Abstract
Background
Inflammation plays a central role in pathogenesis of diabetic nephropathy (DN), a major cause of morbidity and mortality in type 2 diabetes mellitus (T2DM). Neutrophil lymphocyte ratio (NLR) is a novel and easily available inflammatory marker that can be used to predict DN.
Objective
The objective was to evaluate NLR as a predictive and prognostic marker for DN.
Material and methods
It was an observational cross-sectional study. A total of 324 T2DM patients and 212 healthy controls (HC) were selected by consecutive sampling between June 2019 and June 2020. Complete blood count, erythrocyte sedimentation rate (ESR), renal function parameters, 24-h urinary protein, and fundoscopy were done. Appropriate statistical analysis was applied using SPSS software.
Results
Of 324 T2DM patients, 146 (45%) had DN and 178 (55%) did not. Mean NLR (± SD) for T2DM without DN, T2DM, with DN and HC was 2.73 ± 0.91, 4.85 ± 1.37, and 2.05 ± 0.73, respectively (p-value < 0.05). Positive correlation between NLR vs ESR (r = + 0.335), creatinine (r = + 0.282), and 24-h urinary protein (r = + 0.508) (p-value < 0.001) and negative correlation with hemoglobin (r = − 0.335) and estimated glomerular filtration rate (r = − 0.163) (p-value = 0.001) was observed. Receiver operating characteristic curve for NLR was highest (0.882) (Std. error − 0.019 and p-value < 0.000), and best cut-off value was 3.28 (sensitivity = 89.7% and specificity = 69.7%).
Conclusion
NLR is a better and reliable inflammatory marker compared to a frequently assayed inflammatory parameter like ESR. Thus, it can be considered as a predictive and prognostic marker for DN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy (DN) is a major microvascular complication occurring in approximately 30% and 40% of type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), respectively [1]. DN is a major cause of morbidity and mortality in diabetes mellitus (DM) patients as it not only leads to end-stage renal disease (ESRD) but also is a major contributor to cardiovascular adverse events. DN is clinically detected by the presence of albuminuria or proteinuria with diabetic retinopathy (DR) in DM patients without evidence of other non-diabetic kidney diseases [2]. Currently, 463 million people worldwide are affected by diabetes and it is expected to increase to 700 million by 2045. After China, India has the world’s largest population living with diabetes approximately 82 million as of 2017, and estimated to be 151 million by 2045 [3]. The prevalence of DN is increasing parallelly with a dramatic worldwide rise in the prevalence of diabetes [4].
Though the exact molecular mechanism of pathogenesis of DN is unknown, various mechanisms have been proposed such as kidney injury by activating several cellular pathways including diacylglycerol (DAG)-protein kinase C (PKC) pathway, advanced glycation end-products (AGE), polyol pathway, hexosamine pathway, and oxidative stress due to direct glucotoxicity. As inflammation is the final common outcome of these cellular pathways, thus, it plays a critical role in developing DN [5]. Moreover, there is growing evidence that chronic inflammation and inflammatory cytokines such as TNF-α, IL-1β, and IL-6 play a central role in endothelial dysfunction, and atherosclerosis contributing to both developments as well as the acceleration of microangiopathy and macroangiopathy in DM patients [6, 7].
Neutrophil–lymphocyte ratio (NLR) stands out as a novel marker of chronic inflammation because it reflects a counterbalance between two complementary components of the immune system; neutrophils being the active nonspecific mediator of inflammation, whereas lymphocytes act as the protective or regulatory component of inflammation [8].
NLR has been demonstrated to be a greater risk factor and better prognostic marker than total leucocyte count (TLC) in the prediction of adverse outcomes in various medical conditions like cancer and cardiovascular diseases [9, 10]. Though microalbuminuria is considered as the earliest marker for DN, a substantial percentage of patients with microalbuminuria may remain microalbuminuric or revert to normoalbuminuria over a period of time [11]. Hence, a reliable biomarker is lacking in these subsets of patients. Thus, there is a quest to find a novel biomarker for the detection of individuals at risk to develop DN.
However, after doing an extensive search using various search engines, only one study was found evaluating the predictive and prognostic value of NLR in our country [12]. Probably, due to this reason, NLR is not being used frequently in clinical practice, hoping that our study would add to the literature that would give more confidence to the clinicians to rely on this parameter especially in a resource-poor setting. Thus, considering these aspects and the dearth of literature present in context to the Indian population, we conducted this study aiming to evaluate NLR as a novel biomarker for DN.
Materials and methods
This was a hospital-based observational cross-sectional study conducted from June 2019 to June 2020. All diagnosed T2DM patients aged > 13 years with a minimum duration of 5 years from the time of diagnosis, attending out-patient, or admitted in medicine department were screened. Age and sex-matched healthy controls (HC) were also taken from the same population.
Among those excluded from the study were diagnosed cases of T1DM; gestational diabetes; secondary diabetes; non-diabetic organic kidney disease; patients with active infections, for example, urinary tract infection (UTI), respiratory tract infections (RTI), gastrointestinal infection, genital infection, skin infection, otitis media, viral hepatitis, pyrexia of unknown origin, tuberculosis, and AIDS; patients with systemic diseases such as cardiovascular disease, obesity, chronic liver disease, blood disorders, autoimmune disorders such as systemic lupus erythematosus; cancer patients, known and unknown poisoning; patients on non-steroidal anti-inflammatory drugs, systemic or topical steroids, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers; alcoholics; smokers; patients with uncontrolled hypertension; and patients having diseases affecting urinary protein excretion as nephritic syndrome, urolithiasis, renal artery stenosis, pregnancy, and dehydration states. At the time of sample collection, patients who performed excessive exercise within 24 h or had fever were excluded. Furthermore, on urine sample, processing those with significant bacteriuria or hematuria were also excluded.
All the participants were subjected to a comprehensive history and clinical examination. Relevant information such as age, sex, duration of diabetes, drug history, family history, alcohol, smoking, and any other medical or surgical illness, height, weight, body mass index (BMI), waist circumference, waist to hip ratio, blood pressure, pulse rate, and the temperature was documented.
Routine blood investigations such as complete blood count (CBC), liver function tests (LFTs), kidney function tests (KFTs), fasting lipid profile, thyroid profile, fasting blood sugar (FBS)/postprandial blood sugar (PPBS), glycated hemoglobin (HbA1c), chest X-ray, and ultrasonography of the abdomen were done. Fundoscopy was done to assess diabetic retinopathy. Absolute neutrophil count (ANC), absolute lymphocyte count (ALC), and NLR were calculated by analyzing differential leukocyte count.
Also, a mid-stream morning urine sample was taken for routine urine analysis as well as for urine albumin to creatinine ratio (UACR) estimation, and 24 h urine was collected for 24-h urinary protein excretion estimation. The estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease-epidemiology collaboration (CKD-EPI) formula. Diabetes was diagnosed as per American Diabetes Association (ADA) 2019 criteria [13]. DN was diagnosed as 24-h urinary albumin excretion > 300 mg supported by the co-existence of DR after excluding other non-diabetic organic kidney diseases [2].
All the data collected were analyzed using Social Sciences (SPSS for Windows, version 23.0 Chicago, SPSS Inc.) software. For the analysis of continuous quantitative data, Student t-test and ANOVA were applied whereas for qualitative data, Chi-square test was used. The reciever operating characteristic (ROC) curve analysis was done to test the ability of various parameters to predict DN risk, while the Spearman correlation coefficient (r) was used for correlation analysis between NLR and other relevant parameters. A p-value of < 0.05 was considered statistically significant.
Results
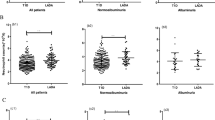
This study included 324 T2DM cases with a mean age (± standard deviation (SD)) 56.21 ± 10.37 years, categorized into 2 groups: 178 (55%) T2DM without DN (DM-DN), and 146 (45%) T2DM with DN (DM + DN), and 212 HC with mean age 55.85 ± 10.52 years. The baseline parameters were compared and projected in Table 1 with their respective p-value. There was a significant difference between the groups with respect to renal parameters, triglyceride, HDL, and LDL-cholesterol (p-value < 0.05). The inflammatory parameters like erythrocyte sedimentation rate (ESR), TLC, ANC, ALC, and NLR were compared among the DM-DN group (group 1), DM + DN group (group 2), and HC group (group 3) and projected in Table 2. The analysis was also done between group 1 vs group 2, group 1 vs group 3, and group 2 vs group 3. Though on comparing all the groups together, we got the p-value < 0.05 in each section but on comparing group 1 vs group 3, TLC was not found to have a significant difference (p-value > 0.05). Similarly, there was no significant difference in ALC when group 1 and group 3 were compared (p = 0.82). The mean value (± SD with 95% confidence interval) of NLR for DM-DN, DM + DN, and HC group was 2.73 ± 0.91, 4.85 ± 1.37, and 2.05 ± 0.73, respectively (Fig. 1). Correlations between NLR and other studied parameters are shown in Table 3. There was a significant positive correlation between NLR vs ESR (r = + 0.335), creatinine (r = + 0.282) and 24-h urinary protein (r = + 0.508) (p-value < 0.001). Significant negative correlation was seen between NLR vs hemoglobin (r = − 0.335) and eGFR (r = − 0.163) (p-value = 0.001). A ROC curve was plotted for NLR, ESR, creatinine, total WBC count (TC), ANC, and ALC (Fig. 2). The area under the curve (AUC) for NLR was 0.882 (Std. error – 0.019 and p 0.000). Based on the graph, 3.28 was found to be the best cut-off value (sensitivity = 89.7% and specificity = 69.7%) which predicts the presence of DN in T2DM patients (CI 95% 0.846–0.919). The AUC for ESR and creatinine was 0.765 (Std. error – 0.027 and p-value = 0.000) and 0.773 (Std. error – 0.026 and p-value = 0.000), respectively. The AUC values for TC, ANC, and ALC were 0.606 (Std. error – 0.032 and p-value = 0.001), 0.751 (Std. error – 0.028 and p-value = 0.000), 0.225 (Std. error 0.026 and p-value = 0.000), respectively.
Discussion
In this study, high NLR levels were found to be significantly associated with T2DM with DN as compared to T2DM without DN, as well as HC. DN being the most common and dreaded complication of DM needs to be detected at the earliest to decrease mortality and morbidity. A major role of inflammation and endothelial dysfunction in diabetes and progression to DN has been well established in various studies [14]. In several studies, higher TLC was related to increased urinary albumin excretion rates [15, 16]. The exact biological mechanisms by which leukocytes and their subtypes play a role in mediating increased protein and albumin excretion are not completely understood. The increased spontaneous adherence of neutrophils to endothelial cells was described as a possible mechanism of DN and proteinuria [17].
NLR is considered superior to other leukocyte parameters such as TLC, ALC, and ANC as its stability is less influenced by physiological, pathological, and physical factors [18]. Moreover, it is a dynamic marker of inflammation representing a combination of two parameters of uncontrolled chronic inflammatory condition (i.e., high neutrophil and low lymphocyte) [19]. Estimation of NLR is simple, easy, and relatively cheap compared to other inflammatory markers, e.g., C-reactive protein (CRP), cytokines, such as IL-6, IL-1b, and TNF-α. In our study, the mean NLR among T2DM with DN patients was significantly higher as compared to T2DM without DN (p-value = 0.001) and healthy controls (p-value < 0.001). In concordance with our study, Huwang et al. also reported significantly higher NLR values in diabetic patients with evidence of nephropathy (2.48 ± 0.59) than in diabetic patients without nephropathy (2.20 ± 0.62) and HC subjects (1.80 ± 0.64) [20]. Similarly, Khandare et al. found the mean NLR among Indian diabetic patients with proteinuria (2.83 ± 0.85) to be significantly higher than those without proteinuria (1.94 ± 0.65) [12]. Asfar et al. reported that NLR could be associated with DN as increased NLR was independently associated with both 24-h urinary protein (p-value < 0.001) and urinary albumin excretion (p-value < 0.001) in newly diagnosed Turkish patients with type 2 diabetes [21]. Recently, Onalan et al. reported NLR to be a predictive hematological parameter for microvascular complications in T2DM [22].
Ashar et al. found NLR values to be higher in patients with DN though it did not reach the significant level (p-value > 0.05). However, a significant association was observed with retinopathy and peripheral neuropathy (p-value < 0.0001) which upholds the evidence that NLR has got an association with microvascular complications in diabetics. The difference can be explained by the fact the author had excluded ESRD patients who might have severe inflammation with higher NLR [23].
We found that NLR had a positive correlation with ESR, serum creatinine, and 24-h urinary protein excretion and a negative correlation with hemoglobin and eGFR. These findings strongly advocate NLR to be considered as a novel biomarker for DN. In agreement with the above finding, a positive correlation between NLR and ESR was reported by Moursy et al. [24]. Similarly, Kahraman et al. reported that NLR was significantly negatively correlated with eGFR, and positively with albuminuria and CRP [25].
As we excluded the potential causes of inflammation and active infection, results of our study reflect the inflammation associated with diabetic nephropathy. In our study, NLR showed better predictive value for DN with AUC of 0.882 as compared to other inflammatory parameters such as TLC, ANC, ALC, and ESR as evident from the ROC curve. Moreover, NLR showed a better predictive value for renal dysfunction than creatinine. Similar findings were reported in a study by Huang et al. where the performance of NLR was higher (AUC 87.2%) than TLC, ANC, ALC, and creatinine [26].
There were several limitations to our study. As it was a single-centered cross-sectional study comprising a relatively small sample size, it did not provide any direct evidence for a cause-effect association between NLR and DN. Multicenter, prospective studies with larger sample size are required for establishing the direct association between NLR and DN. Furthermore, the analyses in this study were based on a single measurement of TLC and NLR, the results of this study might not reflect the relationship between NLR and DN over time. It would be fruitful to measure the serial changes of TLC and NLR to further clarify the prognostic role of NLR in DN. We were unable to correlate the prognostic value of NLR with other inflammatory markers such as IL-6, TNF-α, CRP, and fibrinogen as they were costly and not routinely done.
Conclusion
We conclude that NLR is a reliable marker of inflammation and can be considered as a novel predictive as well as a prognostic marker for diabetic nephropathy. Further studies are required to support the findings of this study.
References
Park C. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab J. 2014;38(4):252.
Rossing P, Fioretto P, Feldt-Rasmussen B, Parving H-H. Brenner & Rectors the kidney. In: Skorecki K, Chertow G, Marsden P, Brenner B, Rector F, editors. 10th ed. Philadelphia: Elsevier, 2016. p. 1283 1320.
International Diabetes Federation Eighth Edition. 2017. Available from: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed 17 Jul 2020.
Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273–80.
Lin Y-C, Chang Y-H, Yang S-Y, et al. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117(8):662–75.
Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19(3):433–42.
Rivero A, Mora C, Muros M, Garcia J, Herrera H, Navarro- Gonzalez JF. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond). 2009;116(6):479–92.
Bhutta H, Agha R, Wong J, Tang TY, Wilson YG, Walsh SR. Neutrophil lymphocyte ratio predicts medium-term survival following elective major vascular surgery: a cross-sectional study. Vasc Endovascular Surg. 2011;45:227–31.
Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2.
Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer. 2013;109:401–7.
Gaede P, Tarnow L, Vedel P, et al. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant. 2004;19(11):2784–8.
Khandare SA, Chittawar S, Nahar N, Dubey TN, Qureshi Z. Study of neutrophil-lymphocyte ratio as a novel marker for diabetic nephropathy in type 2 diabetes. Indian J Endocrinol Metab. 2017;21(3):387–92.
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in Diabetes-2019. Diabetes Care. 2019;42(Suppl. 1):S13–28.
Pitsavos C, Tampourlou M, Panagiotakos DB, et al. Association between low-grade systemic inflammation and type 2 diabetes mellitus among men and women from the ATTICA study. Rev Diabet Stud. 2007;4:98–104.
Tong PC, Lee KF, So WY, et al. White blood cell count is associated with macro- and microvascular complications in Chinese patients with type 2 diabetes. Diabetes Care. 2004;27:216–22.
Cavalot F, Massucco P, Perna P, Traversa M, Anfossi G, Trovati M. White blood cell count is positively correlated with albumin excretion rate in subjects with type 2 diabetes. Diabetes Care. 2002;25:2354–5.
Takahashi T, Hato F, Yamane T, et al. Increased spontaneous adherence of neutrophils from type 2 diabetic patients with overt proteinuria: possible role of the progression of diabetic nephropathy. Diabetes Care. 2000;23(3):417–8.
Lou M, Luo P, Tang R, et al. Relationship between neutrophil-lymphocyte ratio and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr Disord. 2015;15(1):4–9.
Woo SJ, Ahn SJ, Ahn J, Park KH, Lee K. Elevated systemic neutrophil count in diabetic retinopathy and diabetes: a hospital-based cross-sectional study of 30,793 Korean subjects. Invest Ophthalmol Vis Sci. 2011;52:7697–703.
Huang W, Huang J, Liu Q, et al. Neutrophil-lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol (Oxf). 2015;82(2):229–33.
Asfar B. The relationship between neutrophil-lymphocyte ratio with urinary protein and albumin excretion in newly diagnosed patients with Type 2 Diabetes. Am J Med Sci. 2013;347(3):217–20.
Onalan E, Gozel N, Doder E. Can hematological parameters in type 2 diabetes predict microvascular complication development? Pak J Med Sci. 2019;35(6):1511–5.
Fawwad A, Butt AM, Siddiqui IA, Khalid M, Sabir R, Basit A. Neutrophil-to-lymphocyte ratio and microvascular complications in subjects with type 2 diabetes: Pakistan’s perspective. Turk J Med Sci. 2018;48(1):157–61.
Youssef ME. Relationship between neutrophil-lymphocyte ratio and microvascular complications in Egyptian patients with type 2 diabetes. Am J Intern Med. 2015;3(6):250.
Kahraman C, Kahraman NK, Aras B, Coşgun S, Gülcan E. The relationship between neutrophil-to- lymphocyte ratio and albuminuria in type 2 diabetic patients: a pilot study. Arch Med Sci. 2016;12(3):571–5.
Huang L, Xie Y, Dai S, Zheng H. Neutrophil-to-lymphocyte ratio in diabetic microangiopathy. Int J Clin Exp Pathol. 2017;10(2):1223–32.
Acknowledgements
We hereby would like to acknowledge our study subjects without whom this study would not have been possible, laboratory technicians for doing the tests promptly, my seniors, and colleagues for their valuable guidance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
Ethical approval was granted by the Instructional Ethics Committee (ref no- PMC/EC/2019/05–015) dated 13/05/2019.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, A., Jha, A.K., Kalita, B.C. et al. Neutrophil lymphocyte ratio: a reliable biomarker for diabetic nephropathy?. Int J Diabetes Dev Ctries 42, 523–528 (2022). https://doi.org/10.1007/s13410-021-01000-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-01000-z