Abstract
Purpose
Neutrophil-to-lymphocyte ratio (NLR) was widely studied as a prognostic marker in various medical and surgical specialties, but its significance in diabetic kidney disease is not yet established.
Methods
The subjects comprised 199 men aged 73 ± 11 (mean ± standard deviation) years and 187 women aged 77 ± 10 years from a rural hospital. We examined the relationship between NLR calculated by analyzing differential leukocyte count in complete blood picture and renal function evaluated by estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease Study Group equation and urinary albumin excretion (UAE).
Results
NLR was negatively related to eGFR and positively related to UAE. Multiple linear regression analysis using eGFR and UAE as an objective variables, adjusted for confounding factors as explanatory variables showed that NLR (β = − 0.101, p = 0.009) as well as age, body mass index, serum uric acid, and presence of uric acid lowing medication were significantly and independently associated with eGFR, and NLR (β = 0.113, p = 0.031) as well as prevalence of cardiovascular disease, systolic blood pressure, presence of antihypertensive medication, presence of antilipidemic medication, and eGFR were significantly and independently associated with UAE. The multivariate-adjusted odds ratios (95% confidence interval) of NLR for stage 3a (eGFR < 60 mL/min/1.73 m2), stage 3b (eGFR < 45 mL/min/1.73 m2), and microalbuminuria (UAE ≥ 30 mg/g Cr) were 1.90 (1.02–3.56) and 2.99 (1.28–6.98), and 1.77 (1.04–3.01), respectively. Next, to examine the consistency of the observed association between NLR and eGFR, we performed subgroup analyses. There was a significant interaction (p = 0.006) only between the two groups regarding antihypertensive medication (absence: β = − 0.272, p < 0.001 and presence: β = − 0.029, p = 0.564).
Conclusions
Our data suggested that NLR might be important as a potential factor for evaluating patients with a higher degree of albuminuria among diabetic outpatients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic kidney disease (DKD) is a microvascular complication of diabetes and the leading cause of end-stage renal disease (ESRD) [1] and mortality [2] among diabetic patients. DKD is clinically manifested as increased urinary albumin excretion (UAE) starting from microalbuminuria to macroalbuminuria and eventually ESRD [3]. Approximately 30% of diabetic patients showed microalbuminuria after 15 years of disease onset and less than half develop real nephropathy [3]. Several factors are involved in the pathophysiology of DKD, including hyperglycemia, which is very important, male gender, obesity, chronic inflammation, insulin resistance, hypertension, dyslipidemia, and polymorphisms and some genetic loci in specific genes. Management of its modifiable risk factors might help in reducing its incidence in the nearby future.
Recently, new pathways involved in the development and progression of DKD have been elucidated; many studies have demonstrated the critical role of inflammation in the pathogenesis of DKD [4, 5]. Total white blood cell (TWBC) count is a crude and surrogate indicator of inflammation which can be easily measured in laboratory and is a cost-effective test. The neutrophil–lymphocyte ratio (NLR) in TWBC is studied in many cardiac and non-cardiac diseases as an inflammatory marker and is used to predict the prognosis of diseases such as cardiovascular disease (CVD) [6,7,8,9,10,11,12]. NLR rather than other white cell parameters was found to be a useful inflammatory marker to predict adverse outcomes in medical and surgical conditions. NLR has recently emerged from among inflammatory parameters as a potential indicator of vascular complications and poorer outcome in patients with diabetes [13]. Nevertheless, the value of NLR as a convenient factor in predicting DKD has not been elucidated, and its significance in nephrology is not yet established.
Thus, the aim of this study was to evaluate the relationship between NLR, potential risk factors such as hypertension, hyperglycemia, and lipids, and renal function by examining cross-sectional data from community-dwelling diabetic patients.
Subjects and methods
Subjects
Subjects for this investigation were continuously recruited from diabetic outpatients that visited the medical department of Seiyo Municipal Nomura Hospital from April to June 2017. For all these individuals, patients with an estimated glomerular filtration ratio (eGFR) of < 30 mL/min/1.73 m2 and an UAE of ≥ 300 mg/g creatinine (Cr) were excluded. Patients who had: acute coronary artery disease; myocardial infarction, heart failure; active infection; severe tissue damage; acute massive hemorrhage; acute poisoning; cancer; and blood diseases that affect neutrophil and lymphocyte (e.g., myeloproliferative disease and leukemia) were also excluded. Patients on medication that may affect neutrophil and lymphocyte were excluded as well. All procedures were approved by the Ethics Committee of Seiyo Municipal Nomura Hospital, and written informed consent was obtained from each subject.
Evaluation of confounding factors
Information on medical history, present conditions, smoking status, alcohol consumption, and medications [e.g., antihypertensives, antidyslipidemics, antidiabetics, and serum uric acid (SUA) lowering medications] were obtained by interview using a structured questionnaire. Body mass index (BMI) was calculated by dividing weight (in kilograms) by the square of the height (in meters). We measured systolic blood pressure (SBP) and diastolic blood pressure (DBP) in the right upper arm of patients while in a sedentary position using an automatic oscillometric blood pressure recorder. Smoking status was defined as the number of cigarette packs per day multiplied by the number of years smoked (pack-year), and the participants were classified into never smokers, past smokers, light smokers (< 20 pack-year) and heavy smokers (≥ 20 pack-year). Daily alcohol consumption was measured using the Japanese liquor unit in which a unit corresponds to 22.9 g of ethanol, and the participants were classified into never drinkers, occasional drinkers (< 1 unit/day), daily light drinkers (< 2 units/day), and daily heavy drinkers (≥ 2 units/day). Fasting total cholesterol (T-C), high-density lipoprotein cholesterol (HDL-C), hemoglobin A1c (HbA1c), Cr (enzymatic method), SUA, WBC count, and UAE were measured. Non-HDL cholesterol (non-HDL-C) was calculated by subtracting HDL-C from TC. NLR, a novel potential indicator of inflammation, was the ratio of neutrophil to lymphocyte. eGFR was calculated using CKD-EPI equations modified by a Japanese coefficient (eGFRCKDEPI): Male, Cr ≤ 0.9 mg/dL, 141 × (Cr/0.9)−0.411 × 0.993age × 0.813; Cr > 0.9 mg/dL, 141 × (Cr/0.9)−1.209 × 0.993age × 0.813; Female, Cr ≤ 0.7 mg/dL, 144 × (Cr/0.7)−0.329 × 0.993age × 0.813; Cr > 0.7 mg/dL, 144 × (Cr/0.7)−1.209 × 0.993age × 0.813 [14]. Microalbuminuria is defined as of urinary albumin excretion (UAE) of 30–300 mg/g Cr. Moreover, past ischemic stroke and ischemic heart disease were defined as CVD.
Statistical analysis
All values are expressed as the mean ± standard deviation (SD), unless otherwise specified, and in the cases of parameters with non-normal distribution (such as HbA1c, NLR, and UAE), the data are shown as median (interquartile range) values. In all the analyses, parameters with non-normal distributions were used after log-transformation. Statistical analysis was performed using IBM SPSS Statistics version 21 (Statistical Package for Social Science Japan, Inc., Tokyo, Japan). Differences in means and prevalence among the groups were analyzed by Student’s t test for continuous data and χ2 test for categorical data, respectively. Pearson’s correlations were calculated in order to characterize the associations between various characteristics and eGFR and UAE. Stepwise logistic regression analysis was used to evaluate the contribution of each confounding factor to eGFR and UAE. Subjects were divided into groups based on stage of chronic kidney disease (stages 2, eGFR ≥ 60; stage 3a, 59.9 to 45.0; stage 3b, 44.9 to 30 mL/min/1.73 m2) and microalbuminuria (UAE, 30–300 mg/g Cr), and logistic regression analyses were used to test significant determinants of CKD and microalbuminuria serving as the dichotomous outcome variable. To examine the consistency of the observed association between NLR and eGFR, we performed subgroup analyses by gender, age (< 75 and ≥ 75 years), antihypertensive medication (absence and presence), SUA (first–second tertiles < 5.8 mg/dL and third tertile, ≥ 5.9 mg/dL), and microalbuminuria (absence and presence). Interaction between NLR and the subgroups was analyzed by a general linear model. A value of p < 0.05 was considered significant.
Results
Characteristics of subjects categorized according to eGFR
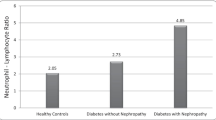
Distribution of NLR in the healthy volunteer and diabetic outpatients is shown in Fig. 1. The neutrophil–lymphocyte ratio (NLR) was significantly increased in subjects compared with in health volunteers (p < 0.001). Characteristics of subjects categorized by eGFR are illustrated in Table 1. The subjects comprised 199 men aged 73 ± 11 years and 187 women aged 77 ± 10 years from a rural hospital. Age, presence of CVD, presence of antihypertensive medication, SUA, presence of SUA lowing medication and NLR were higher in correlation with decreased eGFR, but DBP, HDL-C, HbA1c, and eGFR were lower. There was no inter-group difference regarding gender, BMI, smoking status, drinking status, SBP, non-HDL-C, presence of antilipidemic and antidiabetic medication and UAE.
Distribution of NLR in the healthy volunteer and diabetic outpatients. The neutrophil–lymphocyte ratio (NLR) was significantly increased in diabetic outpatients compared with in health volunteers (p < 0.001). Data for NLR was skewed and presented as median (interquartile range) values and were log-transformed for analysis
Relationship between various confounding factors including NLR and eGFR
As shown Fig. 2, a negative correlation was found between NLR and eGFR. Table 2 shows the simple relationship between various confounding factors and eGFR. Pearson’s correlation coefficient showed that NLR as well as gender, age, smoking status, drinking status, prevalence of CVD, DBP, presence of antihypertensive medication, HDL-C, HbA1c, presence of antidiabetic medication, SUA, presence of SUA lowing medication and UAE was significantly correlated with eGFR. Multiple linear regression analysis using eGFR as an objective variable, adjusted for confounding factors as explanatory variables showed that NLR as well as age, BMI, SUA, and presence of SUA lowing medication were significantly and independently associated with eGFR.
Relationship between various confounding factors including NLR and UAE
As shown Fig. 3, a positive correlation was found between NLR and UAE. Table 3 shows the simple relationship between various confounding factors and UAE. Pearson’s correlation coefficient showed that NLR as well as age, prevalence of CVD, SBP, presence of antihypertensive medication, and eGFR was significantly correlated with UAE. Multiple linear regression analysis using UAE as an objective variable, adjusted for confounding factors as explanatory variables showed that NLR as well as prevalence of CVD, SBP, presence of antihypertensive medication, and presence of antilipidemic medication were significantly and independently associated with UAE.
Prevalence and ORs of NLR for CKD stage 3 and microalbuminuria
The multivariate-adjusted odds ratios (ORs) (95% confidence interval) of NLR for stage 3a (eGFR < 60 mL/min/1.73 m2), stage 3b (eGFR < 45 mL/min/1.73 m2), and microalbuminuria (UAE ≥ 30 mg/g Cr) were 1.90 (1.02–3.56) and 2.99 (1.28–6.98), and 1.77 (1.04–3.01), respectively (Table 4).
Relationship between NLR and eGFR within selected subgroups
To examine the consistency of the observed association between NLR and eGFR, we performed subgroup analyses by gender (men and women), age (< 75 and ≥ 75 years), antihypertensive medication (absent and present), and SUA (first–second tertiles < 5.8 mg/dL and third tertile, ≥ 5.9 mg/dL), and microalbuminuria (absence and presence). NLR was also a significant and independent determinant for eGFR in men, age < 75 years, absence of antihypertensive medication and SUA < 5.8 mg/dL, absence of microalbuminuria. There was a significant interaction only between the two groups regarding antihypertensive medication (Table 5).
Discussion
In this cross-sectional, hospital-based study of 386 diabetic outpatients, we set out to determine renal function, as assessed by eGFR and UAE, and examine potential confounding factors. The key finding of this study was that NLR levels were found to be significantly associated with decreased eGFR and increased UAE, independently of confounding factors, among patients who were diagnosed with early-stage DKD. As NLR values are readily available in routine blood count analysis, NLR may be used as a cost-effective predictor of inflammation. To our knowledge, few studies have suggested that NLR might be important as a potential factor for early DKD.
The NLR has been reported as a novel marker because the NLR is very stable compared with the absolute counts (e.g., total leukocyte, neutrophil, lymphocyte, monocyte, and platelet counts) that could be changed by various physical, physiological, and pathological factors [15]. Several previous studies have reported possible associations between NLR and the development and acceleration of some diabetic complications. In a 3-year follow-up study of 338 diabetic patients, NLR predicted the worsening of the renal function [15]. In 80 patients newly diagnosed patients with type 2 diabetes, NLR was significantly and independently associated with 24-h UAE and also correlated as an indicator of ESRD [16]. In a cross-sectional study involving 200 diabetic patients, NLR with epicardial adipose tissue and platelet-to-lymphocyte ratio (PLR) were found to be independently associated with increased albuminuria [17]. Among 253 patients with type 2 diabetes, 115 of whom have early-stage diabetic nephropathy, NLR (β = 2.088, p = 0·004) levels was significantly and independently associated with diabetic nephropathy [18]. Moreover, a study in Egyptian patients has shown that NLR were significantly higher in diabetic patients with retinopathy (p < 0.001), neuropathy (p = 0.025), and nephropathy defined as UAE of 30–300 mg/g or overt nephropathy with values ≥ 300 mg/g (p < 0.001) than those of diabetic patients without any microvascular complications and healthy controls [19]. Recently published study in Turkish diabetic patients has also shown that NLR significantly increased in parallel to albuminuria levels [20]. Also in our study, NLR was significantly increased in diabetic patients compared with healthy volunteers, and increased in parallel to eGFR levels and albuminuria among diabetic patients.
The mechanisms that lead to increased NLR in individuals with early stage DKD remains to be clarified. Multiple studies demonstrate the important role of inflammatory molecules (e.g., adipokines, Toll-like receptors, chemokines, adhesion molecules and pro-inflammatory cytokines), endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications [21,22,23]. A stage of pathological events, with glomerular damage as an early sign, and which induce microalbuminuria, proteinuria, followed by progressive renal damage, inflammation, fibrosis, and finally loss of functional nephrons, is known to play an important role in the development and progression of DKD [23]. Especially, inflammation plays an essential role in the progression of DKD. Thus, WBC counts and its fraction, which has been recognized to be a novel inflammatory biomarker for systemic inflammation, correlated strongly with DKD. In addition, a lot of studies demonstrates that NLR has been found to have a positive relation with metabolic syndrome [24], the prognosis of diseases such as acute severity of coronary atherosclerosis [25, 26], stroke [27], and pancreatic cancer [28] in which inflammation is significantly involved in onset.
Some limitations of this study must be considered. First, the cross-sectional study design is limited in its ability to eliminate causal relationships between confounding factors and eGFR. Second, estimating GFR using the CKD equation tends to be less accurate in subjects with normal renal function and CKD when inulin clearance is used, but is more accurate than serum creatinine or eGFR when the Modification of Diet in Renal Disease (MDRD) formula [14] is used. Third, confounding factors and eGFR are based on a single assessment of blood, which may introduce a misclassification bias. Fourth, we could not eliminate the possible effects of underlying diseases and medications for hypertension, diabetes, and dyslipidemia on NLR because our subjects were not underfed renal biopsy. However, renal diseases other than DKD and diseases that may affect neutrophil and lymphocyte were excluded as much as possible. Therefore, the demographics and referral source may limit generalizability.
In conclusion, this study suggested that NLR was significantly and independently associated with decreased eGFR and increased UAE among diabetic outpatients. The underlying mechanism behind this relationship is unknown, and these factors seem to be independent of confounding factors, such as age, gender, drinking status, hypertension, lipids, HbA1c, and SUA. Further investigation of the longitudinal data obtained from our study will provide more definitive answers to this issue.
References
Ghaderian SB, Hayati F, Shayanpour S, Beladi SS, Mousavi (2015) Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev 4:28–33
Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M (2008) Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care 31:714–719
Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T (2016) Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol 5:49–56
Duran-Salgado MB, Rubio-Guerra AF (2014) Diabetic nephropathy and inflammation. World J Diabetes 5:393–398
Pichler R, Afkarian M, Dieter BP, Tuttle KR (2017) Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol 312:F716–F731
Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, Meghani M, Akhtar M, Costantino T (2013) Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther 11:55–59
Sen N, Afsar B, Ozcan F, Buyukkaya E, Isleyen A, Akcay AB, Yuzgecer H, Kurt M, Karakas MF, Basar N, Hajro E, Kanbay M (2013) The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long term adverse outcome in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis 228:203–210
Sawant AC, Adhikari P, Narra SR, Srivatsa SS, Mills PK, Srivatsa SS (2014) Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol J 21:500–508
Afari ME, Bhat T (2016) Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther 14:573–577
Yu C, Chen M, Chen Z, Lu G (2016) Predictive and prognostic value of admission neutrophil-to-lymphocyte ratio in patients with CHD. Herz 41:605–613
Bajari R, Tak S (2017) Predictive prognostic value of neutrophil-lymphocytes ratio in acute coronary syndrome. Indian Heart J 69(Suppl 1):S46–S50
Dong CH, Wang ZM, Chen SY (2018) Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: a systematic review and meta-analysis. Clin Biochem 52:131–136
Azab B, Chainani V, Shah N, McGinn JT (2013) Neutrophil-lymphocyte ratio as a predictor of major adverse cardiac events among diabetic population: a 4-year follow-up study. Angiology 64:456–465
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S (2010) Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis 56:32–38
Azab B, Daoud J, Naeem FB, Nasr R, Ross J, Ghimire P, Siddiqui A, Azzi N, Rihana N, Abdallah M, Azzi N, Patel P, Kleiner M, El-Sayegh S (2012) Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study). Ren Fail 34:571–576
Afsar B (2014) The relationship between neutrophil lymphocyte ratio with urinary protein and albumin excretion in newly diagnosed patients with type 2 diabetes. Am J Med Sci 347:217–220
Akbas EM, Demirtas L, Ozcicek A, Timuroglu A, Bakirci EM, Hamur H, Ozcicek F, Turkmen K (2014) Association of epicardial adipose tissue, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with diabetic nephropathy. Int J Clin Exp Med 7:1794–1801
Huang W, Huang J, Liu Q, Lin F, He Z, Zeng Z, He L (2015) Neutrophil-lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol 82:229–233
Youssef Moursy E (2015) Relationship between neutrophil-lymphocyte ratio and microvascular complications in Egyptian patients with type 2 diabetes. Am J Intern Med 3:250–255
Kahraman C, Kahraman NK, Aras B, Cosgun S, Gulcan E (2016) The relationship between neutrophil-to-lymphocyte ratio and albuminuria in type 2 diabetic patients: a pilot study. Arch Med Sci AMS 12:571–575
Rivero A, Mora C, Muros M, Garcia J, Herrera H, Navarro-Gonzalez JF (2009) Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond) 116:479–492
Fujita T, Hemmi S, Kajiwara M, Yabuki M, Fuke Y, Satomura A, Soma M (2013) Complement-mediated chronic inflammation is associated with diabetic microvascular complication. Diabetes Metab Res Rev 29:220–226
Lim AK, Tesch GH (2012) Inflammation in diabetic nephropathy. Mediators Inflamm 2012:146154
Buyukkaya E, Karakas MF, Karakas E, Akcay AB, Tanboga IH, Kurt M, Sen N (2014) Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin Appl Thromb Hemost 20:159–163
Kaya H, Ertas F, Islamoglu Y, Kaya Z, Atilgan ZA, Cil H, Caliskan A, Aydin M, Oylumlu M, Soydinc MS (2014) Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hemost 20:50–54
Balta S, Celik T, Mikhailidis DP, Ozturk C, Demirkol S, Aparci M, Iyisoy A (2016) The relation between atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl Thromb Hemost 22:405–411
Zhang J, Ren Q, Song Y, He M, Zeng Y, Liu Z, Xu J (2017) Prognostic role of neutrophil-lymphocyte ratio in patients with acute ischemic stroke. Medicine 96:e8624
Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG (2015) Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol 21:2807–2815
Acknowledgements
This work was supported in part by a grant-in-aid from the Foundation for Development of Community (2017).
Author information
Authors and Affiliations
Contributions
RK participated in the design of the study, performed the statistical analysis and drafted the manuscript. RK DN, AK, TA, YK, ToK, NO, and TeK contributed to the acquisition and interpretation of data. RK, DN, and TeK contributed to the conception and design of the statistical analysis. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies conducted.
Informed consent
We obtained consent through opt-out procedure from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kawamoto, R., Ninomiya, D., Kikuchi, A. et al. Association of neutrophil-to-lymphocyte ratio with early renal dysfunction and albuminuria among diabetic patients. Int Urol Nephrol 51, 483–490 (2019). https://doi.org/10.1007/s11255-018-02065-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-02065-2