Abstract
Background
Neoadjuvant chemoradiotherapy (nCRT) stands as a pivotal therapeutic approach for locally advanced rectal cancer (LARC), yet the absence of a reliable biomarker to forecast its efficacy remains a challenge. Thus, this study aimed to assess whether the proteomic compositions of small extracellular vesicles (sEVs) might offer predictive insights into nCRT response among patients with LARC, while also delving into the proteomic alterations within sEVs post nCRT.
Methods
Plasma samples were obtained from LARC patients both pre- and post-nCRT. Plasma-derived sEVs were isolated utilizing the TIO2-based method, followed by LC-MS/MS-based proteomic analysis. Subsequently, pathway enrichment analysis was performed to the Differentially Expressed Proteins (DEPs). Additionally, ROC curves were generated to evaluate the predictive potential of sEV proteins in determining nCRT response. Public databases were interrogated to identify sEV protein-associated genes that are correlated with the response to nCRT in LARC.
Results
A total of 16 patients were enrolled. Among them, 8 patients achieved a pathological complete response (good responders, GR), while the remaining 8 did not achieve a complete response (poor responders, PR). Our analysis of pretreatment plasma-derived sEVs revealed 67 significantly up-regulated DEPs and 9 significantly down-regulated DEPs. Notably, PROC (AUC: 0.922), F7 (AUC: 0.953) and AZU1 (AUC: 0.906) demonstrated high AUC values and significant differences (P value < 0.05) in discriminating between GR and PR patients. Furthermore, a signature consisting of 5 sEV protein-associated genes (S100A6, ENO1, MIF, PRDX6 and MYL6) was capable of predicting the response to nCRT, yielding an AUC of 0.621(95% CI: 0.454–0.788). Besides, this 5-sEV protein-associated gene signature enabled stratification of patients into low- and high-risk group, with the low-risk group demonstrating a longer overall survival in the testing set (P = 0.048). Moreover, our investigation identified 11 significantly up-regulated DEPs and 31 significantly down-regulated DEPs when comparing pre- and post-nCRT proteomic profiles. GO analysis unveiled enrichment in the regulation of phospholipase A2 activity.
Conclusions
Differential expression of sEV proteins distinguishes between GR and PR patients and holds promise as predictive markers for nCRT response and prognosis in patients with LARC. Furthermore, our findings highlight substantial alterations in sEV protein composition following nCRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Colorectal cancer (CRC) ranks as the third most prevalent cancer type globally and is the second leading cause of cancer-related deaths [1]. Locally advanced rectal cancer (LARC), which encompass T3 and T4 tumors and/or tumors involving nearby lymph nodes within the rectum, account for approximately 15% of CRC cases [2]. Due to anatomical constraints and the imperative to preserve sphincter and nerve function, treating LARC presents a significant challenge in colorectal surgery [3]. Neoadjuvant chemoradiotherapy (nCRT) has emerged as the standard treatment for LARC to improve the resection rate of tumors and reduce the risk of local recurrence [4]. Studies have shown that LARC patients who achieve a pathological complete response (pCR) following nCRT experience significantly increased disease-free survival (DFS) and overall survival (OS) compared to those who do not achieve pCR [4]. Additionally, patients with low rectal cancer who exhibit clinical complete response (cCR) to nCRT can opt for watchful waiting, avoiding the complications associated with surgery while preserving the affected organ [5]. However, only about 15-27% of patients attain pCR with conventional radiation therapy doses [6]. In the era of personalized medicine, accurately predicting an individual’s response to nCRT is vital [7]. Unfortunately, there is currently no effective biomarker available to predict the efficacy of nCRT in LARC. Therefore, the identification of novel predictive biomarkers holds considerable importance in tailoring LARC treatment and enhancing patient outcomes.
Liquid biopsy refers to the utilization of biomarkers present in bodily fluids, primarily blood, for diagnostic and prognostic purposes [8]. While the technology behind liquid biopsies is still evolving, its non-invasive nature holds immense potential and ample opportunities for application, particularly in scenarios where tissue acquisition poses challenges [9, 10]. Notably, liquid biopsies are already employed to assess disease response and monitor relapse. The primary targets of liquid biopsy encompass circulating tumor DNA, circulating tumor cells, small extracellular vesicles (sEVs), circulating cell-free RNA, and tumor-educated platelets [10]. SEVs, which are released by living cells and contain various bioactive substances such as proteins, nucleic acids, lipids, and metabolites [11], exhibit greater stability and sufficient concentration in the bloodstream compared to circulating tumor DNA and circulating tumor cells [12]. These characteristics give sEVs significant advantages in liquid biopsy, making them promising tools for monitoring tumors and therapeutic responses. For instance, studies have shown that the levels of sEV PD-L1 correlate with tumor burden and treatment response in melanoma patients undergoing PD-1 checkpoint inhibitor therapy [13, 14]. Moreover, sEVs can act as carriers for intercellular communication and play a role in regulating tumor development and treatment sensitivity [15]. A notable example is the discovery that ALK protein present in sEVs can drive tumor growth and compromise the effectiveness of ALK inhibitors in ALK-positive non-small cell lung cancer [16].
In CRC, the nucleic acids and proteins present in sEVs have potential as biomarkers for diagnosis and monitoring [17]. Mutant KRAS and BRAF DNA were identified on the surface of the plasma-derived sEVs of CRC patients [18]. Besides, the mutational status of plasma sEV KRAS has been identified as a prognostic marker for metastatic CRC [19]. Additionally, a combination of tumor markers (EGFR, EpCAM, GPA33, and CD24) on sEVs has demonstrated a diagnostic accuracy of 98% for CRC [20]. Overall, profiling sEVs provides a feasible approach for minimally invasive liquid biopsies, and further analysis of sEV populations shows promise as a strategy to identify potential biomarkers for diagnosis, prognosis, treatment monitoring in CRC [17].
While Strybel et al. [21] have reported an association between molecular components of sEVs and the response of patients with LARC to nCRT, our understanding of the predictive capabilities of sEVs in the context of nCRT response for LARC remains considerably limited. In this study, we collected plasma-derived sEVs from LARC patients both pre- and post-nCRT, followed by LC-MS/MS-based proteomic analysis. sEVs were isolated utilizing TiO2 enrichment technology, which was based on the specific interaction between titanium oxide and the phosphate groups on the lipid bilayer of sEVs [22]. We aimed to determine whether these proteomic profiles of sEVs could serve as predictive markers for the response to nCRT in LARC patients and to investigate the proteomic alterations within sEVs post nCRT.
2 Methods
2.1 Plasma samples collection
Plasma samples were obtained from patients diagnosed with LARC at the Second Affiliated Hospital of Zhejiang University School of Medicine. Inclusion criteria comprised: (1) histological confirmation of rectal adenocarcinoma; (2) tumor staging as T3-T4, and/or lymph node involvement confirmed by MRI; (3) absence of distant metastasis; (4) no prior anti-tumor treatment; (5) undergoing nCRT followed by total mesorectal excision surgical resection. The treatment regimen involved intensity-modulated radiation therapy, with a total radiation dose of either 50 Gy (administered in daily fractions of 2.0 Gy) or 57.5 Gy (administered in daily fractions of 2.3 Gy), given 5 days a week over 5 weeks. Concurrently, chemotherapy was administered using capecitabine at a dose of 825 mg/m2 orally twice daily on radiation therapy days [23]. Plasma samples were collected on one day before commencement of nCRT and on one day after the completion of nCRT. Additionally, patients were categorized as either good responders (GR), defined by achieving pathological complete response (pCR), or poor responders (PR), indicating those without pCR. This project was approved by the Independent Ethics Committee of the Second Affiliated Hospital of Zhejiang University, and we got the informed consent from all patients.
2.2 Isolation of sEVs from plasma
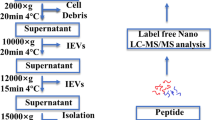
SEVs were extracted from plasma utilizing TiO2 enrichment technology [22, 24]. Initially, cells and dead cells of plasma were eliminated via centrifugation at 2000 g for 30 min, followed by a subsequent removal of cells debris through centrifugation at 12,000 g for 45 min. Subsequently, 200 µL of plasma was combined with 20 mg of TiO2 microspheres (Alson Scientific Instruments, Guangzhou, China) and incubated for 10 min on a shaker. Following this, exosomes adhering to the surface of the TiO2 microspheres were lysed using 40 µL of lysis buffer (composed of 2% SDS, EDTA-free protease inhibitor cocktail, and 0.1 M Tris-HCl, pH 7.5), followed by ultrasonication on ice for 20 min. The sEV proteins were then harvested through centrifugation at 20,000 g for 5 min. Besides, transmission electron microscopy (TEM) (Hitachi HT-7700, Tokyo, Japan) was used to confirm the morphology of sEVs, and sEV concentration and size were determined via Nano-Flow Cytometry device (NanoFCM Inc. N30E, China) [25].
2.3 Western blot (WB) analysis
WB was performed as depicted previously [25, 26]. The sEV protein samples underwent separation through 12% SDS-PAGE, followed by transfer onto a PVDF membrane (Bio-Rad, Hercules, CA, USA). Detection was carried out using primary antibody and peroxidase-conjugated secondary antibody (1:5000, Huabio, Hangzhou, China). Bands were then visualized utilizing enhanced chemiluminescence reagents (YEASEN, Shanghai, China) and captured by scanning with a Tanon 5200 Chemiluminescent Imaging System (Tanon, Shanghai, China). The primary antibodies used were Alix (1:1000, Cell Signaling Technology, Beverly, MA, USA), Calnexin (1:1000, Cell Signaling Technology, Beverly, MA, USA) and TSG101(1:1000, Cell Signaling Technology, Beverly, MA, USA).
2.4 LC-MS/MS-based proteomic analysis
SEV proteins were first treated with 5 mM dithiothreitol (DTT) to reduce disulfide bonds, followed by alkylation with 10 mM iodoacetamide (IAA) to prevent reformation of disulfide bonds. Subsequently, the proteins underwent digestion by trypsin at an enzyme/substrate ratio of 1:100, incubated overnight at 37 °C. The resulting peptides were then subjected to desalting and concentration using C18 StageTip. Fractionation of the peptide samples was performed using a homemade capillary column (75 μm i.d. × 12 cm; ReproSil-Pur C18-AQ, 3 μm) on a Q Exactive HF-X mass spectrometer (Thermo Fisher Scientific, San Jose, CA) equipped with an UltiMate 3000 high-pressure liquid chromatography (UHPLC) system (Thermo Fisher Scientific, San Jose, CA) operating in data independent acquisition (DIA) mode. For the generation of the spectral library, DIA raw data were analyzed using Spectronaut software and a mass spectrometer vendor-independent software from Biognosys. The DIA files were searched against the Swiss-Prot human database (20,353 entries released on Aug 10, 2020).
2.5 Publication search for reported CRC-derived sEVs
We conducted a comprehensive search of PubMed, MEDLINE, Web of Science, and Google Scholar for articles related to CRC and sEVs proteomic profiling. The final search was updated in May 2024, using the following search terms: (“colorectal cancer” or “colorectal carcinoma” or “colorectal tumor” or “colorectal neoplasm” or “colon tumor” or “rectal tumor” or “colon cancer” or “rectal cancer”) AND (“extracellular vesicles” or “vesicles” or “exosomes”) AND (“protein”) AND (“proteomics”). All eligible articles were screened by titles, abstracts, and full texts. The inclusion criteria were: (1) articles focusing on CRC and sEVs proteomic profiling; (2) containing proteomic data for sEVs; (3) providing a detailed list of the sEV proteomes. Subsequently, proteins identified in CRC cell-derived and fibroblast cell-derived sEVs from the selected studies were compiled and analyzed. Additionally, the quality of each study was independently assessed by two authors.
2.6 Small interfering (siRNA) transfection
We transfected human CRC cells (RKO) with siRNAs targeting S100A6, ENO1, MIF, PRDX6, and MYL6 using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to our previous studies [25,26,27].
The siRNA sequences were as follows: S100A6 siRNA-1,sense:5’-CAGGAUGCUGAAAUUGCAA-3’,anti-sense:5’-UUGCAAUUUCAGCAUCCUG-3’;S100A6 siRNA-2, sense:5’-GGCUUUGAUCUACAAUGAA-3’, anti-sense: 5’-UUCAUUGUAGAUCAAAGCC-3’; ENO1 siRNA-1, sense: 5’-GAGCAGAGGUUUACCACAA-3’, anti-sense: 5’-UUGUGGUAAACCUCUGCUC-3’; ENO1 siRNA-2, sense: 5’-CGAGAUGGAUGGAACAGAA-3’, anti-sense: 5’-UUCUGUUCCAUCCAUCUCG-3’; MIF siRNA-1, sense: 5’-CAACUCCACCUUCGCCUAA-3’, anti-sense: 5’-UUAGGCGAAGGUGGAGUUG-3’; MIF siRNA-2, sense: 5’-ACAUGAACGCGGCCAAUGU-3’, anti-sense: 5’-ACAUUGGCCGCGUUCAUGU-3’; PRDX6 siRNA-1, sense: 5’-CCUGGAGCAAGGAUAUCAA-3’, anti-sense: 5’-UUGAUAUCCUUGCUCCAGG-3’; PRDX6 siRNA-2, sense: 5’-GGAACUUUGAUGAGAUUCU-3’, anti-sense: 5’-AGAAUCUCAUCAAAGUUCC-3’; MYL6 siRNA-1, sense: 5’-GAGGCCACCUAUUGUUUCA-3’, anti-sense: 5’-UGAAACAAUAGGUGGCCUC-3’; and MYL6 siRNA-2, sense: 5’-UCUCCAAACCUGCUGCUAA-3’, anti-sense: 5’-UUAGCAGCAGGUUUGGAGA-3’.
2.7 Quantitative real-time PCR (qPCR)
The transfection efficiency of siRNAs was validated by qPCR. Total RNA from RKO cells was isolated using RNAiso reagent (Takara Biotechnology, Dalian, China). And qPCR was performed using a standard SYBR-Green PCR kit protocol (Takara Biotechnology) with the StepOne Plus Real Time PCR System (Life Technologies) [25,26,27].
The primers were synthesized by Sangon Biotech (Shanghai, China), and the sequences were as follows: S100A6 forward: 5′-CTCCCTACCGCTCCAAGC-3′, reverse: 5′- CACCTCCTGGTCCTTGTTCC-3′; ENO1 forward: 5′- CGGGAATCCCACTGTTGAGG-3′, reverse: 5′- CCATGGGCTGTGGGTTCTAA-3′; MIF forward: 5′-AGAACCGCTCCTACAGCAAG-3′, reverse: 5′- GCGAAGGTGGAGTTGTTCCA-3′; PRDX6 forward: 5′- GCTACCACTGGCAGGAACTT-3′, reverse: 5′- CAGGGATGGTTGGAAGGACC-3′; MYL6 forward: 5′- CAGGGATGGTTGGAAGGACC-3′, reverse: 5′- AAGGTCCTCAGCCATTCAGC-3′;GAPDH forward: 5′- AATGGGCAGCCGTTAGGAAA-3′, reverse: 5′-GCGCCCAATACGACCAAATC-3′. GAPDH was used as an internal control.
2.8 Clonogenic survival assay
Six hundred RKO cells pretreated with siRNAs were seeded in 12-well culture plates, followed by radiotherapy at a 6 Gy dose. The plates were then placed in a cell incubator with 5% CO2 for 9 days, with the medium being replaced every three days. After 9 days of culture, the medium was removed, and 0.5 mL of methanol was added to each well to fix the cells. The plates were placed on ice for 30 min. The fixed cells were then stained with crystal violet (Beyotime, China) for 20 min. The surviving fraction was normalized to that of the unirradiated control cells.
2.9 Functional analysis
Differentially expressed proteins (DEPs) were identified as proteins showing a fold change > 1.5 and a statistically significant difference (p < 0.05, Student’s t-test) using the “MSstats” R package between the two groups. Volcano plots, heatmaps, and partial least squares discriminant analysis (PLS-DA) were performed. For the DEPs, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment were annotated and visualized using the “clusterProfiler” R package. The pathway-level p-value was calculated using a hypergeometric test, and values with p < 0.05 were deemed significantly enriched.
2.10 Public database mining
Expression data for sEV protein-associated genes in LARC were sourced from both the Memorial Sloan Kettering Cancer Center (MSKCC) database [28] accessible via http://www.cbioportal.org and the Gene Expression Omnibus [29, 30] (GSE35452) available at http://www.ncbi.nlm.nih.gov/geo/. Access to these databases was granted in accordance with the freedom-to-publish criteria outlined by The Cancer Genome Atlas and the National Center for Biotechnology Information. Patients were randomly assigned to training and testing cohorts at a ratio of 7:3. The Lasso-Logistic regression analysis was employed to identify sEV protein-associated genes correlated with the response to nCRT in LARC. The LASSO algorithm utilized the “glmnet” R package for variable selection and contraction. In the regression model, the independent variable consisted of the normalized expression matrix of sEV protein-associated genes, while the response variable indicated whether patients achieved pCR following nCRT.
2.11 Statistical analysis
Receiver Operating Characteristic (ROC) curves were utilized to assess the specificity and sensitivity of DEPs, with the Area Under the ROC Curve (AUC) calculated for each DEP [31]. These curves and AUC values were generated through the “timeROC” package in R. Additionally, Student’s t-test was employed for comparisons between two groups using Prism 9.0.0. Statistical significance was established at a p-value of < 0.05. All analyses were two-sided, with 95% confidence intervals (CIs) employed.
3 Results
3.1 Isolation and proteomic analysis of plasma-derived sEVs in LARC
The procedure for conducting sEV proteomics from plasma samples is depicted in Fig. 1A. The study included a total of 16 patients diagnosed with LARC, of whom 8 patients achieved a pathological complete response (GR) and the remaining 8 did not achieve a complete response (PR). No statistically significant differences were observed in terms of age, gender, T stage, and N stage between the two groups (Fig. 1B), and the basic clinicopathological characteristics of these included patients were summarized in supplementary Table 1. Plasma samples were collected from these patients both before and after undergoing nCRT. Then, plasma-derived sEVs were isolated using the TIO2-based method and validated by TEM, WB and Nano-Flow. TEM results revealed round and cup-shaped vesicles with diameter ranging from 50 to 150 nm (supplementary Fig. 1A), correspond to size of sEVs. WB analysis demonstrated significant expression of traditional exosomal marker proteins, such as Alix and TSG101, while the intracellular protein contamination marker, calnexin, was absent (supplementary Fig. 1B). Additionally, Nano-Flow analysis showed that the mean size of the purified sEVs was 171.8 ± 11.76 nm (supplementary Fig. 1C). Taken together, these findings collectively indicate the successful isolation of sEVs from plasma samples obtained from patients with LARC.
(A) The methodology for performing sEV proteomics on plasma samples from patients diagnosed with LARC, categorized into GR and PR groups. (B) Comparative analysis of age, gender, T stage, and N stage across the GR and PR groups. (C) Heatmap depicting the expression of sEV proteins frequently found in human-derived sEVs
We next conducted LC-MS/MS-based proteomics of plasma-derived sEVs to identify potentially predictive biomarkers and investigate the proteomic alterations within sEVs post nCRT. Collectively, we quantified 6050, 6288, 6289, and 6359 peptides in sEV samples from GR and PR patients before and after nCRT (supplementary Fig. 2A). Then, a total of 705, 748, 736 and 752 proteins were identified in each respective group (supplementary Fig. 2B). The results for the coefficient of variation (CV%) were shown in supplementary Fig. 2B, illustrating favorable stability and consistency across the samples within each group. We compared the results of GR and PR patients before and after nCRT, and identified 827 sEV proteins that were common to all four groups, which was depicted in Venn diagram (supplementary Fig. 2C). To visualize the overall expression patterns among the groups, a heat map was created to visualize the overall expression patterns among the groups (supplementary Fig. 2D). Moreover, we detected 12 out of 13 (92.3%) sEV proteins (e.g., A2M, B2M, FLNA, FN1, GSN) that are reported by Ayuko et al. [32], which are expressed at high frequency in human-derived sEVs and likely markers of endocytosis/exocytosis (Fig. 1C).
3.2 Identification of differentially-expressed sEV proteins in LARC patients
To identify sEV proteins that could be used as predictive biomarkers for nCRT response in LARC, we first sought to identify DEPs by comparison between GR sEV proteomes and PR sEV proteomes before nCRT. PCA analysis revealed a distinction between GR and PR sEV proteins (Fig. 2A). In total, we identified 67 significantly up-regulated DEPs (e.g., TTC9C, ROA1, UFC1, IGHG2 and SUMO3) and 9 significantly down-regulated DEPs (e.g., Q8IUL9, KV224, GUC2A and CDN1A) (Fig. 2B) (supplementary Table 2). The expression of DEPs was displayed in a heatmap (Fig. 2C). KEGG pathway enrichment analysis demonstrated that DEPs were enriched in pathways including the riboflavin metabolism (ACP1 and BLVRB), malaria (TGFB1, THBS1 and HBB) and TGF-beta signaling pathway (TGFB1, THBS1 and LTBP1) (Fig. 2D). In addition, GO analysis of biological processes showed that DEPs were enriched in cellular process, single-organism process and biological regulation. GO analysis of cellular components revealed that most of the DEPs were associated with organelle, cell and cell part. At the molecular function level, DEPs exhibited enrichment in binding, catalytic activity and molecular function regulator (Fig. 2E).
(A) PCA analysis of sEV proteins from GR and PR groups before nCRT. (B) Significantly upregulated and downregulated DEPs in sEV proteins from GR and PR groups prior to nCRT. (C) Heatmap showing the expression levels of DEPs in the GR and PR groups before nCRT. (D-E) KEGG pathway and GO enrichment analysis of the identified DEPs. (F) DEPs including PROC (AUC: 0.922), F7 (AUC: 0.953), and AZU1 (AUC: 0.906) demonstrated statistically significant differences (P value < 0.05) and high AUC values, effectively distinguishing between GR and PR patients
We identified sEV proteins that were present in ≥ 50% of the GR samples and examined their baseline expression association with the likelihood of being a good responder. PROC (AUC: 0.922), F7 (AUC: 0.953), AZU1 (AUC: 0.906), ALB (AUC: 0.875), ENO1 (AUC: 0.828), APOC4 (AUC: 0.844), LCN2 (AUC: 0.875), CRNKL1 (AUC: 0.859), and HBB (AUC: 0.812) demonstrated statistically significant differences (P value < 0.05) and exhibited high AUC values in discriminating between GR and PR patients in the univariate logistic analysis (Fig. 2F) (supplementary Fig. 3). These findings collectively indicate that the expression of sEV proteins differs between GR and PR patients and can be used to distinguish between different response types.
3.3 SEV protein-associated genes predicted therapeutic response to nCRT in LARC
Plasma-derived SEVs originate from various sources, including tumor, normal tissues and elsewhere. We have identified 76 differentially-expressed sEV proteins between GR and PR patients before nCRT, and then we investigated whether these sEV proteins originated from the tumor. To identify the likely source of differentially-expressed sEV proteins, we compared our plasma-derived sEV proteins with reported CRC cell-derived [33,34,35,36,37] and fibroblast cell-derived [35] sEV proteomes based on the public database (supplementary Table 3). Interestingly, 30 proteins such as SH3BGRL, SOD1, CA2 were present in both plasma- and cancer cell-derived CRC sEVs, but were undetectable in all of the fibroblast cell-derived sEV samples, suggesting that these proteins most likely originate from CRC (supplementary Table 4). Other 46 proteins were absent in cancer cell-derived sEVs, suggesting that these sEV proteins might originate from other normal cells or distant organs. These results reinforce the idea that all the cells can secret sEVs and nCRT response is a systemic procedure that requires not only cancer but also normal organs’ participation.
To investigate the predictive role of cancer source-sEV protein-associated genes in determining radiosensitivity in CRC, we collected gene expression and clinical data of CRC from a public MSKCC database. Our objective was to assess the predictive value of these genes for response to nCRT. In the training cohort, we utilized lasso-logistic regression analysis to develop a signature consisting of 5 sEV protein-associated genes, including S100A6, ENO1, MIF, PRDX6 and MYL6 (Fig. 3A). The predictive performance of this signature was evaluated using ROC curves, yielding an AUC of 0.692 (95% CI: 0.535–0.848) in the training cohort (Fig. 3B), and 0.823 (95% CI: 0.628-1.000) in the testing cohort (Fig. 3C). Additionally, we validated the predictive capability of this signature in an external dataset (GSE35452), where the area under the ROC curve was found to be 0.621(95% CI: 0.454–0.788), indicating favorable predictive value (Fig. 3D). Concludely, sEV protein-associated genes can predict response to nCRT in LARC patients. In addition, based on this 5-sEV protein-associated gene signature, patients can be stratified into low- and high-risk group according to the optimal cut-off value selected by the ROC curve. The low-risk group had a longer OS in both training (P = 0.057) (Fig. 3E) and testing sets (P = 0.048) (Fig. 3F). Furthermore, the knockdown of MIF, ENO1, and S100A6 significantly improves the survival of human CRC cells after irradiation, as validated by the clonogenic survival assay (Fig. 3G) (supplementary Fig. 4). In conclusion, our findings suggest that sEV protein-associated genes can serve as predictors of response to chemoradiotherapy and prognosis in LARC patients.
(A) Establishment of the sEV protein-associated gene signature for predicting the response to nCRT. (B) The 5-sEV protein-associated gene signature achieved an AUC of 0.692 (95% CI: 0.535–0.848) in the training cohort of the MSKCC dataset. (C) The 5-sEV protein-associated gene signature achieved an AUC of 0.823 (95% CI: 0.628-1.000) in the testing cohort of the MSKCC dataset. (D) The 5-sEV protein-associated gene signature achieved an AUC of 0.621 (95% CI: 0.454–0.788) in an external dataset (GSE35452). (E-F) The 5-sEV protein-associated gene signature effectively stratified patients into low- and high-risk groups. The low-risk group exhibited longer overall survival (OS) in both the training (P = 0.057) and testing sets (P = 0.048). (G) Knockdown of PRDX6 and MYL6 did not significantly affect the survival of RKO cells after irradiation. However, knockdown of MIF (P < 0.0001 for siRNA 1 and P = 0.002 for siRNA 2), ENO1 (P = 0.04 for siRNA 1 and P = 0.01 for siRNA 2), and S100A6 (P = 0.0002 for siRNA 1 and P = 0.01 for siRNA 2) significantly improved the survival of RKO cells after irradiation, as assessed by the clonogenic survival assay
3.4 nCRT-induced alteration of sEV proteins in LARC patients
Afterward, we delved into the proteomic changes pre- and post-treatment among 16 patients diagnosed with LARC. We pinpointed 11 significantly up-regulated DEPs, such as IGLV3-22, RAB3IL1, and HPSE, alongside 31 significantly down-regulated DEPs, including IGKV1-9, IGHA2, and AHSP (Fig. 4A) (supplementary Table 5). GO analysis of molecular function unveiled enrichment in the regulation of phospholipase A2 (PLA2) activity, 1-acylglycerophosphocholine O-acyltransferase activity, and calcium-independent PLA2 activity among the DEPs (Fig. 4B).
(A) Identification of significantly upregulated and downregulated DEPs in altered sEV proteins after nCRT. (B) GO analysis of molecular functions associated with DEPs in altered sEV proteins after nCRT.(C) Analysis of significantly upregulated and downregulated DEPs in the GR group among altered sEV proteins before and after nCRT. (D) Analysis of significantly upregulated and downregulated DEPs in the PR group among altered sEV proteins before and after nCRT. (E) Venn diagram showing the overlap of altered sEV proteins after nCRT between GR and PR patients. (F) GO analysis of molecular functions for the unique DEPs identified in the GR group. (G) GO analysis of molecular functions for the unique DEPs identified in the PR group
Within the GR cohort, we identified 16 significantly down-regulated DEPs (e.g., Q5NV74, ADH4, and HPSE) and 23 significantly up-regulated DEPs (e.g., OLFL3, CYTB, and HV70D) (Fig. 4C) (supplementary Table 6). Conversely, in the PR cohort, we noted 19 significantly down-regulated DEPs (e.g., KI67, AEC1, and SBSN) and 89 significantly up-regulated DEPs (e.g., CPNE3, ROA1, and HNPRK) (Fig. 4D) (supplementary Table 7). Subsequently, a comparison of the GR-associated DEPs with the PR-associated DEPs revealed 13 common DEPs across both groups. Furthermore, 26 DEPs were exclusive to the GR group, while 95 DEPs were unique to the PR group (Fig. 4E). GO analysis of molecular function for the unique DEPs in the GR group unveiled significant enrichment in pathways such as protein self-association, extracellular matrix structural constituent, NADPH: quinone reductase activity, and alcohol dehydrogenase (NAD) activity (Fig. 4F). Additionally, GO analysis of molecular function highlighted pathways enriched in the unique DEPs within the PR group, including magnesium ion binding, arylesterase activity, phospholipase A2 activity, and lyase activity (Fig. 4G).
4 Discussion
Identifying predictive biomarkers is crucial for tailoring treatment and improving outcomes for patients with LARC. Previous studies have employed gene mutation [38], DNA methylation [39], transcriptomic or proteomic [40] profiling of human tissue samples to explore potential biomarkers linked to different responses to nCRT [41]. However, the invasive nature of obtaining rectal tissue samples limits their frequency and accessibility. Additionally, tissue profiling may not adequately capture the dynamic changes in biomarkers over time or reflect systemic responses to treatment. In contrast, plasma studies offer a less invasive method and provide a broader view of biomarkers in the circulatory system, potentially allowing for better monitoring of treatment responses. Thus, in this study, we used plasma samples from LARC patients before and after nCRT to identify predictive markers for treatment response.
SEVs are secreted by cells and released into various body fluids, including plasma, urine, and saliva. They carry specific molecular information inherited from their parent cells and possess advantages in stability and enrichment, making them promising cancer biomarkers for liquid biopsy [42].Techniques such as ultracentrifugation and field flow fractionation are used to isolate sEVs from plasma [43, 44]. However, ultracentrifugation usually requires large volumes of plasma, and field flow fractionation requires specialized equipment and is relatively low throughput [43, 44]. Therefore, in this study, we used a TiO2-based method to isolate sEVs. This method leverages the specific interaction between titanium oxide and the phosphate groups on the lipid bilayer of sEVs, which is reported to be stable and reliable s [22, 45].
While many studies on sEVs from human samples primarily focus on nucleic acids, it’s noteworthy that the proteome within sEVs is also enriched and emerging as a potential source of biomarkers. Park et al. [20] discovered that the expression of sEV proteins such as EGFR, EpCAM, CD24, and GPA33 could differentiate plasma samples from patients with CRC and non-CRC controls with accuracies exceeding 96%. Furthermore, the abundance of sEVs was predictive of five-year disease-free survival and strongly associated with tumor burden, showing a decrease after surgery and an increase upon relapse. The aforementioned findings indicate that sEVs have demonstrated excellent predictive capabilities in CRC. Additionally, mass spectrometry-based proteomic profiling is emerging as a strategy to gain insight into the biology and clinical potential of circulating sEVs [32]. However, whether they can forecast the efficacy of nCRT in LARC has not been reported in the literature thus far. Hence, our study aims to explore whether the proteomic profiles of sEVs could function as predictive markers for nCRT response in LARC patients and to investigate proteomic alterations within sEVs following nCRT.
We identified differential expression of sEV proteins before nCRT that distinguishes between patients categorized as GR and PR. Notably, PROC, F7, and AZU1 exhibited high AUC values (AUC > 0.9) and significant differences (P < 0.05). AZU1, functioning as a tumor suppressor, not only inhibits tumor cell proliferation but also facilitates tissue reorganization [46]. Interestingly, we found significantly higher levels of AZU1 in sEVs from GR patients compared to PR patients. The observed differential expression of these proteins in sEVs can serve as a valuable tool in distinguishing between GR and PR patients in LARC. Furthermore, considering that plasma-derived sEVs originate from tissues, and tumors are particularly prone to releasing exosomes on a large scale, sEV-associated genes were found to be dysregulated in tumor tissues among patients with varying response rates. For example, wu et al. [47] utilized tumor-derived sEV-associated genes to establish a TEXscore for predicting prognosis across various cancer types and in patients undergoing immunotherapy. They found that TEXscore serves as a robust biomarker for prognosis and treatment responses in independent cohorts.
Therefore, we investigated the expression of cancer-derived sEV protein-associated genes in LARC tissues. Our analysis identified a signature of five sEV protein-associated genes (S100A6, ENO1, MIF, PRDX6, and MYL6) that can predict responses to nCRT and contribute to OS predictions. Regarding the underlying mechanisms, ENO1 has been reported to reduce reactive oxygen species and apoptosis by modulating mitochondrial homeostasis, which affects radiotherapy sensitivity [48]. Additionally, MIF, secreted by cancer cells, regulates microglia M1 polarization and impacts radiosensitivity [49]. Our in vitro studies further demonstrated that knockdown of MIF, ENO1, and S100A6 significantly improves the survival of human CRC cells post-irradiation, suggesting these genes directly influence radiosensitivity and thus might predict nCRT response. However, the response to radiotherapy is influenced by the complex interplay between cancer cells and their microenvironment, which is intricate and multifactorial. Therefore, the underlying mechanisms of this 5-gene signature warrant further investigation. In summary, sEVs hold substantial promise as predictive markers for nCRT response and prognosis in LARC patients.
In addition to their predictive role in patients with LARC, we also investigated the proteomic changes following nCRT. It has been demonstrated that radiation can alter the cargo of sEVs. We identified 11 significantly up-regulated DEPs alongside 31 significantly down-regulated DEPs after nCRT. GO analysis of molecular function revealed enrichment in the regulation of PLA2 activity. Currently, PLA2 enzymes have emerged as targets in cancer therapy, with elevated PLA2 activities detected in plasma from patients with CRC, exceeding those of healthy controls. Moreover, PLA2 levels were found to be correlated with CRC tumor stage [50]. Additionally, radiation can induce alterations in PLA2 activity, which can mediate critical biological processes such as inflammation, senescence, and apoptosis [51]. Our data demonstrate that radiation-induced alterations in PLA2 activity might be associated with radiotherapy-related toxicity. Furthermore, when stratified by tumor response, GO analysis of unique DEPs within the PR group showed enrichment in magnesium ion binding. Magnesium is an essential cofactor in almost all enzymatic systems involved in DNA processing and is highly required to maintain genomic stability. Apart from its stabilizing effect on DNA and chromatin structure, magnesium is involved in the removal of DNA damage and is required for repairing double-strand breaks arising after radiotherapy [52]. The alterations in the proteome of sEVs in the PR group might be associated with resistance to radiotherapy. However, as pointed out by Jelonek et al., there is a significant gap in understanding how radiation-induced changes in the composition of sEVs translate into their functional importance [53]. Further investigation is needed to elucidate how altered sEVs affect the response to nCRT.
Our study utilized human plasma sEVs to identify potential biomarkers for nCRT response in patients with LARC. However, several limitations must be acknowledged. Firstly, we employed a TiO2-based sEV isolation methodology, and additional technologies will be required to further minimize contaminants in the plasma. Secondly, the sample size is not large enough, limiting the validation and further exploration of the role of biomarkers in relation to other clinicopathological characteristics and prognosis. Lastly, the detailed roles of the predictive biomarker proteins in sEVs require further investigation, and the underlying mechanisms associated with response to nCRT in LARC should be further elucidated and experimentally validated.
In conclusion, differential expression of sEV proteins distinguishes between GR and PR patients and shows promise as predictive markers for nCRT response and prognosis in LARC patients. Furthermore, our findings underscore significant alterations in sEV protein composition following nCRT. However, it is important to note that further studies are necessary to validate and verify these sEV proteins in larger clinical sample sets, and to explore their underlying functions and mechanisms.
Data availability
No datasets were generated or analysed during the current study.
References
R.L. Siegel, K.D. Miller, N.S. Wagle, A. Jemal, Cancer Statistics, 2023. CA. Cancer J. Clin. 73(1), 17–48 (2023). https://doi.org/10.3322/caac.21763
K. He, L. Li, A. Li, Y. Xu, J. Pang, D. Mu, J. Ma, H. Ge, A. Maleki, X. Qin, X. Zhang, Q. Ou, Y. Shao, J. Yu, S. Yuan, Genomic features, evolutionary patterns and minimal residual disease at Surgical margins as novel Prognostic/Predictive biomarkers in locally advanced rectal Cancer. Clin. Transl Med. 13(6), e1286 (2023). https://doi.org/10.1002/ctm2.1286
B.Y. Oh, Advances in surgery for locally advanced rectal Cancer. Ann. Coloproctology. 38(4), 279–280 (2022). https://doi.org/10.3393/ac.2022.00493.0070
W.H. Zwart, A. Hotca, G.A.P. Hospers, K.A. Goodman, J. Garcia-Aguilar, The Multimodal Management of locally advanced rectal Cancer: making sense of the New Data. Am. Soc. Clin. Oncol. Educ. Book. No. 42, 264–277 (2022). https://doi.org/10.1200/EDBK_351411
High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S1470204515001205. Accessed 16 June 2023
T. Millard, P.R. Kunk, E. Ramsdale, O.E. Rahma, Current debate in the oncologic management of rectal Cancer. World J. Gastrointest. Oncol. 8(10), 715–724 (2016). https://doi.org/10.4251/wjgo.v8.i10.715
C. Koulis, R. Yap, R. Engel, T. Jardé, S. Wilkins, G. Solon, J.D. Shapiro, H. Abud, P. McMurrick, Personalized medicine—current and emerging predictive and prognostic biomarkers in Colorectal Cancer. Cancers. 12(4), 812 (2020). https://doi.org/10.3390/cancers12040812
A. Bardelli, K. Pantel, Liquid Biopsies, What we do not know (yet). Cancer Cell. 31(2), 172–179 (2017). https://doi.org/10.1016/j.ccell.2017.01.002
M. Ignatiadis, G.W. Sledge, S.S. Jeffrey, Liquid Biopsy enters the clinic — implementation issues and Future challenges. Nat. Rev. Clin. Oncol. 18(5), 297–312 (2021). https://doi.org/10.1038/s41571-020-00457-x
C. Alix-Panabières, K. Pantel, Liquid Biopsy: from Discovery to Clinical Application. Cancer Discov. 11(4), 858–873 (2021). https://doi.org/10.1158/2159-8290.CD-20-1311
G. Wang, J. Li, L. Bojmar, H. Chen, Z. Li, G.C. Tobias, M. Hu, E.A. Homan, S. Lucotti, F. Zhao, V. Posada, P.R. Oxley, M. Cioffi, H.S. Kim, H. Wang, P. Lauritzen, N. Boudreau, Z. Shi, C.E. Burd, J.H. Zippin, J.C. Lo, G.S. Pitt, J. Hernandez, C.P. Zambirinis, M.A. Hollingsworth, P.M. Grandgenett, M. Jain, S.K. Batra, D.J. DiMaio, J.L. Grem, K.A. Klute, T.M. Trippett, M. Egeblad, D. Paul, J. Bromberg, D. Kelsen, V.K. Rajasekhar, J.H. Healey, I.R. Matei, W.R. Jarnagin, R.E. Schwartz, H. Zhang, Lyden, D. Tumour Extracellular vesicles and particles induce liver metabolic dysfunction. Nature. 618(7964), 374–382 (2023). https://doi.org/10.1038/s41586-023-06114-4
D. Yu, Y. Li, M. Wang, J. Gu, W. Xu, H. Cai, X. Fang, X. Zhang, Exosomes as a New Frontier of Cancer Liquid Biopsy. Mol. Cancer. 21(1), 56 (2022). https://doi.org/10.1186/s12943-022-01509-9
S.M. Morrissey, J. Yan, Exosomal, PD-L1: roles in Tumor Progression and Immunotherapy. Trends Cancer. 6(7), 550–558 (2020). https://doi.org/10.1016/j.trecan.2020.03.002
S. Serratì, M. Guida, Di R. Fonte, De S. Summa, S. Strippoli, R.M. Iacobazzi, A. Quarta, De I. Risi, G. Guida, A. Paradiso, L. Porcelli, A. Azzariti, Circulating Extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma. Mol. Cancer. 21(1), 20 (2022). https://doi.org/10.1186/s12943-021-01490-9
S.N. Lone, S. Nisar, T. Masoodi, M. Singh, A. Rizwan, S. Hashem, W. El-Rifai, D. Bedognetti, S.K. Batra, M. Haris, A.A. Bhat, M.A. Macha, Liquid biopsy: a step closer to transform diagnosis, prognosis and future of Cancer treatments. Mol. Cancer. 21(1), 79 (2022). https://doi.org/10.1186/s12943-022-01543-7
H. Wu, C. Zeng, Y. Ye, J. Liu, Z. Mu, Y. Xie, B. Chen, Q. Nong, D. Wu, Exosomes from irradiated Nonsmall Cell Lung Cancer cells reduced sensitivity of recipient cells to anaplastic lymphoma kinase inhibitors. Mol. Pharm. 15(5), 1892–1900 (2018). https://doi.org/10.1021/acs.molpharmaceut.8b00059
S.E. Glass, R.J. Coffey, Recent advances in the study of Extracellular vesicles in Colorectal Cancer. Gastroenterology. 163(5), 1188–1197 (2022). https://doi.org/10.1053/j.gastro.2022.06.039
S. Galbiati, F. Damin, D. Brambilla, L. Ferraro, N. Soriani, A.M. Ferretti, V. Burgio, M. Ronzoni, R. Vago, L. Sola, M. Chiari, Small EVs-Associated DNA as complementary biomarker to circulating Tumor DNA in plasma of metastatic colorectal Cancer patients. Pharm. Basel Switz. 14(2), 128 (2021). https://doi.org/10.3390/ph14020128
D. Lucchetti, I.V. Zurlo, F. Colella, C. Ricciardi-Tenore, Di M. Salvatore, G. Tortora, De R. Maria, F. Giuliante, A. Cassano, M. Basso, A. Crucitti, I. Laurenzana, G. Artemi, A. Sgambato, Mutational status of plasma Exosomal KRAS predicts outcome in patients with metastatic colorectal Cancer. Sci. Rep. 11(1), 22686 (2021). https://doi.org/10.1038/s41598-021-01668-7
J. Park, J.S. Park, C.-H. Huang, A. Jo, K. Cook, R. Wang, H.-Y. Lin, Van J. Deun, H. Li, J. Min, L. Wang, G. Yoon, B.S. Carter, L. Balaj, G.-S. Choi, C.M. Castro, R. Weissleder, H. Lee, An Integrated Magneto-Electrochemical device for the Rapid profiling of Tumour Extracellular vesicles from blood plasma. Nat. Biomed. Eng. 5(7), 678–689 (2021). https://doi.org/10.1038/s41551-021-00752-7
U. Strybel, L. Marczak, M. Zeman, K. Polanski, Ł. Mielańczyk, O. Klymenko, A. Samelak-Czajka, P. Jackowiak, M. Smolarz, M. Chekan, E. Zembala-Nożyńska, P. Widlak, M. Pietrowska, A. Wojakowska, Molecular composition of serum exosomes could discriminate rectal Cancer patients with different responses to Neoadjuvant Radiotherapy. Cancers. 14(4), 993 (2022). https://doi.org/10.3390/cancers14040993
F. Gao, F. Jiao, C. Xia, Y. Zhao, W. Ying, Y. Xie, X. Guan, M. Tao, Y. Zhang, W. Qin, X. Qian, A novel strategy for facile serum exosome isolation based on specific interactions between Phospholipid Bilayers and TiO2. Chem. Sci. 10(6), 1579 (2019). https://doi.org/10.1039/c8sc04197k
C. Li, H. Chen, B. Zhang, Y. Fang, W. Sun, D. Wu, Z. Su, L. Shen, Q. Wei, Radiomics signature based on support Vector machines for the prediction of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in locally advanced rectal Cancer. Cancers. 15(21), 5134 (2023). https://doi.org/10.3390/cancers15215134
L. Zhao, J. Shi, L. Chang, Y. Wang, S. Liu, Y. Li, T. Zhang, T. Zuo, B. Fu, G. Wang, Y. Ruan, Y. Zhang, P. Xu, Serum-derived exosomal proteins as potential candidate biomarkers for Hepatocellular Carcinoma. ACS Omega. 6(1), 827–835 (2021). https://doi.org/10.1021/acsomega.0c05408
K. Jiang, H. Chen, Y. Fang, L. Chen, C. Zhong, T. Bu, S. Dai, X. Pan, D. Fu, Y. Qian, J. Wei, K. Ding, Exosomal ANGPTL1 attenuates colorectal Cancer Liver Metastasis by regulating Kupffer Cell Secretion Pattern and Impeding MMP9 Induced Vascular Leakiness. J. Exp. Clin. Cancer Res. 40(1), 21 (2021). https://doi.org/10.1186/s13046-020-01816-3
H. Chen, Q. Xiao, Y. Hu, L. Chen, K. Jiang, Y. Tang, Y. Tan, W. Hu, Z. Wang, J. He, Y. Liu, Y. Cai, Q. Yang, K. Ding, ANGPTL1 attenuates Colorectal Cancer Metastasis by Up-Regulating microRNA-138. J. Exp. Clin. Cancer Res. 36(1), 78 (2017). https://doi.org/10.1186/s13046-017-0548-7
H. Chen, L. Chen, X. Wang, X. Ge, L. Sun, Z. Wang, X. Xu, Y. Song, J. Chen, Q. Deng, H. Xie, T. Chen, Y. Chen, K. Ding, J. Wu, J. Wang, Transgenic overexpression of ITGB6 in intestinal epithelial cells exacerbates Dextran Sulfate Sodium-Induced Colitis in mice. J. Cell. Mol. Med. 25(5), 2679–2690 (2021). https://doi.org/10.1111/jcmm.16297
W.K. Chatila, J.K. Kim, H. Walch, M.R. Marco, C.-T. Chen, F. Wu, D.M. Omer, D.N. Khalil, K. Ganesh, X. Qu, A. Luthra, S.-H. Choi, Y.-J. Ho, R. Kundra, K.I. Groves, O.S. Chow, A. Cercek, M.R. Weiser, M. Widmar, I.H. Wei, E.P. Pappou, G.M. Nash, P.B. Paty, Q. Shi, E. Vakiani, S. Duygu Selcuklu, M.T.A. Donoghue, D.B. Solit, M.F. Berger, J. Shia, R. Pelossof, P.B. Romesser, R. Yaeger, J.J. Smith, N. Schultz, F. Sanchez-Vega, J. Garcia-Aguilar, Genomic and transcriptomic determinants of response to neoadjuvant therapy in rectal Cancer. Nat. Med. 28(8), 1646–1655 (2022). https://doi.org/10.1038/s41591-022-01930-z
J. Gao, B.A. Aksoy, U. Dogrusoz, G. Dresdner, B. Gross, S.O. Sumer, Y. Sun, A. Jacobsen, R. Sinha, E. Larsson, E. Cerami, C. Sander, N. Schultz, Integrative Analysis of Complex Cancer Genomics and Clinical profiles using the cBioPortal. Sci. Signal. 6(269), pl1 (2013). https://doi.org/10.1126/scisignal.2004088
E. Cerami, J. Gao, U. Dogrusoz, B.E. Gross, S.O. Sumer, B.A. Aksoy, A. Jacobsen, C.J. Byrne, M.L. Heuer, E. Larsson, Y. Antipin, B. Reva, A.P. Goldberg, C. Sander, N. Schultz, The cBio Cancer Genomics Portal: an Open platform for exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2(5), 401–404 (2012). https://doi.org/10.1158/2159-8290.CD-12-0095
H. Chen, C. Li, L. Zheng, W. Lu, Y. Li, Q.A. Wei, Machine learning-based survival prediction model of high Grade Glioma by Integration of Clinical and dose-volume histogram parameters. Cancer Med. 10(8), 2774–2786 (2021). https://doi.org/10.1002/cam4.3838
A. Hoshino, H.S. Kim, L. Bojmar, K.E. Gyan, M. Cioffi, J. Hernandez, C.P. Zambirinis, G. Rodrigues, H. Molina, S. Heissel, M.T. Mark, L. Steiner, A. Benito-Martin, S. Lucotti, Di A. Giannatale, K. Offer, M. Nakajima, C. Williams, L. Nogués, F.A. Pelissier Vatter, A. Hashimoto, A.E. Davies, D. Freitas, C.M. Kenific, Y. Ararso, W. Buehring, P. Lauritzen, Y. Ogitani, K. Sugiura, N. Takahashi, M. Alečković, K.A. Bailey, J.S. Jolissant, H. Wang, A. Harris, L.M. Schaeffer, G. García-Santos, Z. Posner, V.P. Balachandran, Y. Khakoo, G.P. Raju, A. Scherz, I. Sagi, R. Scherz-Shouval, Y. Yarden, M. Oren, M. Malladi, M. Petriccione, De K.C. Braganca, M. Donzelli, C. Fischer, S. Vitolano, G.P. Wright, L. Ganshaw, M. Marrano, A. Ahmed, J. DeStefano, E. Danzer, M.H.A. Roehrl, N.J. Lacayo, T.C. Vincent, M.R. Weiser, M.S. Brady, P.A. Meyers, L.H. Wexler, S.R. Ambati, A.J. Chou, E.K. Slotkin, S. Modak, S.S. Roberts, E.M. Basu, D. Diolaiti, B.A. Krantz, F. Cardoso, A.L. Simpson, M. Berger, C.M. Rudin, D.M. Simeone, M. Jain, C.M. Ghajar, S.K. Batra, B.Z. Stanger, J. Bui, K.A. Brown, V.K. Rajasekhar, J.H. Healey, de M. Sousa, K. Kramer, S. Sheth, J. Baisch, V. Pascual, T.E. Heaton, La M.P. Quaglia, D.J. Pisapia, R. Schwartz, H. Zhang, Y. Liu, A. Shukla, L. Blavier, Y.A. DeClerck, M. LaBarge, M.J. Bissell, T.C. Caffrey, P.M. Grandgenett, M.A. Hollingsworth, J. Bromberg, B. Costa-Silva, H. Peinado, Y. Kang, B.A. Garcia, E.M. O’Reilly, D. Kelsen, T.M. Trippett, Jones, D. R.; Matei, I. R.; Jarnagin, W. R.; Lyden, D. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell. 182(4), 1044–1061.e18 (2020). https://doi.org/10.1016/j.cell.2020.07.009
D.-S. Choi, J.-S. Yang, E.-J. Choi, S.C. Jang, S. Park, O.Y. Kim, D. Hwang, K.P. Kim, Y.-K. Kim, S. Kim, Y.S. Gho, The Protein Interaction Network of Extracellular vesicles derived from human colorectal Cancer cells. J. Proteome Res. 11(2), 1144–1151 (2012). https://doi.org/10.1021/pr200842h
B.J. Tauro, D.W. Greening, R.A. Mathias, S. Mathivanan, H. Ji, R.J. Simpson, Two distinct populations of Exosomes are released from LIM1863 Colon Carcinoma Cell-Derived organoids *. Mol. Cell. Proteom. 12(3), 587–598 (2013). https://doi.org/10.1074/mcp.M112.021303
C.-H. Lee, E.-J. Im, P.-G. Moon, M.-C. Baek, Discovery of a diagnostic biomarker for Colon cancer through proteomic profiling of small extracellular vesicles. BMC Cancer. 18(1), 1058 (2018). https://doi.org/10.1186/s12885-018-4952-y
W. Suwakulsiri, A. Rai, R. Xu, M. Chen, D.W. Greening, R.J. Simpson, Proteomic profiling reveals Key Cancer Progression modulators in Shed Microvesicles released from Isogenic Human Primary and metastatic colorectal Cancer cell lines. Biochim. Biophys. Acta BBA - Proteins Proteom. 1867(12), 140171 (2019). https://doi.org/10.1016/j.bbapap.2018.11.008
A. Montero-Calle, I. Aranguren-Abeigon, M. Garranzo-Asensio, C. Poves, M.J. Fernández-Aceñero, J. Martínez-Useros, R. Sanz, J. Dziaková, J. Rodriguez-Cobos, G. Solís-Fernández, E. Povedano, M. Gamella, R.M. Torrente-Rodríguez, M. Alonso-Navarro, de los V. Ríos, J.I. Casal, G. Domínguez, A. Guzman-Aranguez, A. Peláez-García, J.M. Pingarrón, S. Campuzano, R. Barderas, Multiplexed Biosensing Diagnostic platforms detecting autoantibodies to Tumor-Associated antigens from Exosomes released by CRC Cells and Tissue Samples showed high diagnostic ability for Colorectal Cancer. Engineering. 7(10), 1393–1412 (2021). https://doi.org/10.1016/j.eng.2021.04.026
M.P. Duldulao, W. Lee, R.A. Nelson, W. Li, Z. Chen, J. Kim, J. Garcia-Aguilar, Mutations in specific codons of the KRAS Oncogene Are Associated with Variable Resistance to Neoadjuvant Chemoradiation Therapy in patients with rectal adenocarcinoma. Ann. Surg. Oncol. 20(7), 2166–2171 (2013). https://doi.org/10.1245/s10434-013-2910-0
Y.J. Ha, C.W. Kim, S.A. Roh, D.H. Cho, J.L. Park, S.Y. Kim, J.H. Kim, E.K. Choi, Y.S. Kim, J.C. Kim, Epigenetic regulation of KLHL34 predictive of pathologic response to preoperative chemoradiation therapy in rectal Cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 91(3), 650–658 (2015). https://doi.org/10.1016/j.ijrobp.2014.11.013
A. Stanojevic, M. Samiotaki, V. Lygirou, M. Marinkovic, V. Nikolic, S. Stojanovic-Rundic, R. Jankovic, A. Vlahou, G. Panayotou, R.J.A. Fijneman, S. Castellví-Bel, J. Zoidakis, M. Cavic, Data-Independent Acquisition Mass Spectrometry Analysis of FFPE rectal Cancer samples offers In-Depth proteomics characterization of the response to Neoadjuvant Chemoradiotherapy. Int. J. Mol. Sci. 24(20), 15412 (2023). https://doi.org/10.3390/ijms242015412
E. Smolskas, G. Mikulskytė, E. Sileika, K. Suziedelis, A. Dulskas, Tissue-based markers as a Tool to assess response to Neoadjuvant Radiotherapy in rectal Cancer—systematic review. Int. J. Mol. Sci. 23(11), 6040 (2022). https://doi.org/10.3390/ijms23116040
Z. Zhang, X. Liu, X. Yang, Y. Jiang, A. Li, J. Cong, Y. Li, Q. Xie, C. Xu, D. Liu, Identification of Faecal Extracellular vesicles as novel biomarkers for the non-invasive diagnosis and prognosis of Colorectal Cancer. J. Extracell. Vesicles. 12(1), 12300 (2023). https://doi.org/10.1002/jev2.12300
K. Takov, D.M. Yellon, S.M. Davidson, Comparison of small extracellular vesicles isolated from plasma by Ultracentrifugation or size-exclusion chromatography: yield, purity and functional potential. J. Extracell. Vesicles. 8(1), 1560809 (2019). https://doi.org/10.1080/20013078.2018.1560809
Q. Zhang, D.K. Jeppesen, J.N. Higginbotham, J.L. Franklin, R.J. Coffey, Comprehensive isolation of Extracellular vesicles and nanoparticles. Nat. Protoc. 18(5), 1462–1487 (2023). https://doi.org/10.1038/s41596-023-00811-0
Y. Pang, J. Shi, X. Yang, C. Wang, Z. Sun, R. Xiao, Personalized detection of circling exosomal PD-L1 based on Fe3O4@TiO2 isolation and SERS Immunoassay. Biosens. Bioelectron. 148, 111800 (2020). https://doi.org/10.1016/j.bios.2019.111800
AZU-1:, A Candidate Breast Tumor Suppressor and Biomarker for Tumor Progression| Molecular Biology of the Cell. https://www.molbiolcell.org/doi/full/. Accessed 02 April 2024
J. Wu, D. Zeng, S. Zhi, Z. Ye, W. Qiu, N. Huang, L. Sun, C. Wang, Z. Wu, J. Bin, Y. Liao, M. Shi, W. Liao, Single-cell analysis of a Tumor-Derived Exosome signature correlates with prognosis and immunotherapy response. J. Transl Med. 19(1), 381 (2021). https://doi.org/10.1186/s12967-021-03053-4
J. Ma, J. Zhu, J. Li, J. Liu, X. Kang, J. Yu, Enhanced E6AP-Mediated ubiquitination of ENO1 via LINC00663 contributes to radiosensitivity of breast Cancer by regulating mitochondrial homeostasis. Cancer Lett. 560, 216118 (2023). https://doi.org/10.1016/j.canlet.2023.216118
L. Liu, J. Wang, Y. Wang, L. Chen, L. Peng, Y. Bin, P. Ding, R. Zhang, F. Tong, X. Dong, Blocking the MIF-CD74 Axis augments Radiotherapy Efficacy for Brain Metastasis in NSCLC via synergistically promoting Microglia M1 polarization. J. Exp. Clin. Cancer Res. 43(1), 128 (2024). https://doi.org/10.1186/s13046-024-03024-9
H. Cai, E.G. Chiorean, M.V. Chiorean, D.K. Rex, B.W. Robb, N.M. Hahn, Z. Liu, P.J. Loehrer, M.L. Harrison, Y. Xu, Elevated phospholipase A2 activities in plasma samples from multiple cancers. PLOS ONE. 8(2), e57081 (2013). https://doi.org/10.1371/journal.pone.0057081
S.-R. Kim, J.-I. Heo, J.-W. Park, C.-M. Kang, K.S. Kim, Radiation-Induced Lipoprotein-Associated phospholipase A2 increases Lysophosphatidylcholine and induces endothelial cell damage. Toxicology. 458, 152841 (2021). https://doi.org/10.1016/j.tox.2021.152841
Role of magnesium in genomic stability - ScienceDirect. https://www.sciencedirect.com/science/article/abs/pii/S0027510701000744. Accessed 02 April 2024
K. Jelonek, P. Widlak, M. Pietrowska, The influence of Ionizing Radiation on Exosome Composition, Secretion and Intercellular Communication. Protein Pept. Lett. 23(7), 656–663 (2016). https://doi.org/10.2174/0929866523666160427105138
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 82103498, No.82072624, No.82203704), CSCO-Roche research funding (Y-Roche2019/2–0088) and Key R&D Program of Zhejiang (2023C03049).
Author information
Authors and Affiliations
Contributions
H.C., Y.F. and K.D. wrote the main manuscript text. S.D., L.S. and J.Z. collected the samples from patients. K.H. and X.Z. participated in analyzing data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This project was approved by the Independent Ethics Committee of the Second Affiliated Hospital of Zhejiang University, and we got the informed consent from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
. (A) TEM images displaying round and cup-shaped sEVs with diameters ranging from 50 to 150 nm. Sample 1 and sample 2 are plasma-derived sEVs from patients with LARC. (B) Western Blot analysis for the sEV proteins Alix, TSG101, and intracellular protein contamination marker calnexin. Sample 1, sample 2, sample 3 and sample 4 are plasma-derived sEVs from patients with LARC. Control 1 and control 2 are positive loading controls for antibodies. (C) Nano-flow cytometry results for sEVs. Sample 1 and sample 2 are plasma-derived sEVs from patients with LARC.
Supplementary Figure 2
. (A) Peptide profiles in sEV samples from GR and PR patients before and after nCRT. (B) Protein profiles in sEV samples from GR and PR patients before and after nCRT. (C) Venn diagram illustrating the overlap of sEV proteins in GR and PR patients before and after nCRT. (D) Heatmap displaying the overall expression patterns of sEV proteins across these four groups.
Supplementary Figure 3
. ROC analysis for ALB (A), ENO1 (B), APOC4 (C), LCN2 (D), CRNKL1 (E), and HBB (F) demonstrated their effectiveness in differentiating between GR and PR patients.
13402_2024_983_MOESM4_ESM.tif
Supplementary Figure 4. (A) The transfection efficiency of siRNAs targeting S100A6, ENO1, MIF, PRDX6, and MYL6 was validated by qPCR. (B) The effects of knocking down S100A6, ENO1, MIF, PRDX6, and MYL6 on cancer cell survival after irradiation were assessed using the clonogenic survival assay
Supplementary Table 1
. Clinicopathological characteristics of included LARC patients
Supplementary Table 2
. List of DEPs by comparison between GR sEV proteomes and PR sEV proteomes before nCRT
Supplementary Table 3
. List of studies investigating the CRC cell-derived and fibroblast cell-derived sEV proteomes
Supplementary Table 4
. List of DEPs present in both plasma- and cancer cell-derived CRC sEVs
Supplementary Table 5
. List of DEPs by comparison between sEV proteomes before and after nCRT
Supplementary Table 6
. List of DEPs by comparison between GR sEV proteomes before and after nCRT
Supplementary Table 7
. List of DEPs by comparison between PR sEV proteomes before and after nCRT
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, H., Fang, Y., Dai, S. et al. Characterization and proteomic analysis of plasma-derived small extracellular vesicles in locally advanced rectal cancer patients. Cell Oncol. (2024). https://doi.org/10.1007/s13402-024-00983-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s13402-024-00983-1