Contributions
Burak Erdem contributed to material preparation and data collection, prepared a study draft, and wrote the manuscript. Yasemin Kaya collected data and performed data analysis. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Ordu University Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erdem, B., Kaya, Y. Prediction of diabetic retinopathy in patients with type 2 diabetes mellitus by using monocyte to high-density lipoprotein-cholesterol ratio. Int J Diabetes Dev Ctries 42, 741–746 (2022). https://doi.org/10.1007/s13410-021-01024-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-01024-5