Abstract
This paper reports the synthesis of positively charged gold nanoparticles [(+)AuNPs] for the colorimetric detection of hydrogen peroxide (H2O2). (+)AuNPs were synthesized using an electrochemically active biofilm in an aqueous solution, which is a novel, simple, and green approach. The as-synthesized (+)AuNPs were characterized by ultraviolet-visible (UV-Vis) spectroscopy, dynamic light scattering (DLS), X-ray diffraction (XRD), transmission electron microscopy (TEM), and high-resolution TEM (HRTEM). UV-Vis spectroscopy confirmed the synthesis of AuNPs and DLS showed that the as-synthesized AuNPs had a charge of +32.72 mV. XRD confirmed the formation of AuNPs as well as the purity, crystallinity, and fcc structure. TEM and HRTEM showed that (+)AuNPs were 15–21 nm in size and spherical in shape. The as-synthesized (+)AuNPs were used for the colorimetric detection of H2O2 using 3,3,5,5-tetramethylbenzidine dihydrochoride. This study provides a simple, fast, and sensitive colorimetric method for the detection of H2O2 in the linear range from 1.0 × 10−3 to 2.5 × 10−3 M. The (+)AuNPs possessed extraordinary intrinsic peroxidase-like activity (peroxidase mimic) compared to citrate-capped negatively charged AuNPs. This approach to the colorimetric detection of H2O2 is novel and simple because it uses positively charged gold nanoparticles, which may provide new areas for further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the last decade, gold nanoparticles (AuNPs) are in prodigious demand by researchers in several fields. Since the first report of gold colloids by Faraday [1], there have been numerous studies on the synthesis, properties, and applications of gold clusters, colloids, and nanoparticles [2,3,4,5,6,7]. Bond [8], Haruta [2, 9], and Hutchings [10] reported gold to be the best catalyst for their catalytic reactions. The interest in AuNPs has increased rapidly over the past few years because of their intriguing properties, such as strong surface plasmon [11], catalytic [12,13,14], redox behavior [15], applications in medical diagnostics [13, 16], imaging [17], optical [18], and forensic sciences [19].
Recently, positively charged gold nanoparticles [(+)AuNPs] have attracted considerable attention for their potential use in biotechnology and biological mimicking [20]. Most AuNPs have been synthesized using chemical methods, which were generally neutral or negatively charged. On the other hand, there are few reports on the synthesis of positively charged AuNPs. In most cases, positively charged gold nanoparticles were synthesized by coating or capping [21, 22]. Therefore, there is a need to synthesize positively charged gold nanoparticles without the use of a capping agent.
Precise measurements of hydrogen peroxide (H2O2) are very important because it is not only the product of reactions catalyzed by a number of oxidizing enzymes, but it is also a mediator in food, pharmaceutical, clinical, industrial, and environmental analyses [20, 22]. Among the techniques for H2O2 analysis, such as photometry, chemiluminescence, titrimetry, high-performance liquid chromatography, colorimetry, and electrochemistry, colorimetry has attracted considerable interest because this technique is simple, sensitive, convenient, and highly selective [20, 22].
This paper reports the extension of a previous study, in which the biogenic extracellular synthesis of positively charged gold nanoparticles [(+)AuNPs] using an electrochemically active biofilm (EAB) was performed in an aqueous solution, which is a novel, simple, and green approach [23]. The as-synthesized AuNPs showed a high positive charge, which plays an important role in its catalytic activity. The high positive charge (+ 32.72 mV) of the AuNPs highlight its potential use in colorimetric assays. Therefore, the as-synthesized (+)AuNPs were used as a peroxidase mimic for the colorimetric detection of H2O2. The proposed colorimetric detection method is simple and precise.
2 Experimental Section
2.1 Materials
Hydrogen tetrachloroaurate(III) hydrate (HAuCl4.nH2O; n = 3.7) and 3,3,5,5-tetramethylbenzidine dihydrochoride (TMB) were purchased from Kojima Chemicals, Japan, and Sigma-Aldrich, respectively. Sodium citrate (Na3C6H5O7.2H2O), sodium acetate, extra pure NaOH (98%), NaBH4, and H2O2 were obtained from Duksan Pure Chemicals Co. Ltd., South Korea, and used as received. The stainless steel mesh of type SUS 304 (Ildong Wire Cloth, Korea), which is a commercial alloy containing 68–72% Fe, 18–20% Cr, 8–10% Ni, and very small amounts of C, Mn, and Si, was used. All solutions were prepared from deionized water obtained using a PURE ROUP 30 water purification system.
2.2 Characterization Techniques
The as-synthesized AuNPs were characterized by ultraviolet-visible-near infrared (UV-Vis-NIR) spectrophotometry (VARIAN, Cary 5000, USA). X-ray diffraction (XRD, PANalytical, X’pert-PRO MPD, Netherlands) was performed using Cu Kɑ radiation (λ = 0.15405 nm). The XRD peaks of the crystalline phases were compared with those of standard compounds reported in the JCPDS data file (21-1272). The morphology and particle size of the AuNPs of the colloidal solution were measured by transmission electron microscopy (TEM) and high-resolution TEM (HRTEM, Tecnai G2 F20, FEI, USA) operating at an accelerating voltage of 200 kV. The zeta potential and particle size of the AuNPs in aqueous solution were measured using a Delsa™ Nano zeta potential apparatus (Beckman Coulter, USA).
2.3 Preparation of Electrochemically Active Biofilm
An EAB on a stainless steel mesh was prepared as reported earlier [23,24,25]. Briefly, a stainless steel mesh (2.5 cm × 4.5 cm) was dipped into a mineral salt medium containing sodium acetate (0.2 g/200 mL) as a substrate [26]. A 10-mL sample of anaerobic sludge (Biogas plant in Paju, South Korea) was added under anaerobic conditions by sparging N2 gas for 5 min. All media, including the bacterial inoculum, were changed every 2 days under anaerobic conditions. This process was repeated for 2 weeks. The living EABs formed on the stainless steel mesh were used for the synthesis of AuNPs.
2.4 Synthesis of AuNPs
A 200-mL sample of a 1 mM aqueous HAuCl4.nH2O solution was prepared in a 250-mL reaction bottle. Subsequently, 0.2 g sodium acetate was added as an electron donor and food for the EAB. A pH of 4 was maintained using 0.1 M NaOH. The vessel was sparged with N2 gas for 5 min to maintain the anaerobic and inert conditions. An EAB developed on a stainless steel mesh was hung and the system was sealed. The entire reaction mixture was stirred magnetically at 30 °C. Within 15 min, the initial golden yellow color of the solution changed to a ruby red color, indicating the formation of gold nanoparticles. The reaction mixture was stirred further for 15 min to complete and stabilize the reaction. Overall, the AuNPs were synthesized within 30 min. The formation of AuNPs was confirmed by UV-visible spectroscopy. The synthesized AuNPs colloidal solution was centrifuged, and a AuNP powder was isolated for further characterization.
The synthesis of AuNPs at pH 4 was chosen because it was found to be the optimal pH from syntheses conducted at pH 4, 7, and 9. The synthesis of AuNPs at pH 4 produced better results than that at pH 7 and 9, which was apparent from the progress of the reaction, i.e., the change in the color of the reaction mixture and observed UV-visible spectra.
2.5 Synthesis of Citrate-Capped Gold Nanoparticles
A citrate-capped gold nanoparticle colloidal solution was prepared, as reported earlier [27]. Briefly, in a 500-mL two-neck round-bottom flask, 300 mL of a 0.01% solution of HAuCl4.nH2O was boiled under reflux with constant stirring. Subsequently, 10.5 mL of 1% trisodium citrate in water was added quickly. After the color change was complete (within the first 3–5 min), the mixture was kept boiling for another 20 min. The heating source was removed, and kept stirring until the solution cooled to room temperature. The citrate-capped gold nanoparticle colloid was stored in a refrigerator at 4 °C until needed.
2.6 Detection of H2O2 by UV-Vis Spectroscopy
Table 1 lists the different reagents, such as (+)AuNPs, H2O2, acetate buffer (pH 4), and peroxidase substrate 3,3,5,5-tetramethylbenzidine (TMB), which were mixed at various ratios for the colorimetric detection of H2O2. To examine the peroxidase-like activity of the as-synthesized (+)AuNPs, the catalytic oxidation of a peroxidase substrate, TMB, was tested in the presence of H2O2. (+)AuNPs could catalyze the oxidation of TMB by H2O2 to produce a blue color. The resulting reaction mixture was analyzed by UV-vis spectrophotometry. For a comparative study, negatively charged citrate-capped AuNPs were used instead of the (+)AuNPs and the same procedure was followed to determine its mimicking ability compared to (+)AuNPs.
2.7 Calculation of the Average Number of Gold Atoms Per Nanoparticle
The mean number of gold atoms per nanoparticle was calculated using the following formula [22, 28]:
where ρ is the density of fcc gold (19.3 g/cm3), M stands for the atomic weight of gold (197 g/mol), and D is the average core diameter of the particles in nm from TEM.
2.8 Determination of the Molar Concentration of the Nanoparticle Solution
The molar concentration of the nanoparticle solution was calculated using the following formula [22, 28]:
where NTotal is the equivalent to the initial amount of gold salt added to the reaction solution, N is the mean number of gold atoms per nanosphere according to Eq. (1), V is the volume of the reaction solution in liters, and NA is the Avogadro’s constant. The reduction from Au3+ to Au atoms was assumed to be 100% complete.
3 Results and Discussion
An electrochemically active biofilm (EAB) is a biogenic and green system that provides electrons by biologically decomposing sodium acetate [23,24,25]. Stainless steel is well known for its reduction behavior by the corrosion of its surface by Cl− ions, which provide electrons that can be used for reduction purposes [22, 29, 30]. Taking the advantage of these two phenomena exhibited by the EAB and stainless steel, these two principles were combined as an EAB on stainless steel mesh. After successful formation of the EAB on the stainless steel mesh, it was exploited for the synthesis of gold nanoparticles, as reported elsewhere [23].
UV-visible spectroscopy of the colloidal aqueous solution was performed (Fig. 1a) and the appearance of an absorbance maximum at 535 nm, which is typically ascribed to the plasmon resonance band and characteristic of AuNPs, confirmed the synthesis of AuNPs [23, 31, 32]. According to Mie theory, metal nanoparticles, such as Au, show a surface plasmon band within the range of 500–550 nm [33, 34]. The absorbance spectra of the as-synthesized (+)AuNPs colloidal solution was measured after 3 h and later after 1 and 2 months, showing the same spectra. This suggests that the as-synthesized AuNPs were stable for a long time, even without any capping or stabilizing agents in solution (data not shown) [23, 35].
The average hydrodynamic zeta potential of the (+)AuNPs in aqueous solution were measured by dynamic light scattering (DLS) using a Delsa™ Nano zeta potential analyzer. The zeta potential measurements of the AuNPs colloid revealed a surface potential of +32.72 mV suggesting that (+)AuNPs were highly positively charged. The high positive charged acquired by the AuNPs was attributed to H+ ions produced by the EABs [23, 25, 35].
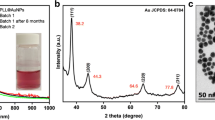
The phase of the as-synthesized (+)AuNPs was determined by XRD. The XRD pattern (Fig. 1b) confirmed the formation of crystalline AuNPs from four prominent XRD peaks in the range, 30–90° 2θ for polycrystalline gold. The peaks at 38°, 46.33°, 64.58°, and 77.24° 2θ were assigned to the 111, 200, 220, and 311 planes of face-centered cubic (fcc) gold, respectively (JCPDS powder diffraction file no. 21-1272) [22, 23, 35]. Figure 1b shows that the AuNPs are dominated mainly by the 111 plane. No impurity peaks were observed in the XRD pattern, which showed that the as-synthesized AuNPs had high purity and crystallinity. The mean crystallite size was calculated by the Scherrer equation using the peak at 38° 2θ and the full width at half maximum (fwhm) [36]. The mean crystallite size of the AuNPs was ~ 20 nm, which is very close to the TEM measurements.
The morphology of the AuNPs was determined by TEM at low magnification, as shown in Fig. 2a. This suggests that the as-synthesized AuNPs were 15–25 nm in size, highly monodispersed, and discrete. Figure 2b shows a HRTEM image of the AuNPs. The AuNPs were 15–21 nm in size with an almost spherical shape. The 111 planes with a lattice spacing of 0.31 nm suggest that the AuNPs were polycrystalline. Figure 2c presents the particle size distribution of the as-synthesized (+)AuNPs, which shows that the majority are in the range, 15–21 nm.
From the characterization techniques, discussion, and previous reports [22, 23], the as-synthesized AuNPs using EABs were well synthesized, pure, monodispersed, and crystalline. In addition, the as-synthesized (+)AuNPs were highly positively charged compared to previous reports, which involved various chemicals and physical methods [13, 14, 20, 22]. Therefore, the results show that this biogenic method involving an EAB on a stainless steel mesh can be a superior tool for the synthesis of highly positively charged AuNPs without chemical contamination and ambiguity.
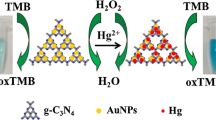
Taking advantage of the high positive charge (+ 32.72 mV) of the as-synthesized AuNPs, a comparative study (Table 1) of (+)AuNPs, negatively charged citrate-capped AuNPs and previous reports on the colorimetric detection of hydrogen peroxide were made [20, 22]. Table 1 lists the different arrangements and ratios of acetate buffer (pH 4), TMB solution, AuNPs colloidal solutions, and H2O2 used. Setups A to K showed different intensities of blue color except for setup C, where AuNPs were not used. This shows that AuNPs are necessary to catalyze the reactions. The highly positively charged AuNPs catalyzes the oxidation of the peroxidase substrate, 3,3,5,5-tetramethylbenzidine (TMB) by H2O2 to develop a blue color in an aqueous solution, which provides the colorimetric detection of H2O2 at 655 nm.
Figure 3 shows the different color developed after the peroxidase mimic reactions. The blue color that developed in the case of TMB-(+)AuNPs-H2O2 was much darker and faster than that of TMB-citrate AuNPs-H2O2, whereas no color developed in the case of TMB-H2O2. Figure 3 shows that the performance of TMB-(+)AuNPs-H2O2 is much better than the TMB-citrate AuNPs-H2O2 and TMB-H2O2, which was attributed to the high positive charge acquired by the AuNPs. Therefore, the (+)AuNPs and citrate-capped AuNPs exhibited different catalytic behavior. This shows that the (+)AuNPs have much higher catalytic activity than the citrate-capped AuNPs, which were negatively charged. This could be because of the high positive charge on the as-synthesized (+)AuNPs [20, 22, 23]. In this case, the response of the (+)AuNPs was much better than that reported elsewhere, which confirms the higher positive charge (+ 32.72 mV) acquired by the AuNPs than AuNPs (+ 24.2 mV) synthesized by other methods [20, 22].
Images of a TMB–H2O2 mixed solution in the absence of (+)AuNPs, b TMB–(+)AuNPs mixed solution, c TMB–H2O2 mixed solution in the presence of 1 mL (+)AuNPs, and d TMB–H2O2 mixed solution in the presence of 1 mL citrate-capped AuNPs. Experimental conditions: 700 μL of 0.5 M acetate buffer (pH 4.0), 500 μL of 5 mM TMB, 200 μL of 10 mM H2O2
The resulting TMB-H2O2, TMB-H2O2-citrate AuNPs, and TMB-H2O2-(+)AuNPs reaction mixture was analyzed by UV-vis spectrophotometry, which revealed a maximum absorbance at 655 nm (Fig. 4) that was assigned to the oxidation of TMB [20]. The UV-visible absorption spectra (Fig. 4) show the absorbance of the reaction mixture solution after the peroxidase-mimicking reaction. The absorbance of TMB-H2O2-(+)AuNPs reached a maximum compared to the TMB-H2O2-citrate AuNPs and TMB-H2O2. This shows that the peroxidase-mimicking catalytic activity of the as-synthesized (+)AuNPs was much higher than that of the citrate-capped AuNPs. This further confirmed the catalytic activities of (+)AuNPs, which was attributed to the higher charge (+ 32.72 mV) acquired by the AuNPs using this synthesis method.
The as-synthesized (+)AuNP solution was centrifuged at 13000 rpm at 30 °C, and the precipitate was re-dispersed in DI water. The resulting (+)AuNPs solution exhibited similar catalytic activity to the original (+)AuNPs colloidal solution. This confirmed that the catalytic activity originated from the (+)AuNPs themselves. The control experiments (set A-K as shown in Table 1) showed that the catalytic activity of the as-synthesized (+)AuNPs was H2O2 concentration-dependent (Fig. 5). Therefore, the system discussed above could be used to detect H2O2. From Fig. 5, which is a plot of the absorbance versus H2O2 concentration (mol/L), it is clear that the absorbance increases with increasing H2O2 concentration. The calibration graph of the absorbance at 655 nm as a function of the H2O2 concentration was linear in the range, 1.0 × 10−3 to 2.5 × 10−3 M, suggesting that this approach could be used effectively for the precise colorimetric detection of H2O2 in the concentration range tested. This colorimetric method is superior to the non-enzymatic and potentiometric methods of H2O2 detection reported elsewhere [37,38,39,40].
4 Conclusion
This paper reported an extension of the biogenic synthesis of (+)AuNPs by an EAB, which is novel, simple, fast, controlled, cost effective, and environmentally friendly, and its application to colorimetric H2O2 detection. A comparative study of the synthesized (+)AuNPs and negatively charged AuNPs for H2O2 detection was also performed. The (+)AuNPs possessed unique peroxidase-like activity (peroxidase mimic) and provided a simple and fast colorimetric assay for H2O2 detection in comparison to the negatively charged AuNPs. The results showed that the (+)AuNPs act as an effective and sensitive peroxidase mimic in the linear range of 1.0 × 10−3 to 2.5 × 10−3 M owing to the high positive charge (+ 32.72 mV), robustness, and stability of the AuNPs. These outcomes indicate a wide variety of new prospective applications of (+)AuNPs in future in the field of biotechnology, medical sciences, and environmental chemistry.
References
Faraday, M. (1857). The Bakerian lecture: experimental relations of gold (and other metals) to light. Philosophical Transactions. Royal Society of London, 147, 145–181.
Haruta, M. (1997). Size- and support-dependency in the catalysis of gold. Catalysis Today, 36, 153–166.
Prasad, B. L. V., Sorensen, C. M., & Klabunde, K. J. (2008). Gold nanoparticle superlattices. Chemical Society Reviews, 37, 1871–1883.
Burda, C., Chen, X., Narayanan, R., & El-Sayed, M. A. (2005). Chemistry and properties of nanocrystals of different shapes. Chemical Reviews, 105, 1025–1102.
Katz, E., & Willner, I. (2004). Integrated nanoparticle–biomolecule hybrid systems: synthesis, properties, and applications. Angewandte Chemie, International Edition, 43, 6042–6108.
Pissuwan, D., Valenzuela, S. M., & Cortie, M. B. (2006). Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotech., 24, 62–67.
Kogan, M. J., Bastus, N. G., Amigo, R., Grillo-Bosch, D., Araya, E., Turiel, A., Labarta, A., Giralt, E., & Puntes, V. F. (2006). Nanoparticle-mediated local and remote manipulation of protein aggregation. Nano Letters, 6, 110–115.
Bond, G. C., Sermon, P. A., Webb, G., Buchanan, D. A., & Wells, P. B. J. (1973). Hydrogenation over supported gold catalysts. Chem. Soc. Chem. Commun., 444–445.
Haruta, M. (2005). Catalysis: gold rush. Nature, 437, 1098–1099.
Hutchings, G. J. (2005). Catalysis by gold. Catalysis Today, 100, 55–61.
Jain, P. K., Lee, K. S., Sayed, I. H. E., & Sayed, M. A. E. (2006). Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. The Journal of Physical Chemistry. B, 110, 7238–7248.
Corma, A., & Garcia, H. (2008). Supported gold nanoparticles as catalysts for organic reactions. Chemical Society Reviews, 37, 2096–2126.
Daniel, M. C., & Astruc, D. (2004). Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chemical Reviews, 104, 293–346.
Corma, A., Leyva-Perez, A., & Sabater, M. J. (2011). Gold-catalyzed carbon-heteroatom bond-forming reactions. Chemical Reviews, 111, 1657–1712.
Hashmi, A. S. K., & Hutchings, G. J. (2006). Gold catalysis. Angewandte Chemie, International Edition, 45, 7896–7936.
Xia, Y., Li, W., Cobley, C. M., Chen, J., Xia, X., Zhang, Q., Yang, M., Cho, E. C., & Brown, P. K. (2011). Gold nanocages: from synthesis to theranostic applications. Accounts of Chemical Research, 44, 914–924.
Huang, X., Jain, P. K., El-Sayed, I. H., & El Sayed, M. A. (2007). Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicines, 5, 681–693.
Fleischer, M., Bargioni, A. W., Altoe, M. V. P., Schwartzberg, A. M., Schuck, P. J., Cabrini, S., & Kern, D. P. (2011). Gold nanocone near-field scanning optical microscopy probes. ACS Nano, 5, 2570–2579.
Mohamed, A. A. (2011). Gold is going forensic. Gold Bulletin, 44, 71–77.
Jv, Y., Li, B., & Cao, R. (2010). Positively-charged gold nanoparticles as peroxidase mimic and their application in hydrogen peroxide and glucose detection. Chemical Communications, 46, 8017–8019.
Niidome, T., Nakashima, K., Takahashi, H., & Niidome, Y. (2004). Preparation of primary amine-modified gold nanoparticles and their transfection ability into cultivated cells. Chemical Communications, 1978–1979.
Han, T. H., Khan, M. M., Lee, J., & Cho, M. H. (2014). Optimization of positively charged gold nanoparticles synthesized using a stainless-steel mesh and its application for colorimetric hydrogen peroxide detection. Journal of Industrial and Engineering Chemistry, 20, 2003–2009.
Khan, M. M., Kalathil, S., Han, T. H., Lee, J., & Cho, M. H. (2013). Positively charged gold nanoparticles synthesized by electrochemically active biofilm—a biogenic approach. Journal of Nanoscience and Nanotechnology, 13, 6079–6085.
Khan, M. M., Kalathil, S., Lee, J., & Cho, M. H. (2012). Synthesis of cysteine capped silver nanoparticles by electrochemically active biofilm and their antibacterial activities. Bulletin of the Korean Chemical Society, 33, 2592–2596.
Khan, M. M., Ansari, S. A., Lee, J. H., Lee, J., & Cho, M. H. (2014). Mixed culture electrochemically active biofilms and their microscopic and spectroelectrochemical studies. ACS Sustainable Chemistry & Engineering, 2, 423–432.
Han, T. H., Khan, M. M., Kalathil, S., Lee, J., & Cho, M. H. (2013). Simultaneous enhancement of methylene blue degradation and power generation in a microbial fuel cell by gold nanoparticles. Industrial and Engineering Chemistry Research, 52, 8174–8181.
Zhu, T., Vasilev, K., Kreiter, M., Mittler, S., & Knoll, W. (2003). Surface modification of citrate-reduced colloidal gold nanoparticles with 2-mercaptosuccinic acid. Langmuir, 19, 9518–9525.
Liu, X., Atwater, M., Wang, J., & Huo, Q. (2007). Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids and Surfaces B: Biointerfaces, 58, 3–7.
Marichev, V. A. (2008). Kinetics of chloride ion adsorption on stainless alloys by in situ contact electric resistance technique. Electrochimica Acta, 53, 6304–6316.
McCafferty, E. (2010). Introduction to Corrosion Science. New York: Springer.
Das, S. K., Das, A. R., & Guha, A. K. (2009). Gold nanoparticles: microbial synthesis and application in water hygiene management. Langmuir, 25, 8192–8199.
Carregal-Romero, S., Pérez-Juste, J., Hervés, P., Liz-Marzán, L. M., & Mulvaney, P. (2010). Colloidal gold-catalyzed reduction of ferrocyanate (III) by borohydride ions: a model system for redox catalysis. Langmuir, 26, 1271–1277.
Mie, G. (1908). Mie theory. Ann. Phys., 25, 377–445.
Horvath, H. (2009). Gustav Mie and the scattering and absorption of light by particles: historic developments and basics. Journal of Quantitative Spectroscopy & Radiative Transfer, 110, 787–799.
Khan, M. M., Lee, J. H., Lee, J., & Cho, M. H. (2013). Electrochemically active biofilm mediated bio-hydrogen production catalyzed by positively charged gold nanoparticles. International Journal of Hydrogen Energy, 38, 5243–5250.
B. D. Cullity, S. R. Stock, Elements of X-ray diffraction, 3rd Ed. Prentice Hall, 2001.
Liu, Z., Zhao, B., Shi, Y., Guo, C., Yang, H., & Li, Z. (2010). Novel nonenzymatic hydrogen peroxide sensor based on iron oxide–silver hybrid sub microspheres. Talanta, 81, 1650–1654.
Palanisamy, S., Chen, S. M., & Sarawathi, R. (2012). A novel nonenzymatic hydrogen peroxide sensor based on reduced grapheme oxide/ZnO composite modified electrode. Sensors and Actuators, B: Chemical, 166–167, 372–377.
Khan, M. M., Ansari, S. A., Lee, J., & Cho, M. H. (2013). Novel Ag@TiO2 nanocomposite synthesized by electrochemically active biofilm for nonenzymatic hydrogen peroxide sensor. Materials Science and Engineering C, 33, 4692–4699.
Bai, H. P., Lu, X. X., Yang, G. M., & Yang, Y. H. (2008). Hydrogen peroxide biosensor based on electrodeposition of zinc oxide nanoflowers onto carbon nanotubes film electrode. Chinese Chemical Letters, 19, 314–318.
Acknowledgements
M. M. Khan is thankful to the Universiti Brunei Darussalam, Brunei Darussalam, for providing support to complete this article.
Funding
This study was supported by the 2017 Yeungnam University Research Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, M.M., Cho, M.H. Positively Charged Gold Nanoparticles for Hydrogen Peroxide Detection. BioNanoSci. 8, 537–543 (2018). https://doi.org/10.1007/s12668-018-0503-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0503-x