Abstract
To explore the therapeutic effect and possible mechanism of catalpol on type 2 diabetic mice. Twenty-four C57/BL6 male mice were randomly divided into four groups—normal control group (CON, n = 6), diabetic model group (DM, n = 6), the lower dose catalpol treatment (80 mg/kg body weight) group (DM+L, n = 6), and the higher dose catalpol treatment (160 mg/kg body weight) group (DM+H, n = 6). Intraperitoneal glucose tolerance test was performed after 30 days of treatment. Fasting blood glucose (FBG), triglyceride (TG), and total cholesterol (TC) in serum were detected by full automatic biochemical instrument. Enzyme-linked immunosorbent assay was used to detect insulin levels in serum. Insulin resistance level was calculated by trapezoid rule. The morphological changes of pancreatic tissue were observed through HE staining. The protein levels of insulin receptor substrate 1 (IRS1) and glucose transporter type 4 (GLUT4) in the liver and muscle were measured by Western blot (WB). Compared with the DM group mice, the glucose tolerance and insulin resistance in the DM+H group and the DM+L group were improved, the levels of TG, TC, and FBG decreased (p < 0.01). The blood insulin levels were elevated in the DM+H group and the DM+L group (p < 0.01) and the insulin resistance level decreased (p < 0.01, p < 0.05). IRS1 and GLUT4 protein levels in the liver and muscle increased in the DM+H group and the DM+L group (p < 0.01, p < 0.05). Catalpol could be potential medicine to treat type 2 diabetes. Its therapeutic mechanism might be improving insulin-stimulated glucose uptake in the liver and muscle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, diabetes, including type 1 diabetes and type 2 diabetes, troubles an increasing number of people [1]. Diabetes mellitus type 2 is a kind of chronic metabolic disease due to the relatively insufficient insulin secretion with hyperglycemia and insulin resistance as its main characteristics [2]. These characteristics further influence some important organs. Insulin resistance of liver cells causes fatty deposition in the liver, and impairment of liver cells’ function, which further aggravates glucolipid metabolic disorder, promotes the development of insulin resistance [3, 4]. Insulin plays a physiological effect by binding with insulin receptor, which mediated a series of enzymatic actions [5]. This effect of insulin is mediated mainly through insulin receptor substrate (IRS) [6,7,8]. GLUT4, a kind of glucose carrier protein, after regulated by its upstream, transfers extracellular glucose to the cells in the liver and muscle, and finally reduces the blood glucose [9, 10].
Previous research has reported that catalpol may ameliorate type 2 diabetes of mice. Catalpol, extracted from the root of Rehmanniae glutinosa L., can significantly decrease blood glucose and improve glucose tolerance, as well as regulate glucose metabolism of diabetic mice [11,12,13]. Moreover, studies suggest that a certain dose of catalpol can improve mitochondrial function of skeletal muscle which further reduces blood glucose [14]. Meanwhile, other studies presented that hypoglycemic effect of catalpol on streptozotocin (STZ)-induced diabetic mice may be related to the morphology of pancreas and insulin secretion, which promotes utilization of glucose [15,16,17]. However, there are few reports about how catalpol affects insulin activation in type 2 diabetic mice. Therefore, to figure out more specific biochemical mechanisms about hypoglycemic effect of catalpol on type 2 diabetic mice, we studied the changes of insulin resistance as well as IRS-1 in type 2 diabetic mice with treatment of catalpol.
Material and methods
Chemicals and reagent
STZ was purchased from Shanghai Biological Technology Co., Ltd. Catalpol (purity 98%) was provided by Nanjing Zelang Biological Technology Co., Ltd. (Jiangsu, China). Blood glucose meter (GA-3) was obtained from Sannuo Biosensor Inc. (Hunan, China). Insulin (INS) (expressed as mIU/L) enzyme-linked immunosorbent assay kits were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). BCA protein assay kit was obtained from ThermoFisher Scientific (Shanghai, China). IRS1, GLUT4, and β-actin primary antibodies were purchased from Lianke Biotech Co., Ltd. (Hangzhou, China).
Animals and drugs
Eighteen to 25 g C57BL/6 mice were supplied by Shanghai Laboratory Animal Center, China Academy of Sciences (Shanghai, China). All the mice were maintained in the Laboratory Animal Center of Whenzhou Medical University (Zhejiang, China). The mice had free access to water and a standard diet and were kept in a 12-h dark/light room with temperature at 23–25 °C and humidity at 53–57%. Catalpol (purity 98%) was provided by Nanjing Zelang Biological Technology Co., Ltd. (Jiangsu, China).
Experimental protocol
After acclimated to the condition of the Laboratory Animal Center for 1 week, the 24 male C57BL/6 mice were randomly divided into four groups: normal control group (CON, n = 6), diabetic model group (DM, n = 6), the lower dose catalpol treatment (80 mg/kg body weight) group (DM+L, n = 6), the higher dose catalpol treatment (160 mg/kg body weight) group (DM+H, n = 6). The drug dose was chosen according to the previous work [11]. Mice in the NC group were fed regular diet, while DM, DM+L, and DM+H were fed high-fat and high-carbohydrate diet (regular fodder 66.5% + 20% brown sugar + 10% lard + 2.5% cholesterol + 1% cholic acid salt). Six weeks later, mice in DM, DM+L, and DM+H were induced into type 2 diabetes by intraperitoneal injection of 1% streptozotocin at 40 mg/kg body weight after 12-h fasting for solids, following that the DM+L group and the DM+H group were respectively given a gavage of catalpol suspended in distilled water at 80 mg/kg body weight and 160 mg/kg body weight once a day for 4 weeks. The DM group was given a gavage of distilled water of the same volume and time with the drug. After 26 days of treatment, the mice were fasted 12 h and then intraperitoneally injected with 2.0 g/kg glucose dissolved in saline. Then, blood samples were taken from the tip of the tail at 0, 30, 60, and 120 min, which was used to estimate the level of glucose with blood glucometer (WetRust, Taiwan, China). To value the intraperitoneal glucose tolerance of mice, we calculated the blood glucose incremental area under the glucose-time curve (iAUC) according to trapezoid rule. At the 30th day, all the mice were fasted for 8 h and then sacrificed. The blood sample was collected and centrifuged (4 °C, 1000g, 10 min) to get the serum. The pancreatic tissues were taken and fixed in 4% phosphate-buffered paraformaldehyde overnight, and then embedded in paraffin for hematoxylin-eosin (HE) staining. The liver and muscle tissues were quickly frozen by liquid nitrogen and then stored at − 80 °C. All experimental procedures were conducted according to the institutional guidelines for the care and use of laboratory animals in China.
Serum biochemistry
Glucose, triglyceride (TG), and total cholesterol (TC) in serum were detected with automatic biochemistry analyzer (Hitachi, Japan).
Enzyme-linked immunosorbent assay
The concentration of insulin (INS) (expressed as mIU/L) in serum was measured with mice enzyme-linked immunosorbent assay kits (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China).
Histopathological study
The pancreatic tissues were taken and washed in PBS. After being fixed in 4% polyformaldehyde for 24 h, tissues were dehydrated in a graded series of alcohol. Next, xylene was used to clear these tissues which were then infiltrated with molten paraffin (melting point of 52–56 °C) and finally embedded in paraffin (melting point of 58–62 °C). The embedded tissues were sliced up into 4 μm. The paraffin wax slices with tissues were cleared by xylene and then rehydrated in a graded series of alcohol. The slices of tissues were adequately hydrated in distilled water. After that, the slices of tissues were dyed in hematoxylin followed by being washed in distilled water. Again, they were dehydrated by a graded series of alcohol and re-dyed by eosin. At last, the slices of tissues were sealed by resinene and examined under a light microscope, and photomicrographs were taken.
Western blot determination
Three samples of liver tissues from each group (CON, DM, DM+L, DM+H) were randomly selected. Each sample (approximately 50 mg) was grinded and lysed in 500 μL lysis buffer which contained 1% phosphatase inhibitors and 1% PMSF for 30 min. The grinder was cooled on the ice in advance and the whole process of grinding was operated on the ice. After the lysates were centrifuged at 12000g at 4 °C for 10 min, the total proteins were obtained from the supernatants. Concentrations of total protein were determined by BCA protein assay kit (ThermoFisher Scientific). The protein products were used to detect the expressions of IRS1, GLUT4, and β-actin in the liver and muscle using Western blot. Equal amounts of protein product (30 μg) from each sample were electrophoresed through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Afterwards, the separated protein was transferred to nitrocellulose membrane. And then it was blocked with 5% skimmed milk in TBST solution (2.42 g Tris-base, 14.6 g NaCl, and 1 mL Tween-20 in 1 L water) for 1.5 h. Following that, the membrane, with protein on it, was incubated with primary antibodies against IRS1 (1:500), GLUT4 (1:3000), or β-actin (1:6000) at 4 °C for 12 h. Next, the membrane was washed with TBST solution for three times (10 min each time) and then incubated with secondary antibodies for 1 h at room temperature. At last, after washing with TBST solution for three times (8 min each time), the brand was covered with ECL chemiluminescence followed by quantitative grayscale scanning.
Statistical analysis
The results were presented as means ± SEM. The data were analyzed by SPSS 19.0 software and performed by one-way analysis of variance (ANOVA). Individual differences among groups were analyzed using Dunnett’s test. When p < 0.05, the difference was considered statistically significant.
Results
The effect of catalpol on serum biochemistry
As shown in Table 1, the TG, TC, and FBG levels of the DM group were significantly higher than those of CON, DM+L, and DM+H. Moreover, TG, TC, and glucose (GLU) levels of the DM+H group were significantly lower than those of the DM+L group.
The effect of catalpol on intraperitoneal glucose tolerance
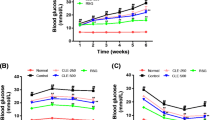
Glucose tolerance is an important indicator of type 2 diabetes. In this experiment, we chose the method of intraperitoneal glucose tolerance test. As shown in Fig. 1, compared with the CON group, the DM group of AUC levels increased significantly (p < 0.01). While compared with the DM group, AUC level of the DM+L group and the DM+H group dropped significantly (p < 0.01). There was no significant difference between the DM+H group and the DM+L group. This result indicated that catalpol (80 and 160 mg/kg) obviously improved glucose tolerance in diabetic mice induced by STZ and high-fat diet.
Effects of catalpol on intraperitoneal glucose tolerance. a Blood glucose levels during intraperitoneal glucose tolerance test. b The incremental area under the curve (iAUC) for intraperitoneal glucose tolerance test. Data were presented as means ± SEM (n = 6). Compared with the CON group, **p < 0.01. Compared with the DM group, ##p < 0.01
Effect of catalpol on INS in blood
As presented in Fig. 2, the blood insulin level of the DM group was significantly higher than that of the CON group (p < 0.01). Compared with the DM group, blood insulin levels of the DM+L group and the DM+H group were significantly higher than that of the DM group (p < 0.01). This result shows that catalpol can obviously reduce the insulin level of diabetic mice induced by STZ and high-fat diet.
Effect of catalpol on insulin resistance
The insulin resistance index was calculated by method of homeostasis model assessment (HOMA-IR) (HOMA-IR=FBG × insulin/405). As shown in Fig. 3, insulin resistance level significantly increased in the DM group (p < 0.01). Compared with the DM group, insulin resistance levels in the DM+L group and the DM+H group were significantly reduced (p < 0.01). The results showed that the catalpol might improve the insulin resistance of diabetic mice induced by STZ and high-fat diet.
Effects of catalpol on pancreas
As presented in Fig. 4, the results of pancreatic HE showed that the 30-day catalpol treatment had brought the pancreas of diabetic mice near normal, which indicated that catalpol might have effects to repair the pancreas.
Effects of catalpol on pancreas in STZ+high-fat diet-induced diabetic mice. a Hematoxylin and Eosin (HE, × 400) staining of normal control mice pancreas. b Hematoxylin and Eosin (HE, × 400) staining of diabetic control mice pancreas showing destroyed islets. c Diabetic+80 mg/kg catalpol-treated mice pancreas. Showing regenerating islet cells (HE, × 400). d Diabetic+160 mg/kg catalpol-treated mice pancreas, showing regenerating islet cells (HE, × 400)
Effects of catalpol on IRS1 and GLUT4 protein expression in the liver and muscle
As presented in Fig. 5, lower dose catalpol (80 mg/kg) and higher dose catalpol (160 mg/kg) significantly reduce the protein level of IRS1 in liver tissue (p < 0.01, p < 0.01). Protein level of IRS1 of higher dose catalpol (80 mg/kg)-treated group was significantly lower than that of lower dose catalpol-treated group (p < 0.05). There was a significant difference of IRS1 protein level between the DM+L group and the DM+H group (p < 0.05). These results indicated that catalpol can improve insulin signaling in the liver. In addition, GLUT4 protein level in the liver was detected. This result shows that both lower dose catalpol and higher dose catalpol significantly increased GLUT4 level of liver tissue in diabetic mice (p < 0.05). There was significant difference between the DM+L group and the DM+H group (p < 0.05). These results provided evidences for increased insulin-stimulated glucose uptake in liver tissues, which may be related to the catalpol dosage.
Western blot analysis for liver and muscle IRS1 and GLUT4 protein expression levels in type 2 diabetic rats. Each value is mean ± SEM for three replicates. **p ≤ 0.01, diabetic mice tissues compared with normal. #p ≤ 0.05 and ##p ≤ 0.01, catalpol-treated mice tissues compared with diabetic control. ▽p ≤ 0.05, Diabetic+160 mg/kg catalpol-treated mice tissues compared with diabetic+80 mg/kg catalpol-treated mice tissues
At the same time, we evaluated IRS1 and GLUT4 protein levels in muscle tissues. IRS1 protein levels in muscle tissues show the same tendency of those in liver tissue. But there were no significant differences between IRS1 protein levels in the DM+L group and the DM+H group. In addition, GLUT4 protein level of catalpol-treated groups was observed higher than that of the DM group, which is similar to GLUT4 protein level in liver tissue. But there were no significant differences between GLUT4 protein levels in the DM+L group and the DM+H group. These results provided evidences for increased insulin-stimulated glucose uptake in muscle tissues, though the effect of catalpol dosage was unknown.
Discussion
The main characteristic of type 2 diabetes is that the pancreatic β cells do not produce abundant insulin to maintain the activity of the enzyme associated with glycemic activity [18]. Diabetic animals can be induced by a single high-dose STZ injection or low dose of STZ. It was reported that catalpol had therapeutic effects on the diabetic mice and db/db mice [12].
In our current study, the catalpol significantly reduced the blood glucose of STZ and high-fat diet mice and improve intraperitoneal glucose tolerance in mice. This result is similar to Yan et al. which indicated that study catalpol significantly reduced the blood glucose and improve oral glucose tolerance in type 2 diabetes [19]. Diabetes is associated with insulin secretion and insulin resistance [4]. Our results showed that the serum insulin levels in serum of mice increased and the insulin resistance index decreased in the treatment of catalpol. The results of elevated serum insulin level were the same as Yan et al. but different from those of Bao et al. whose study showed decreased serum insulin level [11, 19]. This may be method different to inducing diabetes, db/db mice were used in their research, but in this case STZ-induced mice. Moreover, in our study, the results of pancreatic HE showed that the 30-day catalpol treatment had brought the pancreas of diabetic mice near normal, which indicated that catalpol might have effects to repair the pancreas. In one word, catalpol might increase the absorption of glucose by improving insulin secretion and insulin sensitivity.
In addition, we evaluated the effect of catalpol on lipid metabolism in diabetic mice. The lipid metabolism disorder is an important factor in diabetes with many diabetics accompanied by high serum lipid. Studies have shown that hyperlipidemia has adverse effects on blood glucose homeostasis [20]. As a result, a drop in blood lipid may be beneficial in controlling blood glucose. In this study, catalpol significantly reduced the levels of TC and TG in diabetic mice induced by STZ, indicating that catalpol may improve lipid metabolism in diabetic mice.
The IRS protein mediates insulin-metabolic activity, and many studies have shown that insulin signaling and the loss of the IRS gene are associated with the development of diabetes [8]. In our study, compared with the normal group, the protein levels of IRS and GLUT4 in the liver and muscle lowered in diabetic mice induced by STZ and high-fat diet. And, under catalpol treatment of different doses, the protein levels of IRS and GLUT4 in the liver and muscle rose. Therefore, we believe that catalpol may reduce blood glucose of diabetic mice by regulating the insulin signaling pathway which involved IRS1.
In conclusion, the hypoglycemic effect of catalpol may be related to insulin signaling pathway. Current researches show that long-term treatment of catalpol may lower blood glucose, promote insulin secretion, and strengthen the glucose tolerance in diabetic mice induced by STZ and high-fat diet. In addition, we found that the hypoglycemic effect of catalpol in this study may be achieved by raising the expression level of IRS1 and GLUT4 in the liver and the muscles.
References
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53.
Lu H, Hu F, Zeng Y, Zou L, Luo S, Sun Y, et al. Ketosis onset type 2 diabetes had better islet β-cell function and more serious insulin resistance. J Diabetes Res. 2014;2014:1–6. https://doi.org/10.1155/2014/510643.
Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91.
Kolodziejski PA, Pruszyńska-Oszmalek E, Strowski MZ, et al. Long-term obestatin treatment of mice type 2 diabetes increases insulin sensitivity and improves liver function. Endocrine. 2017;56:538–55.
Wang ZQ, Yu Y, Zhang XH, et al. Chromium-insulin reduces insulin clearance and enhances insulin signaling by suppressing hepatic insulin-degrading enzyme and proteasome protein expression in KKAy mice. Front Endocrinol. 2014;5:99.
Xu H, Zhou Y, Liu YX, et al. Metformin improves hepatic IRS2/PI3K/Akt signaling in insulin resistant rats of NASH and cirrhosis. J Endocrinol. 2016;229:133–44.
Sajan MP, Ivey RA III, Farese RV. BMI-related progression of atypical PKC-dependent aberrations in insulin signaling through IRS-1, Akt, FoxO1 and PGC-1α in livers of obese and type 2diabetic humans. Metab Clin Exp. 2015;64:1454–65.
Lavin DP, White MF, Brazil DP. IRS proteins and diabetic complications. Diabetologia. 2016;59:2280–91.
Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5'AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–71.
Nymphayol increases glucose-stimulated insulin secretion by RIN-5F cells and GLUT4-mediated insulin sensitization in type 2 diabetic rat liver. Chem Biol Interact. 2015;226:72–81.
Bao Q, Shen X, Qian L, Gong C, Nie M, Dong Y. Anti-diabetic activities of catalpol in db/db mice. Korean J Physiol Pharmacol. 2016;20:153–60.
Huang WJ, Niu HS, Lin MH, Cheng JT, Hsu FL. Antihyperglycemic effect of catalpol in streptozotocin-induced diabetic rats. J Nat Prod. 2010;73:1170–2.
Wang Z, Wang J, Chan P. Treating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbs. Evid Based Complement Alternat Med. 2013;2013(6):343594.
Li X, Xu Z, Jiang Z, Sun L, Ji J, Miao J, et al. Hypoglycemic effect of catalpol on high-fat diet/streptozotocin-induced diabetic mice by increasing skeletal muscle mitochondrial biogenesis. Acta Biochim Biophys Sin Shanghai. 2014;46:738–48.
Shieh JP, Cheng KC, Chung HH, Kerh YF, Yeh CH, Cheng JT. Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in Streptozotocin-induced diabetic rats. J Agric Food Chem. 2011;59(8):3747–53.
Zhu H, Wang Y, Liu Z, Wang J, Wan D, Feng S, et al. Antidiabetic and antioxidant effects of catalpol extracted from Rehmannia glutinosa (Di Huang) on rat diabetes induced by streptozotocin and high-fat, high-sugar feed. Chin Med. 2016;11(1):25.
Zou G, Zhong W, Xu R, et al. The protective effects of catalpol on the islet cells in rats with type 2 diabetes mellitus. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. 2016;14(15):1727–29.
Aybar M, Sanchez Riera AN, Grau A, Sanchez SS. Hypoglycemic effect of the water extract of Smallanthus soncifolius (yacon) leaves in normal and diabetic rats. Ethnopharmacology. 2002;74:125–32.
Yan J, Wang C, Jin Y, et al. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol Res. 2017;130:466–80.
Biden TJ, Boslem E, Chu KY, Sue N. Lipotoxic endoplasmic reticulum stress, β cell failure, and type 2 diabetes mellitus. Trends Endocrinol Metab. 2014;25:389–98.
Funding
The authors would like to extend their sincere appreciation to the Deanship of Wenzhou Medical University for its funding of this research through the 2015 Zhejiang Public Interest Technology Application Research Project No. 2015C37099.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Eighteen to 25 g C57BL/6 mice were supplied by Shanghai Laboratory Animal Center, China Academy of Sciences (Shanghai, China). All the mice were maintained in Laboratory Animal Center of Whenzhou Medical University (Zhejiang, China). The mice had free access to water and a standard diet and were kept in a 12-h dark/light room with temperature at 23–25 °C and humidity at 53–57%.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The experiment was approved by the laboratory animal ethics committee of Wenzhou Medical University.
Rights and permissions
About this article
Cite this article
Xiao, M., Chen, H., Wei, C. et al. Therapeutic effect of catalpol on type 2 diabetic mice induced by STZ and high-fat diet and its possible mechanism. Int J Diabetes Dev Ctries 39, 528–534 (2019). https://doi.org/10.1007/s13410-018-0685-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-018-0685-3