Abstract
Background
Type 2 diabetes is a chronic metabolic disease characterized by vascular complications, the prevalence of which varies widely.
Aim
Determine the prevalence of microvascular and macrovascular complications in type-2 diabetes in North India and their correlation with various risk factors.
Design
Prospective observational study
Setting
Tertiary referral centres
Methods
A total of 6400 patients attending diabetes clinic were screened, out of which 6105 patients were enrolled during April 2018 to February 2021. Data were collected using a standardized electronic case record form. Prevalence estimates of microvascular and macrovascular complications were performed, and multivariate regression was applied for the analysis of correlation with known modifiable and non-modifiable risk factors. ROC analysis was done to find the best cut-offs for predictors of diabetic vascular complications.
Results
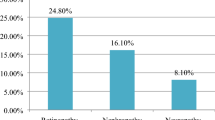
The mean age of the participants was 58.3 ± 9.1 years (57% males). The mean duration of diabetes was 11.3 ± 7 years and HbA1c was 8.4 ± 4%. Prevalent diabetic neuropathy was observed in 30.1%, nephropathy in 18.8% and retinopathy in 13.7%. Coronary artery disease (CAD) was prevalent in 15.7%, cerebrovascular accident (CVA) in 3.6% and foot complications in 2.9%. In the multivariate regression model, all microvascular complications significantly correlated with the longer duration of diabetes but the same was not observed for macrovascular complications. Obesity [BMI cut-off>26.4 kg/m2 (AUC 0.51, p=0.04) and waist circumference>94 cm (male) and >98 cm (female)] significantly predicted prevalent diabetic neuropathy and nephropathy. A BMI of >25.7 kg/m2 had a sensitivity of 55.2% (AUC 0.57), p<0.001 for predicting foot complications.
Conclusion
There is a strong association of obesity and longer diabetes duration with the prevalent microvascular and foot complications irrespective of the glycemic control but not for macrovascular complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the diabetes atlas (9th edition), the global prevalence of diabetes is estimated at 463 million and is expected to reach 578 million by 2030 and 700 million by 2045 [1]. Of all the nations, India leads the world and has earned the distinction of ‘Diabetes capital of the world’. This is possible due to the Asian-Indian phenotype characterized by higher insulin resistance, increased abdominal adiposity, lower adiponectin which predisposes Indians to a higher risk of diabetes and its vascular complications. In the Global burden of disease study, it was shown that among the major non-communicable diseases, diabetes had the highest increase in DALY rate in India from 1990 to 2016, with a crude increase of 80.0% and an age-standardized increase of 39.6% [2]. In 2016, the age-standardized DALY rate of diabetes in India was 1.3 times the global average. Much of this increased morbidity and mortality has been attributed to delayed diagnosis and poor glycemic control leading to long-term damage and diabetic complications mainly affecting the eyes, nerves, kidneys and heart.
The increasing prevalence of diabetes and its vascular complications have burdened the national health systems which are now facing huge economic losses. A study in India in 2008–2009 found that total costs per hospitalization visit comprising consultation charges and medicines for patients without complications were Rs.4493 compared to Rs.14,691.75 (US$ 301.32) for patients with complications. Among the different types of complications investigated, foot complications incur the highest cost [3, 4]. Patients with foot complications spent four times more than patients without such complications along with higher mortality [5, 6]. Patients with renal disease, cardiovascular and retinal complications spent three times more than those without complications. Consultation and hospitalization costs were especially high for patients with complications. Hence, it is imperative to estimate the real burden of vascular complications and their risk factors for the early identification of individuals within a diabetic population and also to formulate preventive measures and realign the healthcare strategies for screening and management of these complications.
A linear relationship between microvascular complications and duration of disease has been established by the authors where they documented the presence of microvasculopathy across different age groups in 25–40% of diabetic patients aged >25 years with more than 5 years duration of diabetes [7].
Previous trials like UKPDS [8] and DCCT [9] have shown that intensive control of hyperglycemia decreases the incidence of microvascular complications, thus proving a cause-and-effect relationship between cumulative glycemic burden and microvascular damage. The Wisconsin Epidemiological Study of Diabetic Retinopathy showed a consistent exponential relationship between worsening glycemic control and prevalence of microvascular complications [10]. The Veterans Affairs (VA) Cooperative Study on Glycemic Control and Complications in Diabetes showed a significant reduction in proteinuria at the end of 2 years with more intensive glycemic control [11]. However, whether the same holds true for development of macrovascular complications like coronary artery disease, stroke or peripheral artery disease still remains controversial.
A favourable beneficial effect between the glycemic control and cardiovascular disease (CVD) is expected but a substantial evidence in the form of outcome studies is still lacking. Also, whether this macrovascular damage is sequential to microvascular complications, with the two representing a continuum or they are isolated events sharing some risk factors (common soil), remains debatable.
Hence, the aim of this study is to estimate the prevalence of microvascular and macrovascular complications in patients with established type 2 diabetes and to identify its relationship with the duration and severity of hyperglycemia.
Materials and methods
Patient enrollment
A total number of 6400 patients attending diabetes clinic of PGIMER, Chandigarh and PGIMS, Rohtak, were screened for the study, out of which 6105 patients were enrolled in duration of April 2018 to February 2021. Two hundred patients were excluded because of the secondary cause of diabetes (steroid-induced, pancreatogenous) or shorter duration of diabetes (<5years). Ninety-five patients were further excluded due to incomplete information about their vascular complications.
Data collection
The variables recorded at baseline included demographic and socioeconomic characteristics, physiological parameters (blood pressure, pulse rate, weight, height, body mass index [BMI] and waist circumference), change in glucose-lowering therapy and reason(s) for change, glycated hemoglobin (HbA1c) level and other laboratory parameters (serum urea, creatinine and urine albumin), occurrence of major and minor hypoglycemic events, comorbidities (including microvascular and macrovascular complications), co-medications and patient-reported outcomes. Duration of diabetes was assessed as time since diagnosis of diabetes until data analysis. Hypertension was defined as blood pressure > 130/80 mm Hg or the history of use of anti-hypertensives. In line with the observational nature of the study, information with regard to glycemic control and other clinical variables was collected as measured in routine clinical practice at each site, according to the prevalent standard of care. The values of Hba1c, blood glucose (self-monitoring of blood glucose) [12] and renal function tests, within last available visit, were considered for analysis.
Definitions
Vascular complications
The presence of vascular complications was assessed by history as well as relevant investigations. Diagnosis and classification of complications were based on the best clinical judgement of the investigators.
Microvascular complications
-
Retinopathy was diagnosed by history of laser therapy or history of anti-VEGF injection or a detailed fundus examination done as a part of routine screening.
-
Nephropathy was diagnosed by presence of chronic kidney disease defined by eGFR < 60 ml/min/1.73 m2 as estimated by MDRD (modification of diet in renal disease) equation. Measurement of urine albuminuria or proteinuria was not available for all participants, in line with the prospective nature of the study.
-
Neuropathy: history of peripheral neuropathy (both positive and negative symptoms — numbness, cotton wool sensation, tingling, paresthesias or burning pain) was taken. Patients with a vibration perception threshold (VPT) test >25 and/or absence of monofilament perception at five standardized plantar sites were considered to have distal symmetric polyneuropathy (DSPN).
Macrovascular complications
-
Coronary artery disease: history of stroke, angina, myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting and mortality from the same were obtained.
-
Cerebrovascular complications: history of stroke, transient ischemic attack, carotid artery stenting or carotid endarterectomy.
-
Peripheral artery disease: history of peripheral artery disease including revascularization procedures, diabetic foot and amputation was recorded. Ankle brachial index (ABI) was recorded and an ABI<0.9 was included for the diagnosis of PAD with or without symptoms.
Foot complications
-
Presence or a history of prior diabetic foot ulcer or Charcot neuroarthropathy with or without amputation.
Additional information on mortality was obtained from hospital medical records in case of in-hospital death.
Statistical analysis
Data analysis was performed using the Statistical Package of Social Sciences (SPSS) version 21. Normality of the data was evaluated by Kolmogorov-Smirnov test and Shapiro-wilk test. In case of normal distribution, mean with SD was used for descriptive statistics. Prevalence of the complications in subgroups was compared by chi-square tests. The significance test was two-tailed and p-value of less than 0.05 was considered statistically significant. Multiple logistic regression analysis with stepwise additions of variables (age, duration of diabetes, Hba1c and hypertension) was performed to assess their association with each of the diabetic complication studied. To determine the relevance of predictors for vascular complications, a receiver operating characteristic curve (ROC) analysis was done to derive sensitivity, specificity and best cut-offs for parameters depicting cumulative glycemic burden, i.e. Hba1c, duration of diabetes and anthropometric parameters of obesity — BMI and waist circumference for early prediction of vascular complications. Youden index (J) was calculated. Waist circumference was analysed separately for males and females, given the gender-specific cut-offs for defining central obesity.
Results
A total of 6105 patients were included for analysis. The mean duration of diabetes was 11.3 ± 7 years and Hba1c was 8.4 ± 3.9 % as shown in Table 1. Majority of the participants (83%) were on at least two or three classes of oral hypoglycemic agents (OHA) and 43.5% were on both OHAs and insulin. The most common prevalent microvascular complication was diabetic neuropathy with estimated prevalence of 30.1% followed by diabetic nephropathy in 18.8% and retinopathy in 13.7% of the study population. Of the macrovascular disease, prevalent CAD was observed in 15.7% of the individuals. CVA was recorded in 3.6% and foot complications were present in 2.9% of the participants. Hypertension was present in 68.6% of the participants.
Correlation analysis for vascular complications with duration of diabetes and prevalent glycemic values
There was a significant correlation between the prevalence of all the microvascular complications (neuropathy, nephropathy and retinopathy) and the duration of the diabetes (p < 0.01 for each) as shown in Table 2. Among macrovascular complications, cardiovascular events increased statistically with increasing duration of diabetes (p<0.01). There was no correlation between the prevalence of stroke and diabetes duration (p=0.483).
With respect to severity of prevalent hyperglycemia, neuropathy (p = 0.01) and retinopathy (p=0.09) showed significant correlation with the increasing HbA1c but the same was not observed for nephropathy (p=0.41) as shown in Table 3. CAD had no correlation (p=0.57), but CVA had significant correlation with increasing HbA1c (p=0.02).
Regression models for predictors of vascular complications
In the multivariate regression model comprising the pertinent risk factors of age, duration of diabetes, HbA1c and hypertension, both the age of the patient and duration of diabetes independently predicted the occurrence of microvascular complications. For retinopathy and nephropathy, presence of hypertension was an equally important risk factor. Similarly, age and presence of hypertension were the most important risk factors for prevalent cardiovascular disease. The risk for CAD was found to be increased only with poorly controlled diabetes, i.e. at HbA1c more than 9.5% and duration of diabetes more than 10 years. The odds of having CAD was 1.43 (95% CI: 1.05–1.94) with diabetes duration of > 10 years and 1.35 times (95% CI: 1.15–2.07) if the HbA1c was > 9.5%. For CVA, none of the risk factors was found to be significantly associated except Hba1c > 7.9% after at which the odds of having stroke increased by twofold (Table 4).
ROC analysis for identifying anthropometric parameter and diabetes characteristics cut-offs to predict vascular complications
ROC analysis highlighted that Hba1c was not a significant predictor for any of the vascular complications whereas duration of diabetes was an important predictor for all complications — neuropathy (sensitivity: 65%, p < 0.001), nephropathy (sensitivity: 55.8 %, p < 0.001), retinopathy (sensitivity: 62.2%, p < 0.001), CAD (sensitivity: 55.9 %, p < 0.001), foot complications (sensitivity: 56%, p = 0.01), except CVA, as shown in Table 5. Among obesity-related parameters, BMI was significant factor for predicting neuropathy (sensitivity: 51.4%, p: 0.04) and foot complications (sensitivity: 55.2%, p: 0.002) while waist circumference was significant for predicting neuropathy (male, sensitivity: 58%, p: <0.001; female, sensitivity: 65.7%, p: <0.001), nephropathy (male, sensitivity: 58.8%, p: <0.001; female, sensitivity: 50 %, p: <0.001) and risk of CVA (male, sensitivity: 57.1%, p: 0.004; female, sensitivity: 54.4%, p: 0.013) as shown in Table 6.
Discussion
This is a large observational cohort study from North India suggesting a significant burden of diabetic complications in people with type 2 diabetes. The mean HbA1c was much higher than recommended by ADA guidelines [13] suggesting an overall poor glycemic control in the studied population. This observation was like that of Diabcare-Asia study in which Indian patients had mean HbA1c level of 8.9 ± 2.1%, with more than 83% of participants having HbA1c levels above 7%. Similarly, ICMR-INDIAB phase I study [14] reported mean HbA1c of 9.1% with 25.3% of patients having HbA1c > 10%. So was observed by Borgharkar et al. [15] in the TIGHT study on real-world evidence of glycemic control among patients with type 2 diabetes in India where out of 55639 participants, two-thirds (76.65%) had poor glycemic control defined by Hba1c > 7%. These studies including ours clearly reflect a gap between the recommended HbA1c target and the real-world observation which may be attributed to poor awareness of targets, ignorance about vascular complications, clinical inertia in timely initiation and intensification of treatment and poor drug adherence.
The prevalence of microvascular complications correlated significantly with duration of diabetes than the glycemic control. The microvascular and macrovascular complications that occur in diabetes contribute substantially to the increased morbidity and mortality. These vascular changes are an outcome of metabolic changes induced by chronic hyperglycemia. Of late, it has been shown that these metabolic changes are not only related to the disease duration but also the severity of hyperglycemia. The acute glycemic excursions also have a significant impact on these metabolic changes leading to cellular damage and metabolic memory that persists even when hyperglycemic crisis resolves.
Despite multiple observational studies on the prevalence of vascular complications in patients with type 2 diabetes, the estimates from these studies vary greatly. This is due to the lack of standardization in the assessment methods used which renders them difficult to compare. Also, this may be due to the heterogeneity of the populations being studied as we know that these vascular complications depend not only on the acquired risk factors but also genetic imprints. A study by Mohan et al. [16] had shown that at any degree of hyperglycemia, Indians have a higher predilection for development of metabolic complications suggesting ethnicity-specific outcomes as described by Neels thrifty gene [17] and thin fat Indians [18] hypothesis. Hence, a better understanding of the burden of vascular complications in patients with type 2 diabetes across the globe is of considerable importance, particularly in countries like India where population characteristics are diverse and available data about diabetic complications is scarce. The Global burden of Disease study had stratified Indian states based on epidemiological transition level (ETL), defined on the basis of the ratio of DALYs from communicable diseases to those from non-communicable diseases and injuries combined and had shown that there was a 2.5 times variation in the prevalence of diabetes between the states in 2016 and hence the subsequent variation in vascular complications [2]. Owing to this considerable heterogeneity, a region-wise study on the prevalence of vascular complications is likely to provide critically important information for making decisions on health policy and strategies for prevention of diabetic complications.
In our study, we observed the prevalence of diabetic retinopathy to be 13.7% at median duration of diabetes of 11.3 years. Previously, retinopathy prevalence of 17.6% (confidence interval 95% CI: 15.8–19.5] is reported by the Chennai Urban Rural Epidemiology Study (CURES) study, which was a population-based large cross-sectional study [19]. The Sankara Nethralaya DR Epidemiology and Molecular Genetic Study had estimated an urban prevalence of 18.0% [20] (95% CI: 16.0–20.1) and a rural prevalence of 10.3% (95% CI: 8.53–11.97%) of DR in South India [21]; Aravind Comprehensive Eye Study reported 10.5% prevalence of DR (in self-reported subjects with diabetes) in the rural South Indian population [22] and Ramchandran et al. [23] showed retinopathy in 23.7% at a diabetes centre in Chennai. In Northern India, Chawla et al. [24] reported DR prevalence of 21.2% and Agrawala [25] reported a prevalence of 32.5%. The prevalence rate in our study was lower than that estimated by other studies because participants with other forms of diabetic retinopathy such as mild to moderate non-proliferative DR may not have experienced sight-threatening complications and hence may not have sought fundus evaluation and treatment. However, this prevalence rate in our study is much higher than the 0.9% prevalence of PDR in South-east Asian cohort of the DISCOVER programme [26], which aimed to estimate and compare the prevalence rates of vascular complications across the globe. In the DISCOVER study, retinopathy was diagnosed on the basis of history of retinopathy or laser photocoagulation. This low prevalence in the DISCOVER study could be due to the shorter duration of diabetes (mean duration 4.6 years compared to 11 years in the present study), with a comparable Hba1c of 8.6% and participants age of 53 years. On multivariate regression analysis, age of the patient, duration of diabetes and hypertension were found to be independent risk factors for the presence of retinopathy. A diabetes duration of >10.1 years significantly predicted the occurrence of DR unlike the presenting Hba1c, suggesting point of care Hba1c may not correlate with the occurrence of retinopathy. This is plausible as the microvasculature damage occurring at the level of retinal capillaries would take time to set in and is observed in 11% of prediabetics as well. However, prevalent retinopathy was independent of anthropometric variable of obesity.
The prevalence of nephropathy in our study was 18.8% as compared to CURES 45 data that reported a prevalence of 2.2% for overt diabetic nephropathy and 26.9% for microalbuminuria [15]. Agrawala et al. [25] had reported prevalence of 30.2% (combined macroalbuminuria and overt nephropathy) from Northwest India. Ramchandran et al. [23] had reported 19.7% prevalence of proteinuria in South Indian diabetes subjects while Gupta et al. had reported microalbuminuria in 26%. The DISCOVER programme showed prevalence of CKD and albuminuria as 0.7% and 5.6%, respectively, in South-east Asian cohort [26]. This low prevalence in the DISCOVER study could be attributed to shorter diabetes duration or may be lesser prevalence of hypertension in their study population. In our study, we did not measure albuminuria and estimated overt nephropathy. Our study showed a fairly high prevalence of overt nephropathy due to higher prevalence of hypertension in our study which is a well-known established risk factor for renal parenchymal disease. Our study was limited by the fact that urine albumin estimation was not available for all participants, while it is well known that diabetic kidney disease may manifest as isolated proteinuria without affecting GFR. Like retinopathy, multivariable regression revealed that increasing age, duration of diabetes > 11.2 years and presence of hypertension significantly predicted the occurrence of nephropathy unlike prevalent Hba1c. Also, a waist circumference of >94.7 cm (male) and 98.3 cm (female) significantly predicted the occurrence of diabetic nephropathy in the present study.
Diabetic neuropathy was the most prevalent microvascular complication in our study, observed in one-third of the participants. This was similar to that reported by Agrawala et al. [25] (26.85%) and Ramachandran et al. [23] (27.5%) while much less than that reported by Shobhana et al. [27] (70%). The increased prevalence noted by Shobhana et al. may be related to referral bias or the modality of assessment. The prevalence of peripheral neuropathy may vary according to the modalities and expertise for assessment as monofilament sensation testing and vibration perception assessment are subjective methods and have significant inter-observer variability unlike objective measures of nerve conduction study (NCS) or electromyography (EMG). In the DISCOVER study, the prevalence of diabetic neuropathy was estimated at 9.6% [26]. On multivariate regression, age and duration of diabetes were the risk factors and hypertension and HbA1c did not predict neuropathy. This correlation estimate was different from the study by Agrawala et al. [25] which revealed a positive association for duration of diabetes, blood pressure, fasting blood sugar, serum cholesterol, serum LDL and serum VLDL. We also observed that diabetes duration of >11.2 years and obesity with waist circumference of >93.5 (male) and 93.1 cm (female) and BMI>26.4 kg/m2 were significantly associated with prevalent neuropathy.
Among the macrovascular complications, clinical CAD was reported by 15.7% of the participants in our cohort. Agrawal et al. [25] found the prevalence of CAD in 25.8% while Ramachandran et al. [23] found the prevalence of coronary artery disease in 11.4%. The occurrence of CAD correlated with duration of diabetes while CVA correlated with Hba1c value at the time of participation in our study. On multivariate regression, age of the patient and duration of diabetes (>10.1 years) were the most important risk factors for CAD occurrence. The odds of having CAD were 1.4 times (95% CI: 1.05–1.94) with diabetes duration > 10 years and 1.3 times (95% CI: 1.15–2.07) for Hba1c > 9.5%. This can be explained by the fact that the causation of atherosclerosis is multifactorial that depends not only on the cumulative glycemic burden in diabetes but also other risk factors like dyslipidemia, hypertension, smoking and familial predisposition. Much of the higher prevalence of ASCVD in diabetes can be explained by the common soil hypothesis, [28] which refers to the amalgamation of risk factors that are similar for the development of diabetes and atherosclerosis. Matheus and Gomes reported also reported that cardiovascular events may not go hand in hand with microvascular complications [29]. For CVA, there was a twofold higher odd of having stroke at HbA1c above 7.9% and the risk was independent of hypertension. However, anthropometric variables of waist circumference and BMI did not corelate with the prevalent CAD in the present study unlike CVA, where a waist circumference >95.2 cm (male) and 97.9 cm (female) significantly predicted the occurrence of CVA.
An assessment of prevalent foot complications among people with diabetes is pertinent considering an increased morbidity (limb amputation) and mortality risk as compared to diabetic patients without foot abnormalities [5, 6]. We observed that only 3% of participants had manifest foot complications in the form of foot ulcers or Charcot neuroarthropathy after a mean diabetes duration of 8 years. This could be an underestimation of foot complications as minor foot abnormalities like claw toes, other deformities or subclinical peripheral arterial abnormalities might not be reported. A study from south India found that more than half of the studied patients had diabetic foot syndrome considering the IWGDF definition of foot at risk [30, 31]. We found that the duration of diabetes >10.1 years and obesity (BMI>25.7 kg/m2) significantly predicted occurrence of foot complications.
Our study included a large number of participants from primarily north-west India. The study population was heterogenous and comprised of both urban and rural population. Since it was a prospective observational study, no specific intervention was planned, and this study represented the real-world scenario of vascular complications. However, there were several limitations. Patients with non-proliferative diabetic retinopathy were not included and a limited information about proteinuria or albuminuria was available which might have underestimated the prevalence of retinopathy and nephropathy. ASCVD was not further confirmed on ECG or 2D echocardiography in asymptomatic individuals and hence might not concur with the true prevalence of CVD. The present analysis is based on electronic registry database which is updated timely but a prospective time bound evaluation of participants was not ensured, limiting the estimation of incidence rate of vascular complications.
Conclusion
This real-world study of people with type 2 diabetes suggests poor glycemic control despite more than 10-year duration of diabetes and on multiple class of oral hypoglycemic agents. Diabetic neuropathy was the most prevalent vascular complication. Diabetic microvascular complications correlated with increasing duration of diabetes but not with the prevalent Hba1c. Obesity also significantly predicted the occurrence of diabetic neuropathy, nephropathy, cerebrovascular and foot complications independent of age and duration of diabetes Fig 1 .
References
International Diabetes Federation.. IDF Diabetes Atlas. 9th ed. Brussels, Belgium: International Diabetes Federation; 2019.
Tandon N, Anjana RM, Mohan V, Kaur T, Afshin A, Ong K, et al. The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health. 2018;6(12):e1352-62.
Bansode B, Jungari DS. Economic burden of diabetic patients in India: a review. Diabetes Metab Syndr. 2019;13(4):2469–72.
Rastogi A, Bhansali A. Diabetic foot infection: an Indian scenario. J Foot Ankle Surg (Asia-Pacific). 2016;3(2):71–9.
Rastogi A, Goyal G, Kesavan R, Bal A, Kumar H, Mangalanadanam, et al. Long term outcomes after incident diabetic foot ulcer: multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study epidemiology of diabetic foot complications study. Diabetes Res Clin Pract. 2020;162:108113. https://doi.org/10.1016/j.diabres.2020.108113.
Chaudhary S, Bhansali A, Rastogi A. Mortality in Asian Indians with Charcot’s neuroarthropathy: a nested cohort prospective study. Acta Diabetol. 2019;56:1259–64.
Chawla A, Chawla R, Bhasin GK, Soota K. Profile of adolescent diabetics in North Indian population. J Clin Diabetol. 2014;1:1–3.
UK Prospective Diabetes Study Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–32.
Levin SR, Coburn JW, Abraira C, Henderson WG, Colwell JA, Emanuele NV, et al. Effect of intensive glycemic control on microalbuminuria in type 2 diabetes. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type 2 Diabetes Feasibility Trial Investigators. Diabetes Care. 2000;23(10):1478–85.
Rao PV, Makkar BM, Kumar A, et al. RSSDI consensus on self-monitoring of blood glucose in types 1 and 2 diabetes mellitus in India. Int J Diabetes Dev Ctries. 2018;38:260–79.
American Diabetes Association. Standards of medical care in Diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S13-27.
Unnikrishnan R, Anjana RM, Deepa M, Pradeepa R, Joshi SR, Bhansali A, et al. ICMR–INDIAB Collaborative Study Group. Glycemic control among individuals with self-reported diabetes in India–the ICMR-INDIAB Study. Diabetes Technol Ther. 2014;16(9):596–603.
Borgharkar SS, Das SS. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diab Res Care. 2019;7:e000654.
Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125(3):217–30.
Neel JV. The “Thrifty Genotype” in 19981. Nutr Rev. 2009;57(5):2–9.
Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. Int J Obes. 2011;35(2):167–87.
Rema M, Premkumar S, Anitha B, Deepa R, Pradeepa R, Mohan V. Prevalence of diabetic retinopathy in urban India: The Chennai Urban Rural Epidemiology Study (CURES) eye study I. Invest Ophthalmol Vis Sci. 2005;46:2328–33.
Raman R, Rani PK, ReddiRachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G, et al. Prevalence of diabetic retinopathy in India: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study report 2. Ophthalmology. 2009;116:311–8.
Raman R, Ganesan S, Pal SS, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study III (SN-DREAMS III) report no 2. BMJ Open Diabetes Res Care. 2014;2:e000005.
Nirmalan PK, Tielsch JM, Katz J, Thulasiraj RD, Krishnadas R, Ramakrishnan R, et al. Relationship between vision impairment and eye disease to vision-specific quality of life and function in rural India: The Aravind Comprehensive Eye Survey. Invest Ophthalmol Vis Sci. 2005;46:2308–12.
Ramachandran A, Snehalatha C, Satyavani K, Latha E, Sasikala R, Vijay V. Prevalence of vascular complications and their risk factors in type 2 diabetes. J Assoc Physicians India. 1999;47(12):1152–6.
Chawla A, Chawla R, Chawla A. Correlation between retinopathy microalbuminuria and other modifiable risk factors. Presented on American Diabetes Association’s 75th Scientific Session; June 5-9; Boston, Massachusetts; 2015.
Agrawal R, Ola V, Bishnoi P, Gothwal S, Sirohi P, Agrawal R. Prevalence of micro and macrovascular complications and their risk factors in type-2 diabetes mellitus. J Assoc Physicians India. 2014;62:6.
Kosiborod M, Gomes MB, Nicolucci A, Pocock S, Rathmann W, Shestakova M, et al. Vascular complications in patients with type 2 diabetes: prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc Diabetol. 2018;17(1):150.
Shobhana R, Ramarao P, Lavanya A, Vijay VV, Ramchandran A. Cost burden to diabetic patients with foot complications – a study from Southern India. JAPI. 2000;48:1147–50.
Stern MP. Diabetes and cardiovascular disease the “common soil” hypothesis. Diabetes Cardiovasc Dis. 1995;44:6.
Matheus AS, Gomes MB. Early aggressive macrovascular disease and type 1 diabetes mellitus without chronic complications: a case report. BMC Res Notes. 2013;6:22.
Bus SA, van Netten JJ, Lavery LA, Monteiro-Soares M, Rasmussen A, Jubiz Y, Price PE. International Working Group on the Diabetic Foot. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):16–24. https://doi.org/10.1002/dmrr.2696.
Vibha SP, Kulkarni MM, Kirthinath Ballala AB, Kamath A, Maiya GA. Community based study to assess the prevalence of diabetic foot syndrome and associated risk factors among people with diabetes mellitus. BMC Endocr Disord. 2018;18(1):43. https://doi.org/10.1186/s12902-018-0270-2.
Acknowledgments
We thank Ms Persis and Ms Reshma for data acquisition and entry.
Funding
This study has been funded by ICMR research grant number 5/4/5-7/Diab.16-NCD-II.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was approved by Institute Ethics Committee vide letter No: PGI/IEC/2014/2341 and a written informed consent was obtained from all the participants. The patients were enrolled during their routine physical appointment in the clinic.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malhotra, B., Hiteshi, P., Rajput, R. et al. Prevalent vascular complications in people with diabetes: a multicentre observational cohort study. Int J Diabetes Dev Ctries 42, 495–504 (2022). https://doi.org/10.1007/s13410-021-01010-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-01010-x