Abstract
The aim of this research was to describe a facile protocol to obtain biocompatible gold nanoparticles (AuNPs) suitable for microbial optical sensing. For this purpose, polycationic poly-L-lysine (PLL) was employed as both reducing and stabilizing agent in order to obtain an optically active microbial nanotag based on the electrostatic interaction with negatively charged cell envelopes. A one-pot procedure was developed to produce homogeneous, positively charged AuNPs. The as-synthesized particles, named PLL@AuNPs, exhibited maximal surface plasmon resonance (SPR) at 532 nm, a FCC crystalline nature, and sizes ranging from 20 to 25 nm, according to spectroscopy, X-ray diffractometry (XRD), transmission electron microscopy (TEM), and dynamic light scattering (DLS) analyses. The reduction of gold ions by PLL was featured by Fourier-transform infrared (FTIR) absorption bands of various functional groups. Zeta potential analysis confirmed the high cationic feature with a value of + 57 mV. The applicability of the particles to tag bacterial cell surfaces was exemplified by their adherence to Escherichia coli, a bacterial species commonly used to monitor fecal pollution in water sources. Finally, the potential of this tagging approach for microbial sensing through surface-enhanced Raman scattering (SERS) was explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gold nanoparticles (AuNPs) represent one of the most promising materials for microbial sensing and diagnostics, mainly due to their unique optical properties [1]. A variety of methods have been proposed for the decoration of AuNPs for tagging microorganisms based on both chemical linkage or physical adsorption of specific ligands, including antibodies, oligonucleotides, aptamers, or phage-displayed polypeptides [2,3,4,5]. Nevertheless, there has been an emergence for the screening of microorganisms in different environments, and the availability of low-cost, functional AuNPs of broader applicability would be beneficial. Given the high negative charge of microbial cell surfaces, the use of electrostatic interactions to unspecifically tag microbial cells seems appealing. The use of poly-L-lysine (PLL), a highly biocompatible, cationic polypeptide of natural origin [6], constitutes an attractive alternative to obtain cationized AuNPs. Incidentally, in recent years, various peptide molecules, including PLL, have been reported to act as reducing agents during the synthesis of metal nanoparticles [7,8,9]. PLL has also been proposed for the immobilization of microorganisms onto inorganic materials, where attached cells can be used for capturing biomolecular ligands or testing antimicrobial agents [10, 11]. Noteworthy, the binding of PLL to bacteria seems to be discriminatory, as distinct degrees of attachment have been found for different cell surface structures [12, 13]. Therefore, a combination of PLL-binding activity with the optical sensing capabilities of nanogold appears very attractive for microbial sensing. Optical features of AuNPs, as used for sensing purposes, arise from the surface plasmon resonance (SPR) excitation, a phenomenon allowing the characterization of surfaces through various techniques, such as SPR-induced color changes or surface-enhanced Raman scattering (SERS) [14, 15]. It has been established that for these techniques to be exploited, nanoparticles must fulfill specific parameters namely sizes above 20 nm [16] and constructs allowing a 10-nm maximal distance between a metal and the tagged analyte for SERS [15, 17]. In this work, we developed a simple, one-pot, highly reproducible and scalable method to obtain functional AuNPs through reduction and functionalization with PLL. This method enables the production of spherical particles of uniform coating thickness, colloidal stability over time, and structural features that are suitable for optical sensing. Furthermore, the potential of the new construct to tag E. coli, leading to differential optical signals, was herein demonstrated.
Experimental
Synthesis
Cationic gold nanoparticles (PLL@AuNPs) were synthesized through a one-pot method, using PLL as a reducing and stabilizing agent. All the reagents were from Sigma-Aldrich (St Louis, MI, USA). The synthesis was carried out in a total volume of 10 ml, using 500 μl of 2.5 mM HAuCl4∙3H2O (≥ 99.9%). The gold precursor was diluted in distilled water and allowed to stir at boiling temperature (95–100 °C). Then, 450 μl of a PLL hydrochloride solution (5 mg/ml, mol wt 15,000–30,000) was added, and the reaction was allowed to proceed for a 10-min period. The formation of particles was followed by color change, from yellow to colorless, then to wine red. After 10 min, the reaction was stopped by ice-quenching. Finally, nanoparticles were centrifuged at 13,000 rpm for 30 min in order to remove the remaining free PLL.
Characterization techniques
UV-visible spectra were measured using a Genesys 10S UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA). The X-ray diffraction (XRD) analysis was performed using an Ultima IV diffractometer (Rigaku Co., Japan) with a Cu-kα radiation source. Transmission electron microscopy (TEM) analyses were carried out in a JEM 1010 (JEOL, Ltd., Japan) at 80 kV, by loading 5 μl of samples onto carbon coated copper grids, and staining with 2% uranyl acetate. The particle size and zeta potential measurements were performed using a Zetasizer Nano ZS (Malvern Panalytical, Ltd, UK). FTIR analyses were carried out in a Spectrum Two FT-IR Spectrometer (PerkinElmer, Inc., Waltham, MA, USA).
E. coli growth and labeling
E. coli XL-Blue was grown in LB nutrient broth at 37 °C under constant agitation. For SERS analyses, 50 μl of an overnight culture was inoculated in 5-ml LB medium at 37 °C until exponential growth phase. Then, cells were collected by centrifugation at 4 °C, 4000 rpm for 5 min, washed thrice with sterile saline solution (0.9% NaCl), and resuspended in 1 ml before labeling. Afterwards, various volumes (250, 500, 1000 μl) of bacterial suspension were incubated with 100 μl of PLL@AuNPs for 15 min at 37 °C under orbital shaking. Finally, the samples were washed twice with phosphate buffered saline (PBS) and resuspended in 1 ml of sterile double-distilled water.
SERS
SERS analyses were carried out using a Senterra II Raman instrument (Bruker, Optik GmbH, Germany), equipped with a 785-nm excitation laser and a × 50 objective. Prior to the measurements, 50 μl of each sample was deposited onto an aluminum foil by drop-casting. Bacteria were inactivated through a 5-min exposure to chloroform vapors within a glass chamber and dried under vacuum. The laser power was set at 10 mW, in order to avoid any sample destruction. Spectra were accumulated with a 5-s exposure time and 10 repetitions. To ensure the accuracy of the results, average spectra were obtained from three independent measurements collected under identical conditions.
Results and discussion
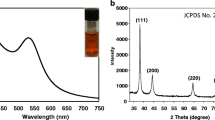
Figure 1a shows the UV-Vis absorption spectrum of the PLL@AuNPs, with an SPR peak at 532 nm, strongly indicating the presence of spherical nanoparticles with a narrow size distribution [18]. Importantly, UV-Vis analysis performed on different PLL@AuNPs batches generated similar spectra, indicating the reproducibility of the synthetic procedure. Also, the SPR profile was found to be unchanged over the 8-month period, thus demonstrating the high stability of the PLL-reduced nanogold. The XRD diffractogram (Fig. 1b) showed peaks for the (111), (200), (220), and (311) hkl planes at 38.2°, 44.3°, 64.6°, and 77.8°, respectively, with peak intensities corroborated with standard gold (Joint Committee on Powder Diffraction Standards-JCPDS card no 04-0784), in agreement with a face-centered cubic (FCC) crystalline nature. TEM was used to analyze the size and morphology of the construct. As suggested by SPR analysis, spherical particles with a narrow size distribution were observed (Fig. 1c). Also, negative staining of the samples indicated the presence of a thin semitransparent layer surrounding the particles [19], as expected for PLL-containing structures.
The possible formation mechanism of PLL@AuNPs is due to acidic character and significant quantity of primary amines from PLL resulting in the reduction and stabilizing the nanoparticles [20]. The average hydrodynamic size distribution was measured using DLS, leading to the detection of 25.6-nm particles (Fig. 2a). In agreement with the presence of protonated amines from the PLL-decorated particles, a zeta potential analysis (Fig. 2b) showed a value of + 57 mV. FTIR analysis demonstrated the presence of absorption bands typifying the aminated polypeptide structure (Fig. 2c, Table 1) [20]. Altogether, these data suggested the suitability of our construct for tagging negatively charged surfaces through simple electrostatic interaction. We, therefore, evaluated whether PLL@AuNPs could adhere to microbial surfaces without tedious functionalization processes and transduce such interaction into optical changes. As a proof of concept, particles were incubated with E. coli viable cells for a 5-min period and washed, and optical spectroscopy data of the pelleted particles were obtained (see experimental details). As depicted in Fig. 3a, TEM analysis confirmed the attachment of PLL@AuNPs to E. coli cells, as expected. A UV-Vis analysis of the formed complexes (see experimental details) displayed a bathochromic shift from 532 to 550 nm, thus indicating the aggregation of nanoparticles onto bacterial cells (Fig. 3b). Finally, to explore the potential applicability of PLL@AuNPs as a microbial sensing tag, a SERS analysis of bacterial-particle complexes was performed. Mid-log E. coli cell cultures were extensively washed, incubated with PLL@AuNPs, and analyzed by Raman spectroscopy. Although there has been concerns regarding the contributions of residual growth media to the SERS signals of bacterial cultures, it has been demonstrated that properly washed cells do not generate SERS signals attributable to growth media components [21]. In this study, the exposure to PLL@AuNPs of E. coli cells led to enhanced “fingerprint” signals previously reported for E coli (Fig. 3c). In Table 2, the assignment of vibrational bands to functional/molecular identities is shown. Most of these signals correspond to the contribution of cell wall components, such as lipids or membrane proteins, or to DNA, typical of E. coli SERS spectra in the presence of different gold substrates [22, 23]. To note, no peaks featuring LB or yeast extract culture media, at ca. 735 cm−1, were found [21]. Therefore, these data support the use of PLL@AuNPs herein described as an easy-to-obtain nanotag for SERS-based detection of microbes (Fig. 4). Further research to study the application of these particles for monitoring microbial populations and for microbial diagnostics is encouraged. Likewise, the exploration of PLL@AuNPs to improve existing nanogold-based antimicrobial procedures, such as photodynamic inactivation [24] or antimicrobial treatments [25], merits further investigation.
Conclusions
A facile method to synthesize optically active, cationic Au nanoparticles using a biocompatible polypeptide (PLL) using one-pot procedure was developed. The characterization techniques which showed a spherical nanogold of about 25 nm were obtained. The suitability of the construct PLL@AuNPs to attach negatively charged bacterial cells was confirmed by their ability to interact with viable E. coli cells upon a short incubation period. Moreover, the size of the obtained particles seemed suitable for the applicability of the construct as a label-free optical tool in microbial analysis. Further research to assess the potential of PLL@AuNPs in microbial sensing is therefore encouraged.
References
Syed MA, Bokhari SHA (2011) Gold nanoparticle based microbial detection and identification. J Biomed Nanotechnol 7:229–237. https://doi.org/10.1166/jbn.2011.1281
Di Pasqua AJ, Ii REM, Ship Y et al (2009) Preparation of antibody-conjugated gold nanoparticles. Mater Lett 63:1876–1879. https://doi.org/10.1016/j.matlet.2009.05.070
Cao X, Ye Y, Liu S (2011) Gold nanoparticle-based signal amplification for biosensing. Anal Biochem 417:1–16. https://doi.org/10.1016/j.ab.2011.05.027
Wang G, Wang Y, Chen L, Choo J (2010) Biosensors and bioelectronics nanomaterial-assisted aptamers for optical sensing. Biosens Bioelectron 25:1859–1868. https://doi.org/10.1016/j.bios.2009.11.012
Peng H, Chen IA (2019) Rapid colorimetric detection of bacterial species through the capture of gold nanoparticles by chimeric phages. ACS Nano 13:1244–1252. https://doi.org/10.1021/acsnano.8b06395
Shima S, Sakai H (1977) Polylysine produced by streptomyces. Agric Biol Chem 41:1807–1809. https://doi.org/10.1271/bbb1961.41.1807
Tan YN, Lee JY, Wang DIC (2010) Uncovering the design rules for peptide synthesis of metal nanoparticles. J Am Chem Soc 132:5677–5686. https://doi.org/10.1021/ja907454f
Prieto M, Arenal R, Henrard L, Gomez L, Sebastian V, Arruebo M (2014) Morphological tunability of the plasmonic response: from hollow gold nanoparticles to gold nanorings. J Phys Chem C 118:28804–28811. https://doi.org/10.1021/jp5096129
Han G, Wu S, Wang J, Geng X, Liu G (2015) Poly-L-lysine mediated synthesis of gold nanoparticles and biological effects. J Nanosci Nanotechnol 15:6503–6508. https://doi.org/10.1166/jnn.2015.10505
Cowan SE, Liepmann D, Keasling JD (2001) Development of engineered biofilms on poly- L -lysine patterned surfaces. Biotechnol Lett 23:1235–1241. https://doi.org/10.1023/A:1010581503842
Rozhok S, Shen CK, Littler PH et al (2005) Methods for fabricating microarrays of motile bacteria. Small 1:445–451. https://doi.org/10.1002/smll.200400072
Liang JF, Kim SC (1999) Not only the nature of peptide but also the characteristics of cell membrane determine the antimicrobial mechanism of a peptide. J Pept Res 53:518–522. https://doi.org/10.1034/j.1399-3011.1999.00051.x
Freer E, Pizarro-Cerdá J, Weintraub A et al (1999) The outer membrane of Brucella ovis shows increased permeability to hydrophobic probes and is more susceptible to cationic peptides than are the outer membranes of mutant rough Brucella abortus strains. Infect Immun 67:6181–6186. https://doi.org/10.1128/iai.67.11.6181-6186.1999
Amendola V, Pilot R, Frasconi M, Maragò OM, Iatì MA (2017) Surface plasmon resonance in gold nanoparticles: a review. J Phys Condens Matter 29:1–48. https://doi.org/10.1088/1361-648X/aa60f3
Le Ru EC, Etchegoin PG (2009) Principles of surface-enhanced raman spectroscopy. Elsevier, Wellington. https://doi.org/10.1016/B978-0-444-52779-0.X0001-3
Hong S, Li X (2013) Optimal size of gold nanoparticles for surface-enhanced raman spectroscopy under different conditions. J Nanomater 2013:790323. https://doi.org/10.1155/2013/790323
Huang J, Mousavi MZ, Zhao Y et al (2019) SERS discrimination of single DNA bases in single oligonucleotides by electro-plasmonic trapping. Nat Commun 10:1–10. https://doi.org/10.1038/s41467-019-13242-x
Liz-Marzán LM (2006) Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 22:32–41. https://doi.org/10.1021/la0513353
Cao B, Xu H, Mao C (2011) Transmission electron microscopy as a tool to image bioinorganic nanohybrids: the case of phage-gold nanocomposites. Microsc Res Tech 74:627–635. https://doi.org/10.1002/jemt.21030
Rozenberg M, Shoham G (2007) FTIR spectra of solid poly-l-lysine in the stretching NH mode range. Biophys Chem 125:166–171. https://doi.org/10.1016/j.bpc.2006.07.008
Premasiri WR, Gebregziabher Y, Ziegler LD (2011) On the difference between SERS spectra of cell growth media and whole bacterial cells. Appl Spectrosc 65:493–499. https://doi.org/10.1366/10-06173
Lemma T, Saliniemi A, Hynninen V, Hytönen VP, Toppari JJ (2016) SERS detection of cell surface and intracellular components of microorganisms using nano-aggregated Ag substrate. Vib Spectrosc 83:36–45. https://doi.org/10.1016/j.vibspec.2016.01.006
Prakash O, Sil S, Verma T, Umapathy S (2020) Direct detection of bacteria using positively charged Ag/Au bimetallic nanoparticles: a label-free surface-enhanced Raman scattering study coupled with multivariate analysis. J Phys Chem C 124:861–869. https://doi.org/10.1021/acs.jpcc.9b09311
Lashkari SM, Kariminezhad H, Safarnezhad N, Amani H (2019) Surface plasmon resonance of naked gold nanoparticles for photodynamic inactivation of Escherichia coli. Gold Bull 52:51–60. https://doi.org/10.1007/s13404-019-00252-2
Demurtas M, Perry CC (2014) Facile one-pot synthesis of amoxicillin-coated gold nanoparticles and their antimicrobial activity. Gold Bull 47:103–107. https://doi.org/10.1007/s13404-013-0129-2
Acknowledgments
R. Manisekaran is a postdoctoral fellow (DGAPA, UNAM). Carlos M. Valdemar-Aguilar is a CONACyT fellow (CVU 665176) at the Programa de Doctorado en Ciencias Biomédicas (UNAM). The authors thank the excellent technical assistance from Lourdes Palma (INB, UNAM), Luz María Avilés (CINVESTAV, Querétaro, IPN) and the Laboratorio Nacional de Caracterización de Materiales (UNAM), especially Antonieta Mondragón and Beatriz Millán. Graphics were created with BioRender.
Funding
This study was funded by Dirección General de Asuntos del Personal Académico, Universidad Nacional Autonoma de Mexico, through Grant PAPIIT No. IT203518.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manisekaran, R., Jiménez-Cervantes Amieva, E., Valdemar-Aguilar, C.M. et al. Novel synthesis of polycationic gold nanoparticles and their potential for microbial optical sensing. Gold Bull 53, 135–140 (2020). https://doi.org/10.1007/s13404-020-00283-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-020-00283-0